Introduction
Chile is rapidly emerging as a pivotal hub for clinical research, underpinned by a robust healthcare infrastructure that combines advanced medical facilities with a skilled workforce. The country's unique blend of public and private health services allows for a diverse patient demographic, essential for conducting high-quality clinical trials. With leading hospitals and research institutions equipped with cutting-edge technology, Chile not only facilitates the execution of trials but also enhances the credibility of the research outcomes.
This article delves into the various facets that make Chile an attractive destination for clinical studies, including its supportive regulatory environment, collaborative international networks, and the commitment to ethical standards, all of which contribute to advancing medical innovations and improving global health.
The Robust Healthcare Infrastructure Supporting Clinical Research in Chile
Chile's healthcare infrastructure is robust, featuring advanced medical facilities, a well-trained healthcare workforce, and a comprehensive healthcare delivery system. The nation provides a combination of public and private health services, ensuring that medical studies can reach a varied array of patient demographics and health conditions. Significantly, top hospitals and research facilities are equipped with cutting-edge technology, promoting an atmosphere favorable to high-quality medical investigations. This framework not only facilitates the implementation of experiments but also boosts the [reliability of research results](https://www.oecd.org/en/publications/oecd-reviews-of-public-health-chile_9789264309593-en.html), establishing Chile as a favored site for carrying out medical investigations.
With bioaccess®’s expertise in comprehensive research management services—including:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
Chile is well-positioned to advance medical device evaluations efficiently. For instance, bioaccess® has successfully facilitated experiments that have led to significant advancements in medical technologies. Their focus on international collaboration drives innovation in Medtech, ultimately contributing to improved global health outcomes.

The Robust Healthcare Infrastructure Supporting Clinical Research in Chile
Chile's healthcare infrastructure is robust, featuring advanced medical facilities, a well-trained healthcare workforce, and a comprehensive healthcare delivery system. The nation provides a combination of public and private health services, ensuring that medical studies can reach a varied array of patient demographics and health conditions. Significantly, top hospitals and research facilities are equipped with cutting-edge technology, promoting an atmosphere favorable to high-quality medical investigations. This framework not only facilitates the implementation of experiments but also boosts the [reliability of research results](https://www.oecd.org/en/publications/oecd-reviews-of-public-health-chile_9789264309593-en.html), establishing Chile as a favored site for carrying out medical investigations.
With bioaccess®’s expertise in comprehensive research management services—including:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
Chile is well-positioned to advance medical device evaluations efficiently. For instance, bioaccess® has successfully facilitated experiments that have led to significant advancements in medical technologies. Their focus on international collaboration drives innovation in Medtech, ultimately contributing to improved global health outcomes.


Key Advantages of Conducting Clinical Trials in Chile
Conducting clinical studies in Colombia offers several advantages that make it an appealing choice for researchers and sponsors. Firstly, the cost-effectiveness of carrying out experiments in Colombia is notable, with lower operational costs compared to many other countries. Secondly, the [diverse population](https://www.linkedin.com/posts/juliomartinezclark_how-chile-is-shaping-medical-device-clinical-activity-7251705851202146305-Z7C-) offers access to various patient pools, facilitating recruitment for research across different demographics. Additionally, Colombia’s regulatory framework supports expedited approval processes, allowing studies to commence more swiftly. The collaborative spirit within the research community enhances the study experience, as investigators and sponsors work together seamlessly to achieve study objectives.
Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a prominent location for research studies in Latin America, backed by Colombia's Minister of Health. Moreover, GlobalCare Clinical Studies' partnership with bioaccess™ emphasizes the improvement of ambulatory services for research in Colombia, achieving over a 50% decrease in recruitment time and a 95% retention rate.
Together, these factors create a flourishing atmosphere for medical device studies, with extensive study management services including:
- Feasibility evaluations
- Site selection
- Compliance assessments
- Setup
- Import permits
- Project management
- Reporting
These services enhance the overall effect on local economies through job creation and healthcare enhancement.

Key Advantages of Conducting Clinical Trials in Chile
Conducting clinical studies in Colombia offers several advantages that make it an appealing choice for researchers and sponsors. Firstly, the cost-effectiveness of carrying out experiments in Colombia is notable, with lower operational costs compared to many other countries. Secondly, the [diverse population](https://www.linkedin.com/posts/juliomartinezclark_how-chile-is-shaping-medical-device-clinical-activity-7251705851202146305-Z7C-) offers access to various patient pools, facilitating recruitment for research across different demographics. Additionally, Colombia’s regulatory framework supports expedited approval processes, allowing studies to commence more swiftly. The collaborative spirit within the research community enhances the study experience, as investigators and sponsors work together seamlessly to achieve study objectives.
Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a prominent location for research studies in Latin America, backed by Colombia's Minister of Health. Moreover, GlobalCare Clinical Studies' partnership with bioaccess™ emphasizes the improvement of ambulatory services for research in Colombia, achieving over a 50% decrease in recruitment time and a 95% retention rate.
Together, these factors create a flourishing atmosphere for medical device studies, with extensive study management services including:
- Feasibility evaluations
- Site selection
- Compliance assessments
- Setup
- Import permits
- Project management
- Reporting
These services enhance the overall effect on local economies through job creation and healthcare enhancement.


Supportive Regulatory Environment for Clinical Research
Chile has created a supportive regulatory framework that promotes medical research. The Chilean regulatory body, Instituto de Salud Publica (ISP), has simplified procedures for research approvals, significantly diminishing bureaucratic delays. For example, the typical approval duration for clinical experiments has dropped by more than 30% in recent years, enabling researchers to commence investigations more swiftly. Additionally, the ISP emphasizes adherence to international standards, ensuring that studies conducted in Chile meet global requirements. This dedication to regulatory excellence not only guarantees patient safety but also boosts the credibility of the research results, making Chile an appealing location for medical device studies.
The proactive engagement between regulatory bodies and researchers further supports a favorable environment for medical innovation. At bioaccess®, we utilize our knowledge in extensive research management services—such as:
- Feasibility assessments
- Site choice
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
to enable expedited medical device research services in Latin America. Our expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up investigations positions us as leaders in Medtech trials, driving innovation and regulatory excellence. By selecting bioaccess®, you guarantee a collaboration dedicated to navigating the complexities of trials in a thriving regulatory landscape.

Supportive Regulatory Environment for Clinical Research
Chile has created a supportive regulatory framework that promotes medical research. The Chilean regulatory body, Instituto de Salud Publica (ISP), has simplified procedures for research approvals, significantly diminishing bureaucratic delays. For example, the typical approval duration for clinical experiments has dropped by more than 30% in recent years, enabling researchers to commence investigations more swiftly. Additionally, the ISP emphasizes adherence to international standards, ensuring that studies conducted in Chile meet global requirements. This dedication to regulatory excellence not only guarantees patient safety but also boosts the credibility of the research results, making Chile an appealing location for medical device studies.
The proactive engagement between regulatory bodies and researchers further supports a favorable environment for medical innovation. At bioaccess®, we utilize our knowledge in extensive research management services—such as:
- Feasibility assessments
- Site choice
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
to enable expedited medical device research services in Latin America. Our expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up investigations positions us as leaders in Medtech trials, driving innovation and regulatory excellence. By selecting bioaccess®, you guarantee a collaboration dedicated to navigating the complexities of trials in a thriving regulatory landscape.


Collaboration with International Research Networks
Chile's medical environment gains significantly from partnerships with global networks, like those created by bioaccess® for expedited medical device trial services. These partnerships enable local researchers to share knowledge, access advanced technologies like telemedicine platforms and data analytics tools, and incorporate best practices from around the world.
Joint initiatives frequently result in multi-center projects that broaden the diversity and range of [clinical investigations](https://www.researchgate.net/publication/361436239_Mapping_Chilean_clinical_research_a_protocol_for_a_scoping_review_and_multiple_evidence_gap_maps) carried out in Chile. Moreover, these global connections can draw funding and investment, enhancing the local academic community and promoting economic growth, job creation, and healthcare enhancement.
By actively participating in global research initiatives, such as the recent multi-national examination on cardiovascular devices, Chile is positioning itself as a key player in the medical device sector, ultimately contributing to better global health outcomes.

Collaboration with International Research Networks
Chile's medical environment gains significantly from partnerships with global networks, like those created by bioaccess® for expedited medical device trial services. These partnerships enable local researchers to share knowledge, access advanced technologies like telemedicine platforms and data analytics tools, and incorporate best practices from around the world.
Joint initiatives frequently result in multi-center projects that broaden the diversity and range of [clinical investigations](https://www.researchgate.net/publication/361436239_Mapping_Chilean_clinical_research_a_protocol_for_a_scoping_review_and_multiple_evidence_gap_maps) carried out in Chile. Moreover, these global connections can draw funding and investment, enhancing the local academic community and promoting economic growth, job creation, and healthcare enhancement.
By actively participating in global research initiatives, such as the recent multi-national examination on cardiovascular devices, Chile is positioning itself as a key player in the medical device sector, ultimately contributing to better global health outcomes.


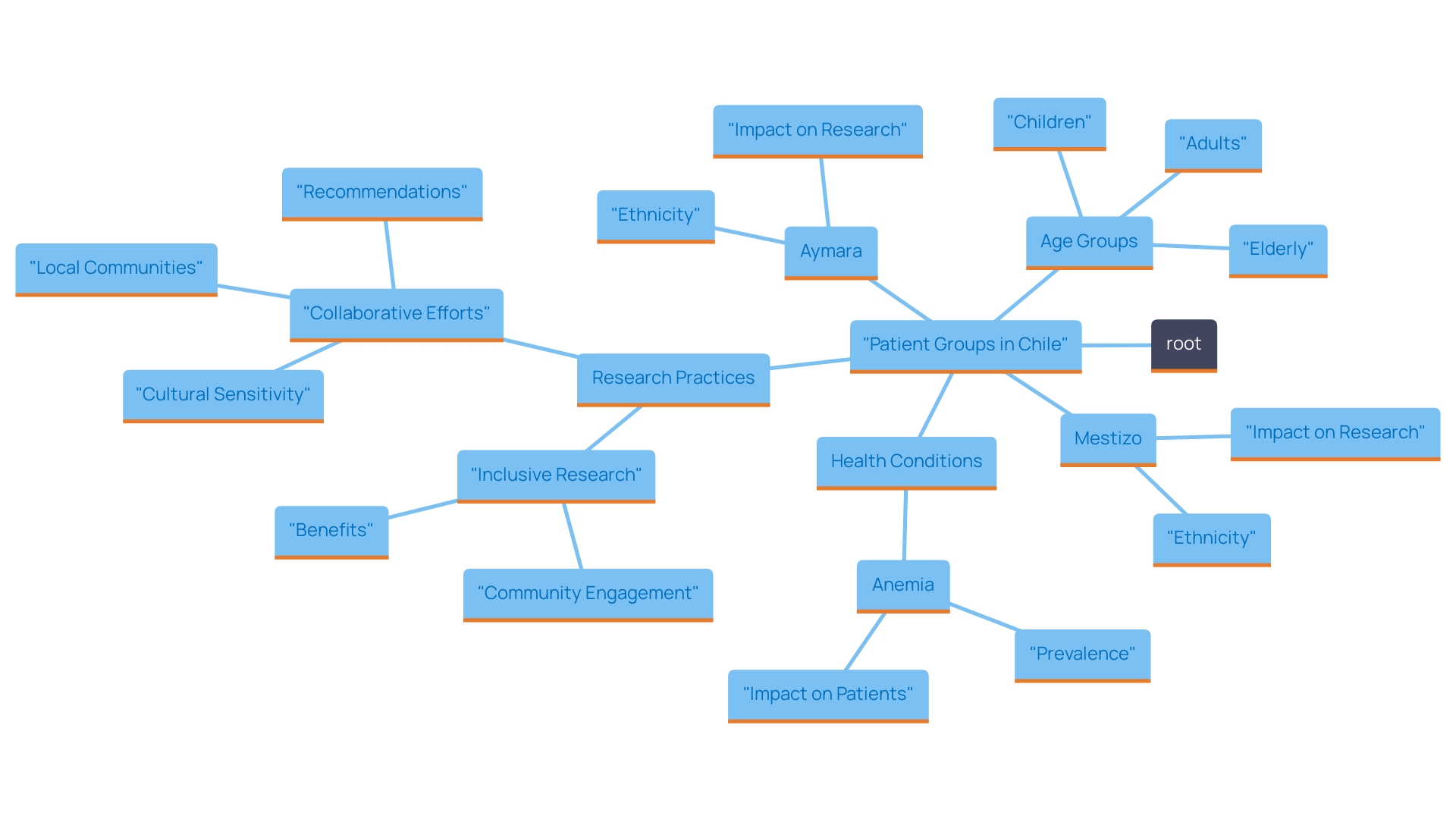
Diverse Patient Populations Enhancing Clinical Trials
The diverse patient groups in Chile play a pivotal role in enhancing the quality and relevance of research trials. This diversity enables researchers to investigate the effects of medical devices and treatments across a spectrum of ethnicities, age groups, and health backgrounds, leading to findings that are more comprehensive and applicable to various demographics.
For instance, recent studies reveal that children from ethnic minorities, particularly Aymara children, exhibit a notable prevalence rate of 1.38 for anemia compared to their mestizo counterparts. Such statistics highlight the necessity of including diverse populations in medical research, as they present unique health challenges and conditions that can inform tailored research efforts.
As Wenting Wang, Global Head of Biomarker Statistics at Sanofi, states, "Involving varied populations in research studies is crucial for producing insights that genuinely represent our global society."
Moreover, the varied health conditions prevalent in the Chilean population allow for an in-depth exploration of specific medical needs. This not only enhances the representativeness of studies but also ensures that the findings are pertinent and advantageous to a broader audience.
By actively engaging with diverse patient populations, trials in Chile—supported by comprehensive trial management services like those offered by bioaccess®, including feasibility studies, site selection, compliance reviews, and project management—are positioned to yield insights that significantly improve outcomes, elevate patient care, and create positive ripples in local economies through job creation and healthcare advancements.
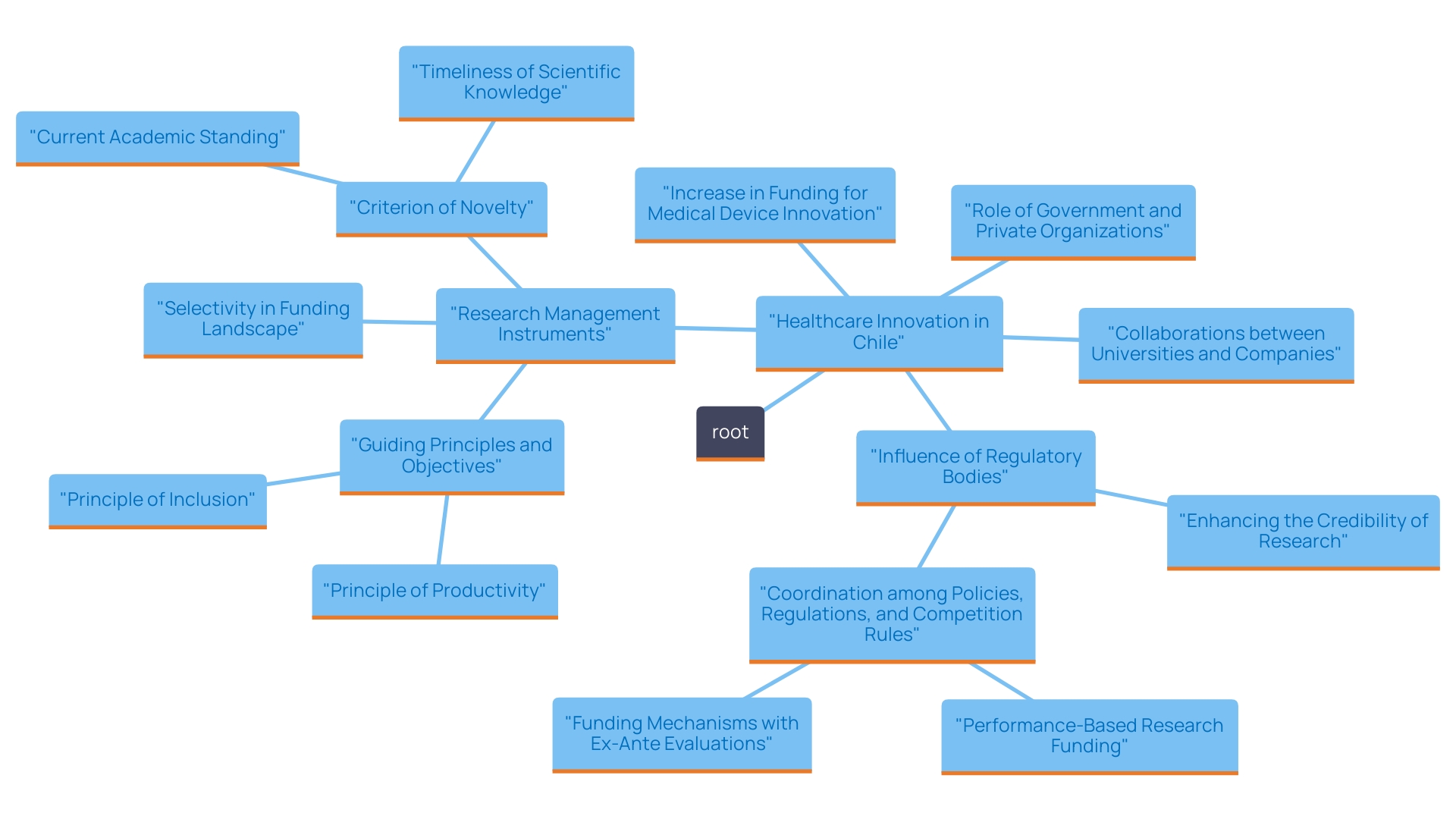
Investment in Research and Development
Chile has witnessed a significant increase in investment in innovation and development, especially in the medical device sector. Both government and private organizations are increasingly acknowledging the essential role that funding research plays in driving innovation and improving healthcare outcomes. Notable collaborations between local universities and international medical device companies have attracted significant venture capital investment, with reports indicating a 30% increase in funding over the past year. This integration of grants, partnerships, and venture capital is becoming more prevalent, empowering researchers to undertake ambitious projects that might have previously been viewed as too risky. This influx of funding is crucial for promoting research studies, establishing a supportive atmosphere for the efficient creation and evaluation of innovative medical devices.
Moreover, the local Ministry of Health, together with the Food and Drug Administration and European Medicines Agency inspections, has confirmed the high standard of Chilean research studies. This validation strengthens the reliability of these medical studies and increases the appeal of Chile as a location for health investigations, thereby promoting additional investments in this field.
At bioaccess®, we provide thorough trial management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Our knowledge in early feasibility, first-in-human, pilot, pivotal, and post-market follow-up trials positions us as a leading CRO in Latin America, fostering trusted partnerships in medical technology and scientific exploration.
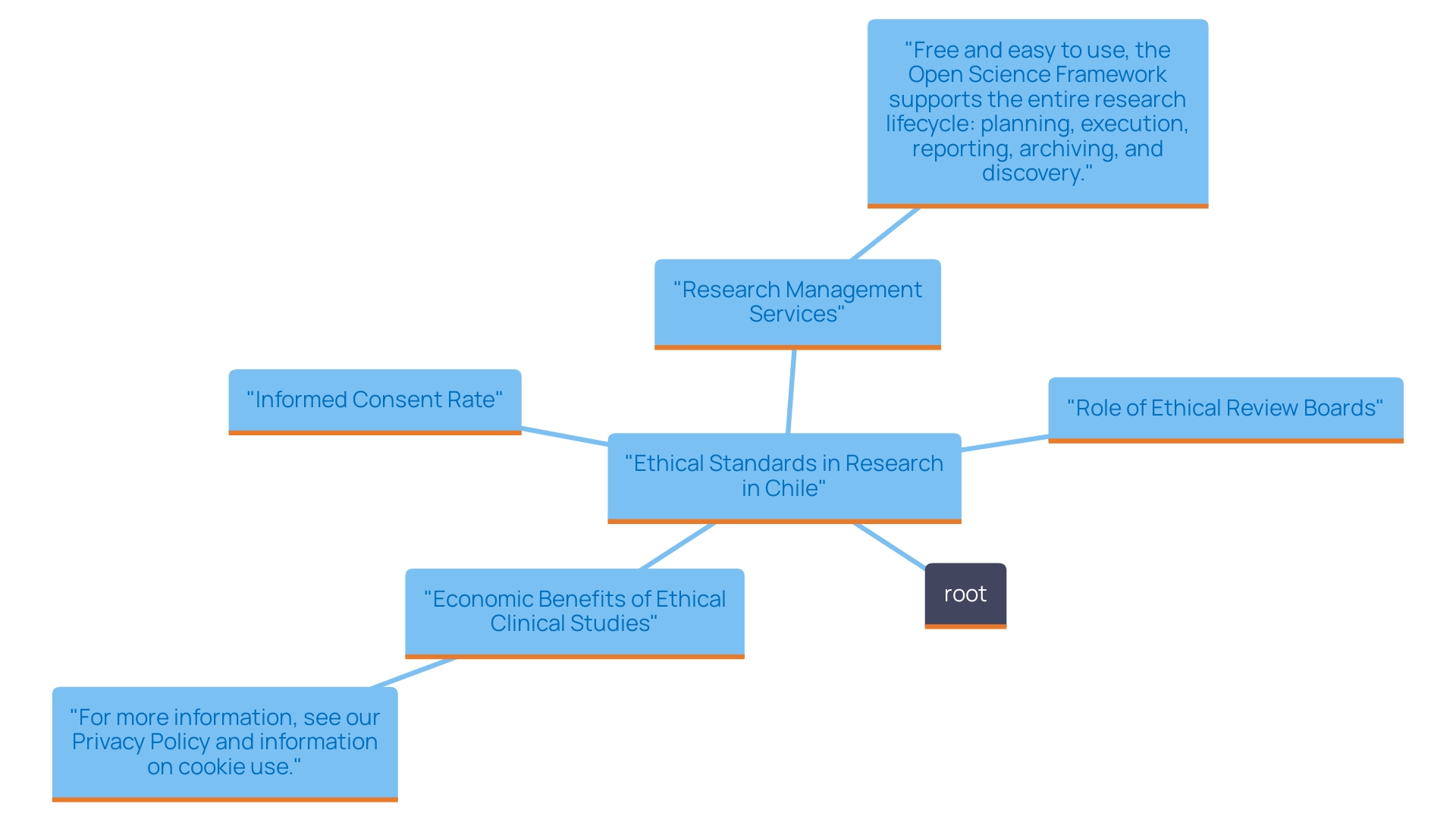
Commitment to Ethical Standards in Clinical Research
Chile shows a strong dedication to upholding high ethical standards in research, essential for protecting the rights and safety of participants. Our extensive research management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
According to recent statistics, the informed consent rate in research studies in Chile for 2024 stands at 85%, reflecting a significant effort to ensure that participants are adequately informed about the studies they are entering. Ethical review boards (ERBs) play a crucial role in this landscape, overseeing the approval process for clinical studies and ensuring adherence to established ethical guidelines, such as those outlined in the World Medical Association's Declaration of Helsinki. This framework emphasizes the importance of informed consent, requiring researchers to provide participants with comprehensive information regarding the associated risks and benefits of their involvement in trials.
Moreover, as Annette Rid, a noted expert in the field, articulates, 'We develop a three-step ethical framework to guide policy-makers in dealing with key ethical considerations involved in navigating this trade-off.' This underscores the continuous endeavor to balance ethical imperatives with practical study needs.
The commitment to ethical practices not only safeguards participants but also boosts the credibility of findings, nurturing a reliable atmosphere crucial for progressing in medical trials. Moreover, when we and our Medtech partners conduct medical studies in the countries where we operate, the effect on the local economy generates ripples with extensive advantages, including:
- Job creation
- Economic growth
- Healthcare enhancement
- Increased innovation
- Achieving international recognition
By prioritizing ethical standards and actively engaging ERBs in the review process, Chile positions itself as a leader in responsible clinical research, ensuring that the welfare of participants remains at the forefront of scientific inquiry.
Conclusion
Chile's emergence as a leading hub for clinical research is underscored by its robust healthcare infrastructure, supportive regulatory environment, and commitment to ethical standards. The combination of advanced medical facilities and a skilled workforce allows for the execution of high-quality clinical trials, attracting both local and international researchers. The diverse patient populations available in Chile further enhance the relevance and applicability of research findings, ensuring that outcomes are beneficial across various demographics.
The country's proactive regulatory framework has significantly streamlined the approval process for clinical trials, reducing bureaucratic delays and fostering an environment conducive to rapid innovation. Collaborative efforts with international research networks not only enhance the scope of studies but also facilitate knowledge exchange and resource sharing, positioning Chile as a key player in global healthcare advancements.
Investment in research and development, particularly within the medical device sector, has seen a notable increase, driven by both government support and private sector initiatives. This influx of funding empowers researchers to pursue ambitious projects, ultimately leading to advancements in medical technology and improved health outcomes.
In summary, Chile stands out as a preferred destination for clinical trials, bolstered by its commitment to ethical practices and the establishment of a trustworthy research environment. As the nation continues to invest in and prioritize clinical research, it will undoubtedly play a crucial role in advancing global health and medical innovation.




