Overview
This article examines best practices and expert insights for designing trials of innovative medical devices, underscoring the critical importance of methodological rigor, regulatory compliance, and the seamless integration of technology. By detailing various strategies—including the use of randomized controlled trials (RCTs), adaptive study designs, and patient-centric approaches—it enhances the efficiency, safety, and effectiveness of clinical research within the medical device sector. Through these insights, the article aims to provide a comprehensive understanding of the Medtech landscape and highlight the role of collaboration in overcoming key challenges in clinical research.
Introduction
In the rapidly evolving landscape of medical device trials, the significance of effective trial design is paramount. Organizations are compelled to navigate intricate regulatory environments and technological advancements, prompting a shift towards robust methodologies that guarantee both compliance and scientific rigor.
The integration of adaptive trial designs and the strategic application of artificial intelligence in patient recruitment exemplify how innovation is reshaping the future of clinical trials, all aimed at enhancing patient outcomes.
This article examines the essential components of trial design, investigates the critical role of early feasibility studies, and underscores best practices for participant engagement.
Ultimately, it provides a comprehensive overview of how the medical device industry is preparing for success in 2025 and beyond.
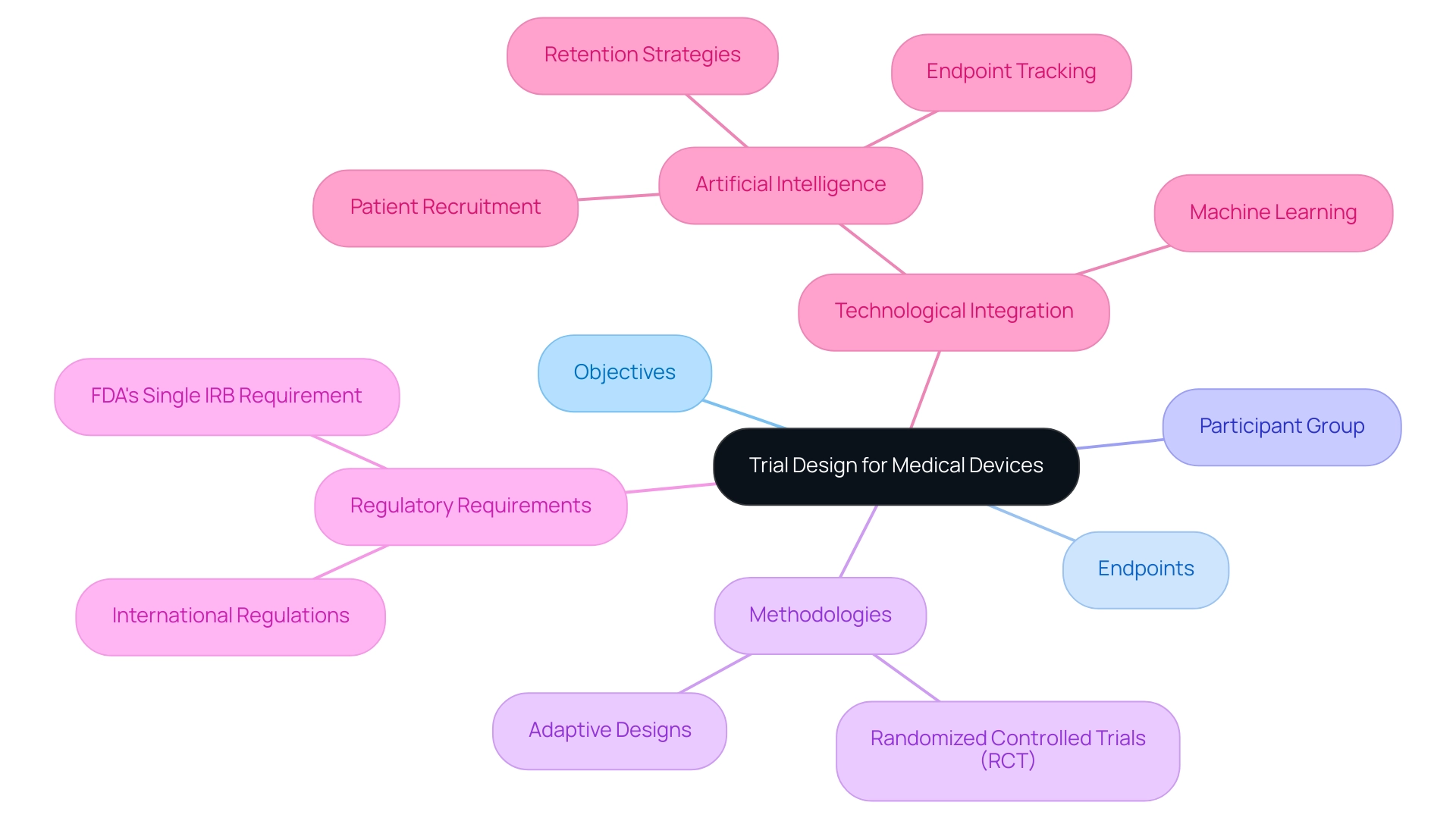
Fundamentals of Trial Design for Medical Devices
The trial design for innovative medical devices encompasses several critical components, including the establishment of clear objectives, the selection of suitable endpoints, and the identification of the participant group. A robust methodology is essential, aligning with both regulatory requirements and scientific rigor. For instance, employing a randomized controlled study (RCT) design is instrumental in minimizing bias and bolstering the validity of results.
Recent advancements underscore that RCTs remain a cornerstone in medical device research, particularly as they facilitate rigorous comparisons between interventions.
In 2025, the integration of adaptive study designs is gaining traction, providing the flexibility to respond to interim findings. This adaptability can lead to modifications that enhance learning efficiency and outcomes, ultimately accelerating the path to commercialization. Furthermore, the integration of artificial intelligence and machine learning in medical studies is revolutionizing patient recruitment and retention strategies. Research indicates that these technologies can shorten study timelines by up to 30% and reduce expenses by as much as 20%.
Notably, 45% of Alcon's data is entered on the same day as the visit date, exemplifying a significant advancement in data management efficiency.
bioaccess® specializes in comprehensive clinical study management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, their expertise ensures that protocol documentation and strict adherence to Good Clinical Practice (GCP) guidelines are upheld, which is crucial for ensuring compliance and preserving the integrity of the research process. As the FDA's Single IRB Requirement is anticipated to be implemented in 2025, it underscores the necessity for organizations to prepare for evolving regulatory landscapes.
This preparedness is echoed by industry leaders, including Dipanwita Das, who emphasizes that "regulations are getting more complex and more prescriptive and more demanding," highlighting the importance of staying current with international regulations to facilitate a smooth commercialization process.
Insights from the case study titled "2025 Trends in Clinical Studies" reveal that the industry faces challenges such as high costs and long timelines. However, technological advancements are expected to optimize study design and patient recruitment. Das forecasts substantial investments in data strategy, patient diversity, and regulatory readiness, underscoring the necessity for organizations to strengthen their data infrastructure and foster connections with underserved communities to enhance accessibility and effectiveness.
In summary, best practices for trial design for innovative medical devices in 2025 revolve around a commitment to methodological rigor, regulatory compliance, and the strategic use of technology to optimize study outcomes. By concentrating on these aspects, organizations like bioaccess® can improve the effectiveness and accessibility of clinical studies in Latin America, ultimately benefiting both the industry and patients.
The Role of Early Feasibility and Pilot Studies in Device Development
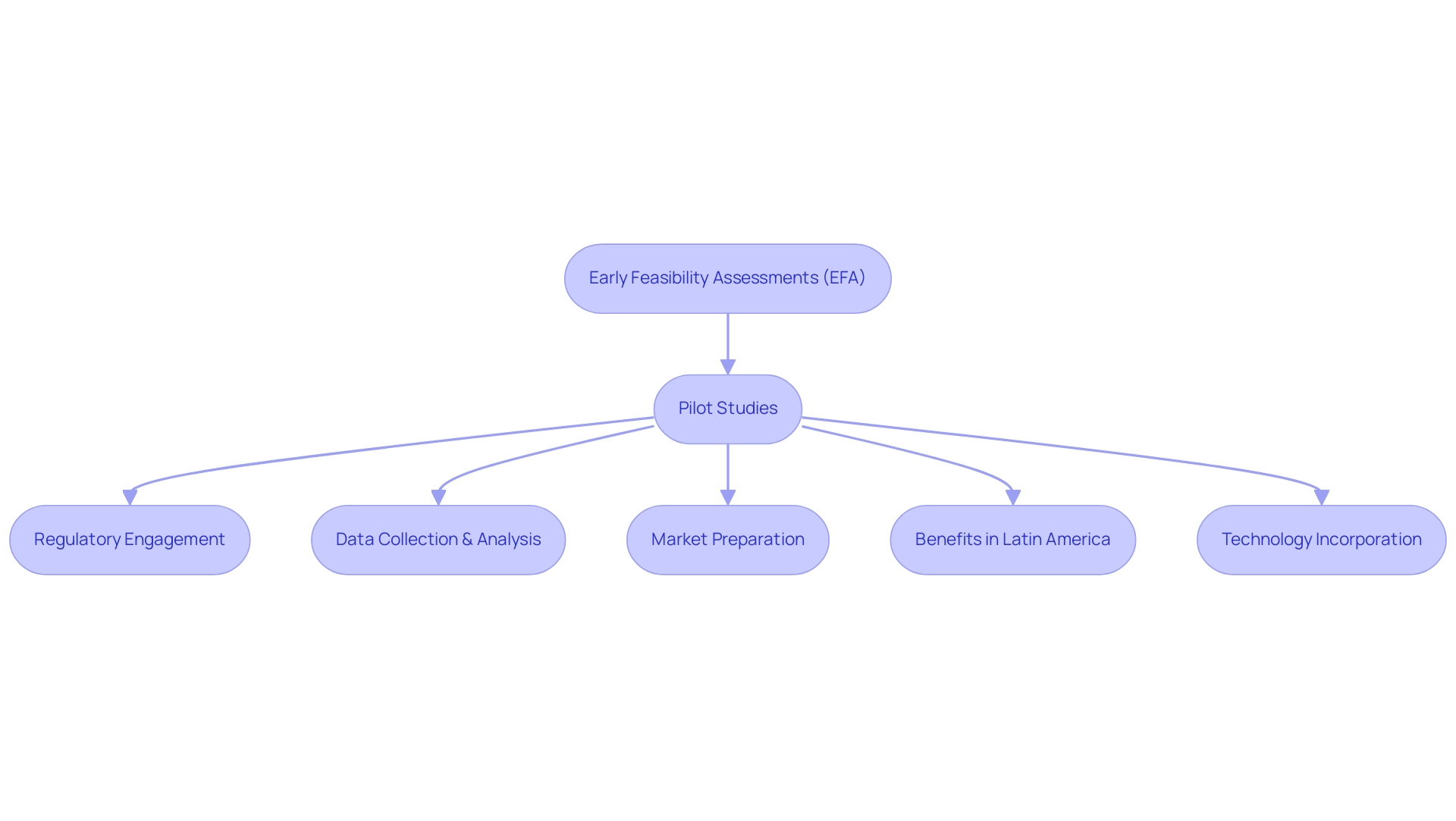
Early feasibility assessments (EFA) and pilot experiments play a pivotal role in the trial design of innovative medical devices, serving as essential precursors to larger pivotal trials. These investigations enable researchers to evaluate both the safety and functionality of a device within a controlled, small group, yielding critical insights before advancing to more extensive testing. For instance, a pilot examination involving a limited participant group can provide invaluable data regarding device performance and user experience, informing necessary modifications that enhance the product's design and usability.
The significance of engaging with regulatory bodies during these initial phases cannot be overstated. Such collaboration streamlines the transition to subsequent learning stages, ensuring that all safety and efficacy concerns are proactively addressed. This strategic approach not only increases the likelihood of successful outcomes but also significantly accelerates the overall development timeline.
In 2025, the landscape for initial feasibility assessments is evolving, with a pronounced emphasis on creative experimental designs that leverage advancements in technology. Statistics reveal that implementing EFS programs necessitates substantial organizational and financial investments, alongside robust performance monitoring mechanisms. As the medical device market continues to expand, particularly in regions like Latin America, the advantages of conducting these studies—such as reduced study costs and access to bilingual, U.S. board-certified physicians—become increasingly apparent.
Notably, the growth of medtech clinical trials in Latin America, exemplified by Avantec Vascular's first-in-human research supported by bioaccess™, underscores these benefits, showcasing the region's potential as an attractive site for clinical research.
Success stories from pilot projects in trial design for innovative medical devices further illustrate their value. Recent case analyses emphasize how early feasibility assessments have led to significant breakthroughs in device development, demonstrating the potential for improved patient outcomes and enhanced market readiness. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his experience with bioaccess® during its initial human study in Colombia, highlighting the critical role of expert support in navigating these complex processes.
bioaccess® provided essential services, including the selection of a principal investigator and assistance with regulatory dossier submissions, which were crucial for the project's success. Expert insights from industry leaders reinforce the importance of these analyses in navigating the complexities of medical device trials, especially as the sector adapts to new regulatory frameworks and evolving patient needs. Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We did a small AI initiative to see if we can generate meaningful test information for setting up and validating our systems."
It turns out we can. An algorithm we created examines previous research, learns from the actual information gathered, and employs it to produce insights for new investigations. This innovative approach illustrates the incorporation of technology in experiment design and information management.
Furthermore, the focus on MDR solutions is vital for enhancing research design, data gathering, analysis, and submission, further advancing the conversation on innovative experimental designs. Overall, the role of trial design for innovative medical devices, particularly through pilot studies and EFS, is more critical than ever, driving innovation and ensuring that new technologies meet the highest standards of safety and efficacy.
Navigating Regulatory Requirements in Medical Device Trials
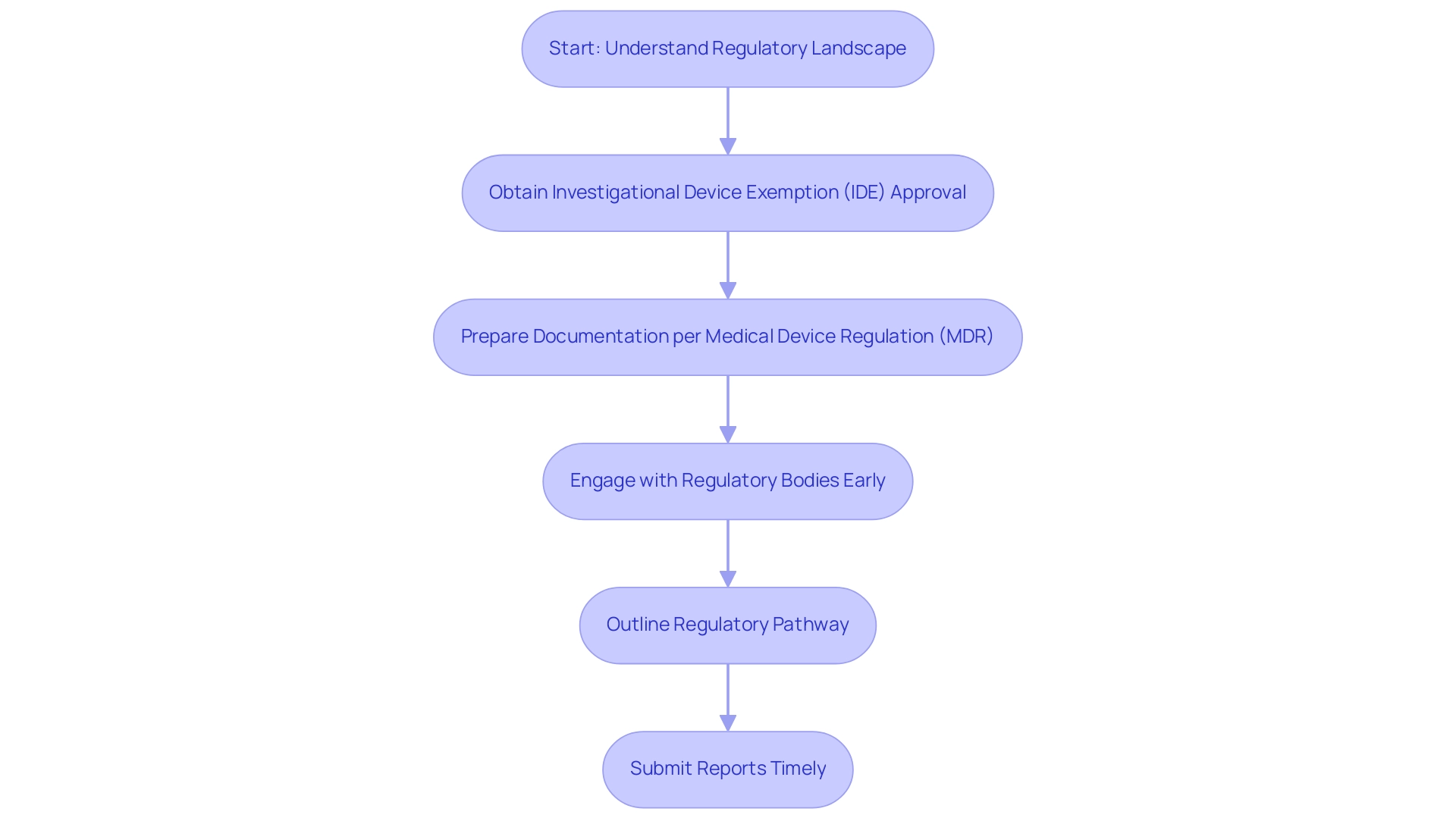
Navigating the regulatory landscape for medical device evaluations in 2025 necessitates a comprehensive grasp of the requirements established by regulatory agencies such as the FDA and the European Medicines Agency (EMA). A crucial element of this process is acquiring Investigational Device Exemption (IDE) approval for significant risk devices, which permits manufacturers to carry out studies without the requirement for complete premarket approval. In 2025, the statistics indicate that the approval rate for IDE applications has shown a steady increase, reflecting a growing confidence in innovative medical technologies.
Key considerations include:
- Strict adherence to the Medical Device Regulation (MDR) in Europe, which mandates that manufacturers prepare meticulous documentation detailing the trial design for innovative medical devices, including study objectives and methodologies.
- Manufacturers or sponsors must outline the regulatory pathway and establish the purpose of the clinical investigation to conduct clinical studies in Europe.
- Engaging with regulatory bodies early in the process is essential; this proactive strategy not only helps identify potential hurdles but also streamlines the approval process.
For example, bioaccess has shown proficiency in feasibility assessments, site selection, and adherence to national regulations, guaranteeing setup and approval procedures, including ethics committee and health ministry authorizations. Furthermore, bioaccess provides critical services such as obtaining import permits and nationalization of investigational devices, as well as comprehensive reporting on study status and adverse events.
Moreover, expert insights emphasize the importance of understanding the evolving regulatory landscape. As noted by Abhishek Pratap, Senior Clinical Program Leader at Boehringer Ingelheim, "This talk will focus on the use of digital health technologies (DHTs) to advance medical product development—from assessment to interventions in real-world settings." This underscores the need for innovative approaches to meet regulatory expectations.
The NIH Framework for validating digital health technologies in clinical studies, recently presented at a conference, further highlights this necessity.
In summary, the current regulatory challenges in medical device studies necessitate a strategic approach to trial design for innovative medical devices, which combines thorough documentation, early engagement with regulatory agencies, and a commitment to data integrity. Additionally, it is important to note that a report on an interrupted study should be submitted within 3 months, emphasizing the need for timely compliance. By following these best practices and utilizing extensive research management services provided by bioaccess, including project oversight and monitoring, companies can enable quicker market access for their innovative devices, ultimately benefiting patients and healthcare systems alike.
Incorporating Patient Perspectives in Clinical Trial Design
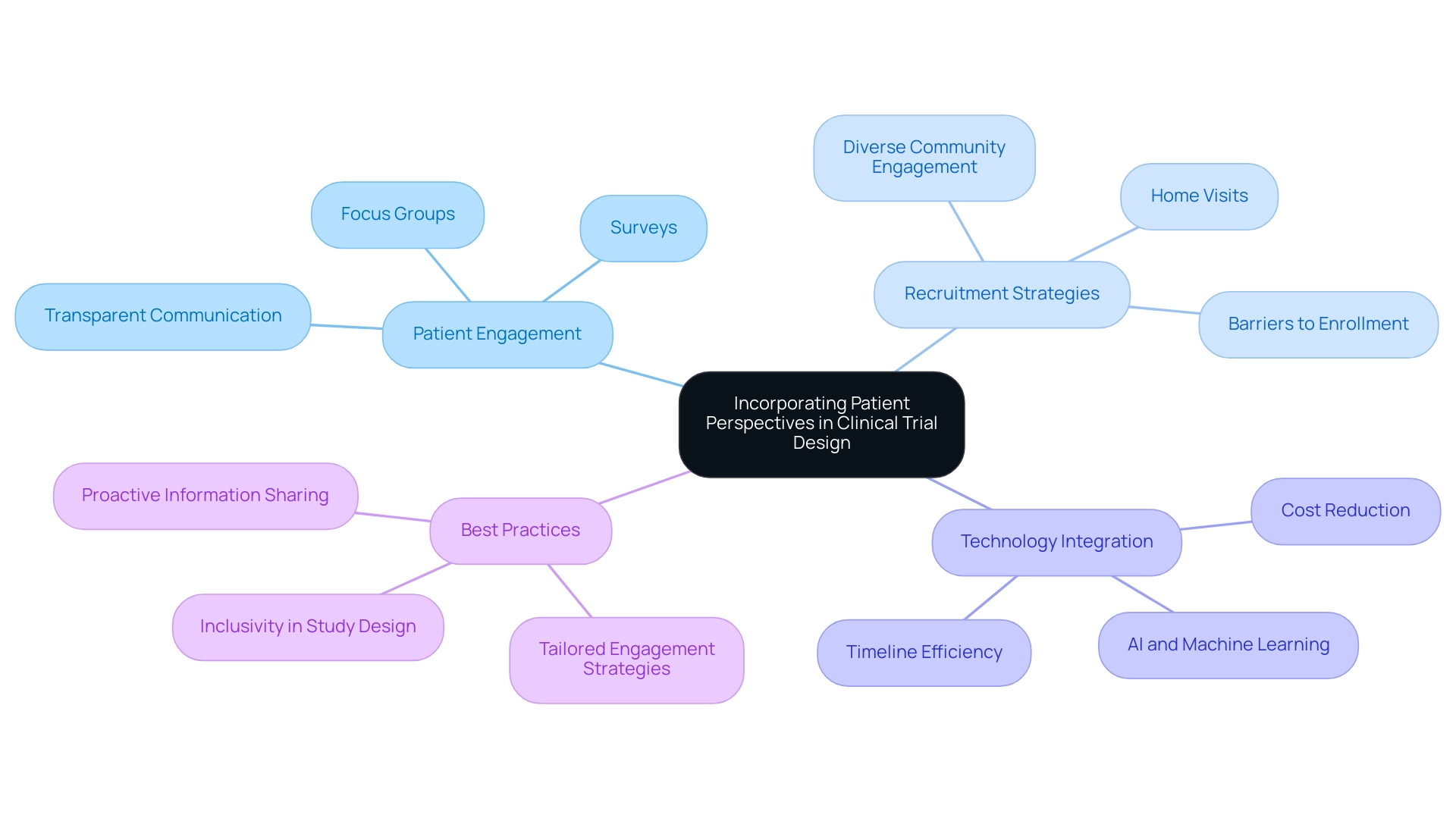
Integrating patient viewpoints into the trial design for innovative medical devices has become a crucial best practice that significantly enhances the quality and significance of research results. Engaging patients throughout the design process yields invaluable insights into their needs, preferences, and experiences, which can directly inform critical elements such as recruitment strategies, intervention delivery, and outcome measures. For example, utilizing focus groups or surveys with potential participants can effectively identify barriers to enrollment and retention, enabling researchers to tailor their approaches to better meet patient expectations.
Moreover, fostering a culture of transparency and open communication with participants is essential for building trust and satisfaction. This approach not only enhances participant engagement but also contributes to improved data quality and overall study outcomes. Significantly, statistics show that only 32% of patients indicated receiving information about research studies from their healthcare providers, highlighting the necessity for proactive engagement strategies to ensure that patients are well-informed and motivated to participate.
Best practices for patient involvement in research studies in 2025 include actively engaging diverse communities in observational studies, which can serve as a gateway to trial design for innovative medical devices and enhance wider research participation. By comprehending and tackling the unique viewpoints of different demographic groups, researchers can improve recruitment efforts and ensure that study designs are more inclusive and representative. For example, research by Antidote showed that home visits attracted more Hispanic and non-white individuals compared to their non-Hispanic and white counterparts, emphasizing the significance of customizing engagement strategies for various populations.
The incorporation of cutting-edge technologies, such as artificial intelligence and machine learning, is also revolutionizing trial design for innovative medical devices. As reported by the Tufts Center for the Study of Drug Development, these advancements can streamline operations, reduce costs by up to 20%, and shorten research timelines by as much as 30%, ultimately enhancing patient recruitment and retention. Furthermore, bioaccess provides extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
These services not only enable the effective implementation of medical studies but also aid in economic development and healthcare enhancement in local communities. By ensuring that patient engagement strategies are aligned with these services, bioaccess improves the overall effectiveness of medical studies. As the global market for precision oncology approaches $98 billion, the significance of patient involvement in research studies becomes increasingly evident, as it directly affects study results and the successful progress of medical devices.
Best Practices for Participant Recruitment and Trial Management
Effective participant recruitment and study management are crucial to the success of research initiatives, particularly in the rapidly evolving Medtech sector. With over 15 years of experience in the Medtech industry, bioaccess® understands the nuances of effective recruitment strategies. Establishing a clear recruitment strategy is essential; this involves identifying target populations and leveraging diverse outreach channels, including social media, healthcare providers, and patient advocacy groups.
In 2025, the effectiveness of social media in recruiting participants for research studies has become increasingly clear, with studies indicating that patient-centered studies can attract up to double the number of individuals compared to conventional methods.
In a significant move to enhance medical research in Latin America, bioaccess® has partnered with Caribbean Health Group (CHG) to position Barranquilla as a leading destination for research studies. This collaboration, supported by Colombia's Minister of Health, Juan Pablo Uribe, aims to attract more clinical research projects to the region, thereby improving recruitment and retention rates. Transparency is crucial in fostering trust among potential participants.
As Elisa Cascade, chief product officer at Advarra, observes, "While both data and trust relationships are important, they serve different purposes and likely differ based on the indication/design." Providing comprehensive information about the trial—its purpose, procedures, and potential risks—can significantly enhance participant engagement. Once individuals are enrolled, maintaining regular communication is vital for retention.
This includes updates on study progress and personalized support, which can mitigate participant burden and enhance overall satisfaction. Prioritizing patient retention is essential to avoid budget excesses, delayed timelines, and reduced quality of outcomes in clinical studies.
Moreover, implementing robust data management systems streamlines operations, ensuring accurate and efficient data collection. This is not only essential for regulatory compliance but also for preserving the integrity of the research. As emphasized in the case analysis titled "Enhancing Patient Recruitment and Retention," the research community encounters difficulties in recruitment and retention, which are essential for the success of research experiments.
Innovative approaches such as digital tools and patient-centric designs have proven effective in enhancing recruitment and retention rates. By prioritizing these strategies, organizations can navigate the intricacies of trial design for innovative medical devices more effectively, ultimately leading to enhanced results and expedited progress in medical device development. Furthermore, bioaccess® provides extensive management services for research projects, encompassing feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring a thorough approach to medical research.
Furthermore, bioaccess® specializes in trial design for innovative medical devices, managing accelerated medical device research study services such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), which are essential for advancing Medtech innovations.
Leveraging Technology to Enhance Clinical Trial Efficiency
Leveraging technology in clinical trials is pivotal for enhancing both efficiency and effectiveness, particularly within the context of bioaccess's comprehensive clinical trial management services in Latin America. Innovations such as electronic data capture (EDC) systems, remote monitoring tools, and telemedicine platforms are revolutionizing data collection and participant engagement. For example, mobile applications enable real-time information entry and communication, significantly reducing participant burden and enhancing accuracy.
Furthermore, the integration of artificial intelligence (AI) and machine learning algorithms is transforming patient recruitment processes. As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We conducted a small AI initiative to determine if we can produce meaningful test information for setting up and validating our systems." The results confirmed that we can.
An algorithm we developed examines previous research, learns from the actual information gathered, and employs it to generate insights for new investigations. This illustrates how technology is being utilized to improve testing processes, particularly in relation to bioaccess's expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
The influence of EDC systems on study efficiency is especially significant in 2025, as they facilitate the collection of various information sources, aligning with contemporary study methods that emphasize streamlined experiences for both locations and participants. Current trends indicate that the use of EDC in medical device studies is increasingly prevalent, with case studies demonstrating how technology enhances operational workflows and accelerates study timelines. Additionally, regulatory readiness is crucial due to the growing complexity and demands of regulations, including privacy requirements, underscoring the importance of compliance in leveraging technology.
As the industry continues to embrace these advancements, particularly through the innovative trial design for medical devices offered by bioaccess, the overall participant experience is expected to improve, leading to more successful trial outcomes and ultimately advancing the development of innovative medical devices. The trend of utilizing human cells and tissues for drug discovery, as highlighted in the case study "Innovative Approaches to Drug Development," reflects a deeper understanding of biological responses and is anticipated to accelerate the development of new treatments, enhancing patient outcomes by providing more accurate data. With over 20 years of experience in Medtech, bioaccess is well-positioned to drive these innovations in Latin America, contributing to local economies and healthcare improvements.
Conclusion
Effective trial design in the medical device industry is essential for navigating the complexities of regulatory requirements, technological advancements, and patient engagement. Establishing clear objectives, selecting appropriate endpoints, and utilizing adaptive trial designs are critical for enhancing study efficiency and outcomes. The integration of artificial intelligence and machine learning is revolutionizing patient recruitment, demonstrating significant reductions in study timelines and costs. Organizations must prioritize early feasibility studies and pilot studies to assess safety and functionality, ultimately ensuring a smoother transition to larger trials.
As the regulatory landscape evolves, proactive engagement with agencies like the FDA and EMA is vital for compliance and successful trial execution. Best practices underscore the importance of thorough documentation, timely reporting, and innovative strategies to meet regulatory expectations. Incorporating patient perspectives into trial design further enhances the relevance and quality of research outcomes, fostering trust and improving recruitment and retention rates.
In summary, the future of medical device trials hinges on a commitment to methodological rigor, strategic use of technology, and a focus on patient engagement. By embracing these principles, organizations can optimize trial outcomes, ultimately advancing medical innovations and improving patient care. As the industry prepares for 2025 and beyond, the emphasis on collaboration, adaptability, and inclusivity will be key to ensuring that clinical trials meet the needs of diverse populations while navigating an increasingly complex regulatory environment.




