Overview
Trial design optimization for Medtech is essential for enhancing the efficiency and success of clinical evaluations. It focuses on key components such as:
- Clearly defined objectives
- Measurable endpoints
- Appropriate patient populations
A well-structured trial design not only facilitates regulatory approval but also accelerates market entry for innovative medical devices. This is supported by insights into emerging technologies and best practices that streamline processes and improve stakeholder collaboration.
Introduction
In the dynamic realm of Medtech, optimizing trial design has become a critical factor for the success of clinical studies. As the industry faces unique challenges—from regulatory complexities to patient recruitment hurdles—the demand for innovative and efficient trial methodologies is more pressing than ever.
This article delves into the essential components of trial design optimization, underscoring the significance of:
- Clear objectives
- Relevant endpoints
- Appropriate patient populations
It further explores the transformative impact of technology, such as artificial intelligence and remote monitoring, in enhancing trial execution. Additionally, collaboration among stakeholders, including regulatory bodies and clinical investigators, is emphasized as a cornerstone for achieving favorable outcomes.
With the landscape of clinical research continuously evolving, understanding and implementing best practices will be paramount for Medtech professionals aiming to navigate the complexities of trial design and accelerate the path to market for groundbreaking medical devices.
Understanding Trial Design Optimization in Medtech
Trial design optimization for Medtech is essential for improving the structure of Medtech studies, thereby boosting the efficiency and effectiveness of clinical evaluations while ensuring adherence to regulatory standards. This process encompasses several key components that contribute to the success of a trial:
- Objectives: Clearly defining the trial's goals is crucial. This clarity aligns the study's design with the desired outcomes, ensuring that all stakeholders understand the purpose of the experiment.
- Endpoints: Selecting relevant and measurable outcomes is vital. These endpoints must accurately reflect the performance of the medical device, providing significant information that can influence regulatory decisions.
- Population: Identifying the appropriate patient population is critical. Focusing on the right group guarantees that the information gathered is pertinent and useful, ultimately enhancing the study's validity.
In 2025, the anticipated incorporation of wearables for daily information gathering from participants is expected to greatly enhance the evaluation of endpoints, offering deeper insights into device performance. Furthermore, the trend of sponsors conducting studies internally is gaining momentum, allowing for greater control over information quality and enhancing transparency. This shift is driven by the need for sponsors to have direct access to real-time information, which is essential for making informed decisions throughout the trial process.
As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We did a small AI initiative to see if we can generate meaningful test information for setting up and validating our systems." It turns out we can. An algorithm we created examines previous studies we established, learns from the actual information gathered, and utilizes it to produce something applicable for new studies.
Moreover, the case study titled "Increased Control and Transparency by Sponsors" emphasizes the shift in the research environment towards sponsors pursuing greater ownership and clarity of their information. This case discusses the implications of ICH E6 R2 and R3 revisions on data management practices, highlighting how sponsors are moving towards insourced models for data management, allowing them direct access to live data and the ability to operationalize studies in-house, enhancing data quality and control over outcomes.
Trial design optimization for Medtech is essential, as a well-optimized study structure not only enhances the chances of regulatory approval but also accelerates the route to market for innovative medical devices. For instance, recent progress in clinical study formulation has demonstrated that clear objectives and well-defined endpoints can lead to higher approval rates. Successful instances from the Medtech field illustrate how careful preparation and implementation of studies can yield positive results.
Furthermore, with the FDA's Single IRB Requirement anticipated to be enacted in 2025, it is vital for Medtech professionals to consider how this regulatory change may influence study design and operational practices.
In summary, concentrating on these key elements—objectives, endpoints, and population—enables researchers to achieve trial design optimization for Medtech, establishing a robust framework for their studies and ensuring compliance with both scientific rigor and regulatory standards. As the landscape of medical research continues to evolve, staying updated on best practices and emerging trends will be crucial for Medtech professionals seeking to conduct successful studies. At bioaccess®, we leverage over 20 years of expertise in overseeing research studies across Latin America, ensuring that your projects are crafted for success.
Challenges Unique to Medtech Trial Designs
Medtech clinical studies encounter a range of distinct obstacles that complicate both planning and execution. Key issues include:
-
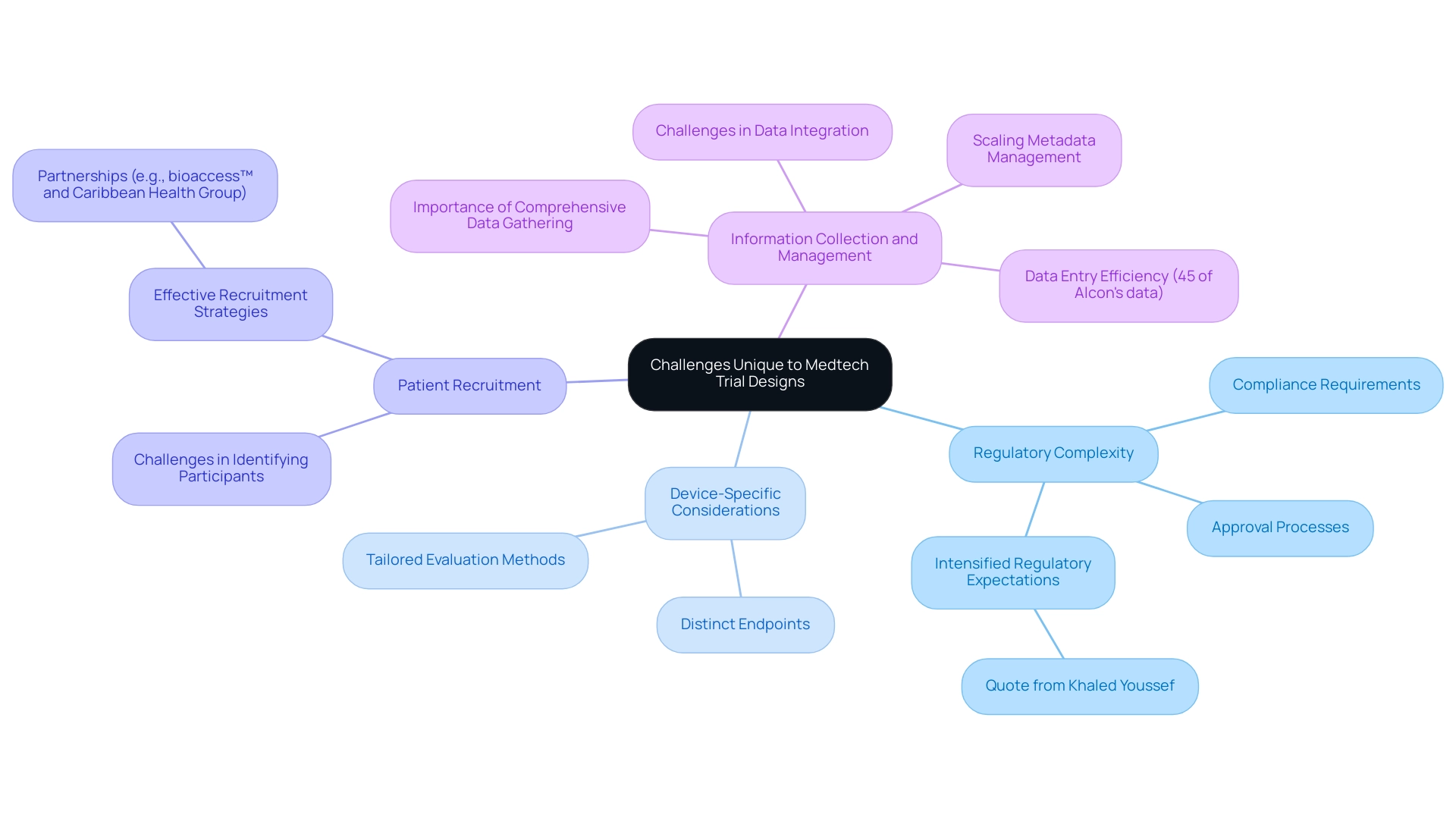
Regulatory Complexity: The intricate regulatory landscape presents significant hurdles, as requirements can vary widely across different regions. This complexity necessitates a proactive approach to ensure compliance and streamline the approval process. As Khaled Youssef, Ph.D., noted, "Pressure to increase efficiency in medical device development has been prevalent over the past year, especially with the intensified regulatory expectations."
-
Device-Specific Considerations: Unlike pharmaceuticals, medical devices often necessitate distinct endpoints and evaluation methods. This divergence complicates study design, requiring tailored strategies that align with the specific characteristics of the device.
-
Patient Recruitment: Identifying suitable participants poses a considerable challenge, particularly for niche devices aimed at specific medical conditions. Effective recruitment strategies are essential to ensure that studies are adequately powered and representative of the target population. Partnerships, like the one between bioaccess™ and Caribbean Health Group, have been crucial in establishing Barranquilla as a prominent location for medical studies in Latin America, backed by Colombia's Minister of Health, which can improve recruitment efforts.
-
Information Collection and Management: Precise and comprehensive information gathering is critical for the success of clinical trials. However, device-specific factors can hinder this process, making it imperative to implement robust management systems that facilitate seamless integration of diverse sources. For instance, 45% of Alcon's information is entered on the same day as the visit date, illustrating the importance of efficient management systems. Additionally, the challenge of scaling metadata management continues to be an issue, as MDR solutions are intended to incorporate study structure, data collection, analysis, and submission.
To effectively address these challenges, trial design optimization for medtech is essential. Early interaction with regulatory agencies can offer valuable insights into compliance requirements, while a comprehensive understanding of the device's intended use and market can facilitate trial design optimization for medtech. For instance, bioaccess® utilizes over 20 years of expertise in managing various types of medical device research studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), to enhance site experiences by streamlining processes and reducing the burden on research sites.
These innovations not only enhance site performance but also tackle recruitment challenges by ensuring that studies are more efficient and user-friendly. Furthermore, bioaccess's comprehensive clinical study management services include setup, ethics committee approvals, and detailed reporting on adverse events, ensuring a thorough approach to clinical studies. As the pressure to enhance efficiency in medical device development escalates, especially considering changing regulatory expectations, adopting best practices in testing becomes more essential than ever.
Leveraging Technology for Enhanced Trial Design
Technology is fundamentally transforming the landscape of trial design optimization for medtech and its execution within the sector. The following key technologies are at the forefront of this transformation:
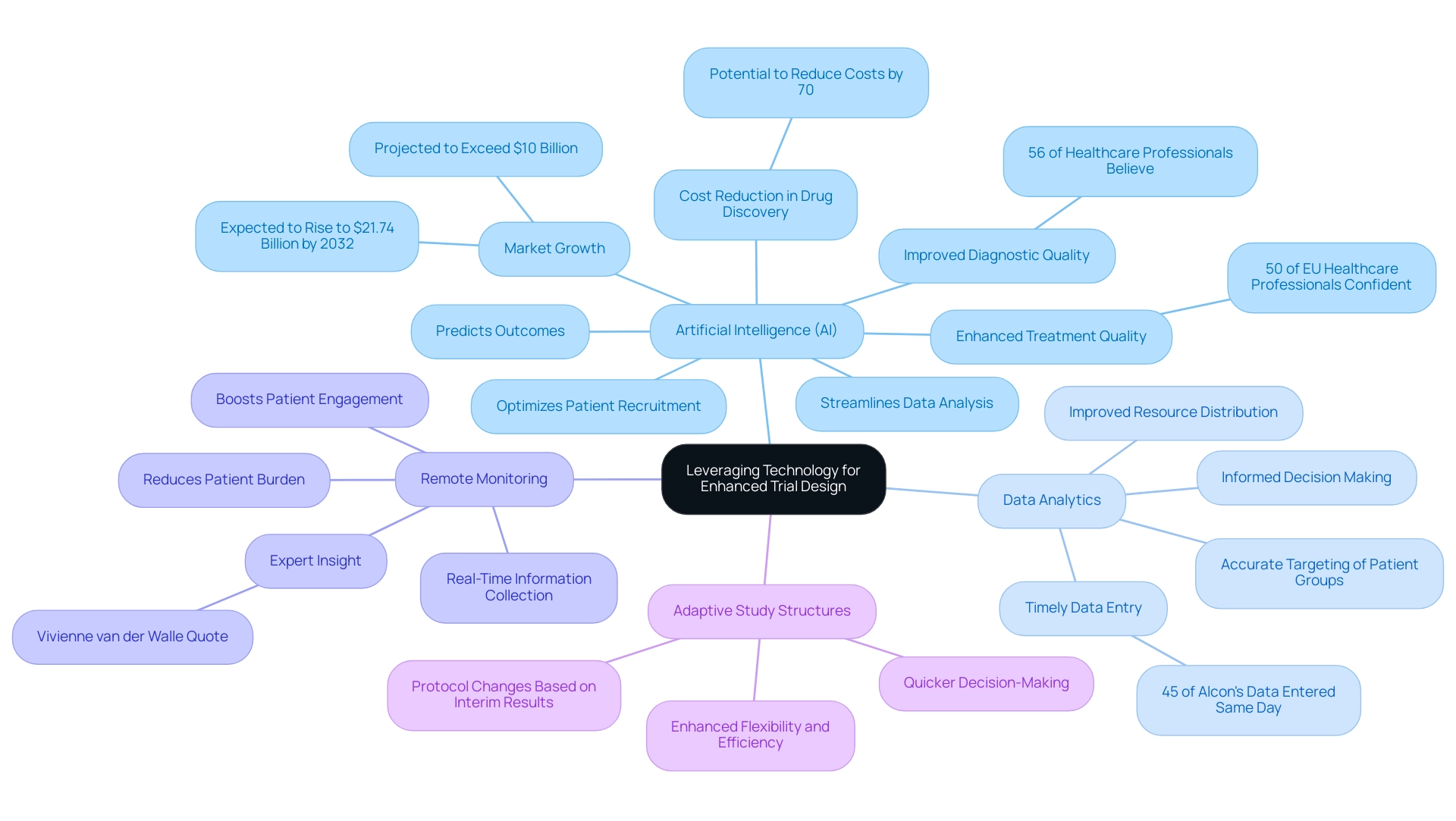
- Artificial Intelligence (AI): AI is revolutionizing clinical trials by optimizing patient recruitment, predicting outcomes, and streamlining data analysis. This not only improves efficiency but also significantly decreases the time and expenses related to testing. For instance, the generative AI healthcare market is projected to exceed $10 billion, with expectations to rise to $21.74 billion by 2032, underscoring the growing reliance on AI in the industry. Furthermore, 56% of healthcare professionals believe AI will improve diagnostic quality, and 50% are confident it will enhance treatment quality, highlighting the technology's potential impact on Medtech.
- Data Analytics: Advanced analytics tools empower researchers to make informed, data-driven decisions, thereby enhancing both study design and execution. The incorporation of analytics can result in more accurate targeting of patient groups and improved resource distribution, ultimately enhancing study outcomes. Significantly, 45% of Alcon's information is recorded on the same day as the visit date, showcasing the effectiveness that prompt information entry can provide to research studies.
- Remote Monitoring: The advent of wearable devices and other remote monitoring technologies facilitates real-time information collection, which significantly boosts patient engagement and compliance. This method not only improves the quality of data gathered but also reduces the burden on patients, addressing a critical pain point in clinical studies. As noted by industry expert Vivienne van der Walle, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care."
- Adaptive Study Structures: These innovative frameworks permit changes to the protocol based on interim results, thereby enhancing flexibility and efficiency. This adaptability can lead to quicker decision-making and a more responsive approach to management of experiments.
By utilizing these technologies along with bioaccess®'s extensive management services for studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—Medtech companies can achieve trial design optimization for medtech, thereby significantly improving the design and implementation of their research. Bioaccess® specializes in accelerated medical device research study services in Latin America, ensuring expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Carrying out studies in Latin America provides unique benefits like cost-effectiveness and access to varied patient groups, which can result in high-quality research outcomes.
The integration of AI, for example, has shown potential in drug discovery, with estimates suggesting it could reduce costs by up to 70%, thereby expediting the introduction of new therapies to the market. As the industry progresses, the role of technology in enhancing medical studies will be crucial, paving the way for more effective and efficient Medtech innovations.
Collaboration Among Stakeholders: A Key to Success
Successful Medtech clinical trials are fundamentally rooted in the collaboration among diverse stakeholders, each playing a pivotal role in the trial's success.
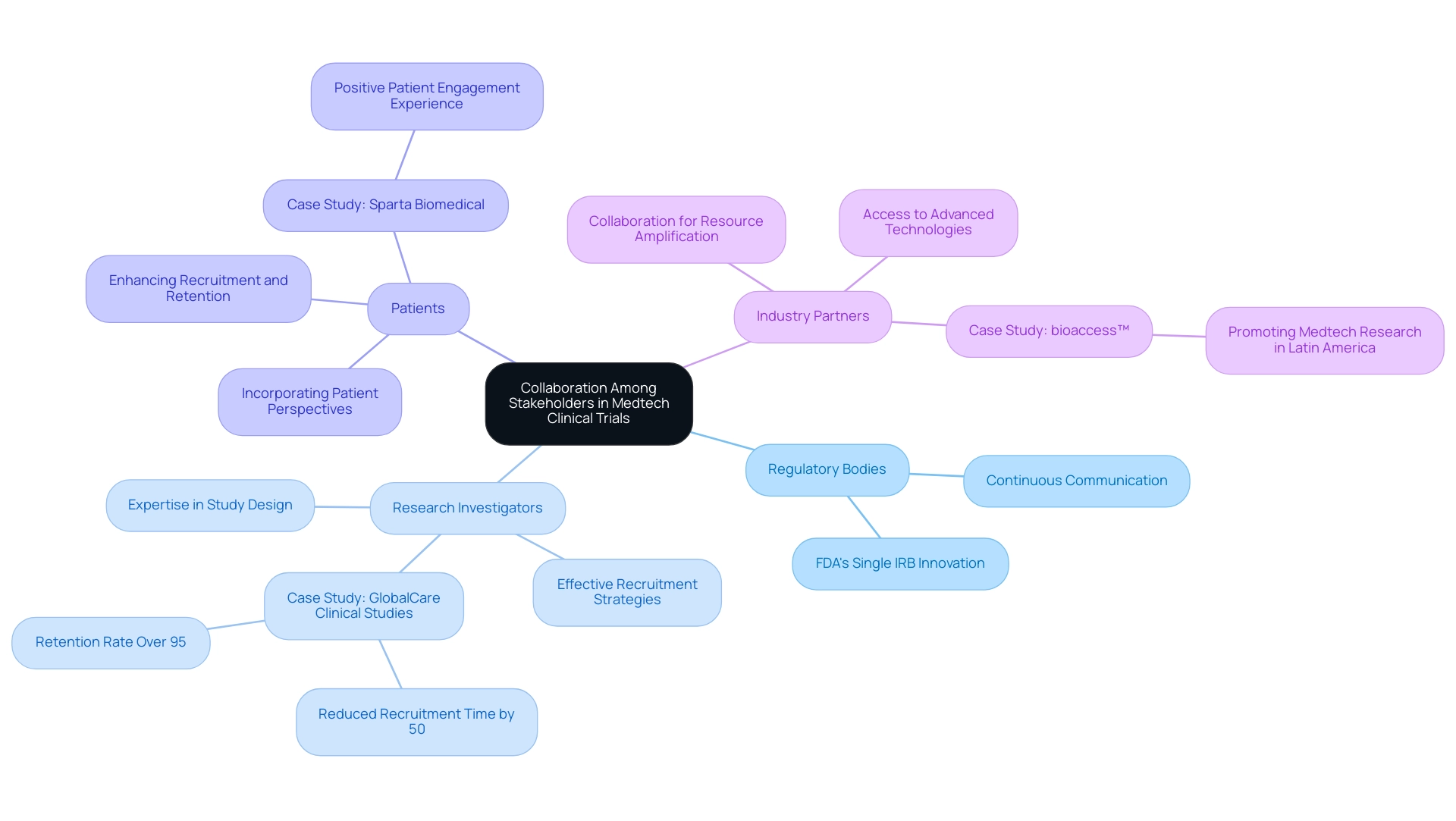
- Regulatory Bodies: Establishing early and continuous communication with regulatory authorities is essential. This proactive engagement not only ensures compliance with evolving regulations but also streamlines the approval process, ultimately expediting the path to market. The FDA's push for single IRB reviews exemplifies a regulatory innovation aimed at streamlining processes and enhancing collaboration among institutions, which is vital for protecting participant rights while expediting study initiation.
- Research Investigators: Involving experienced research investigators can greatly improve study design and execution. Their expertise and established networks can lead to more effective recruitment strategies and enhanced study management, which are critical for meeting study objectives. For instance, GlobalCare Clinical Studies' partnership with bioaccess™ has resulted in a remarkable reduction in clinical study subject recruitment time by over 50% and an impressive subject retention rate of over 95% in Colombia, showcasing the impact of effective investigator collaboration. This partnership not only broadens ambulatory services but also establishes a standard for future experiments in the region.
- Patients: Actively incorporating patient perspectives during the design phase yields invaluable insights into their needs and preferences. This involvement not only enhances recruitment and retention rates but also aligns the objectives with patient-centered outcomes, thereby increasing the relevance of the research. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its first human study in Colombia, emphasizing the importance of patient engagement in study success.
- Industry Partners: Collaborating with other companies or research institutions can amplify resources and expertise. Such collaborations can enhance access to advanced technologies and methodologies, ultimately contributing to the success of the study. Julio Martinez-Clark, CEO of bioaccess™, promotes Medtech research in Latin America, concentrating on implementing studies and guiding startups, which underscores the significance of industry cooperation in addressing the challenges of research projects.
The importance of fostering robust relationships among these stakeholders is crucial for trial design optimization for Medtech and cannot be overstated. As the environment of Medtech studies becomes increasingly intricate, effective cooperation is crucial for overcoming challenges and achieving trial design optimization for Medtech to attain successful results. Recent insights indicate that trial design optimization for Medtech through a well-coordinated approach can lead to improved efficiency in studies and higher success rates, underscoring the critical nature of stakeholder engagement in the Medtech sector.
For example, as highlighted by Peng Lu, "Standardizing the application of specific outcomes and outcome measures for studies will aid in the development of guidelines and future indirect comparisons among interventions." Moreover, the statistic that 45% of Alcon's data is entered on the same day as the visit date illustrates effective data management practices that improve efficiency in the study. Furthermore, as emphasized by industry specialists, the incorporation of particular results in research studies will aid in the formulation of practice guidelines and enable future comparisons among interventions, strengthening the necessity for a cohesive strategy in study methodology.
Moreover, Max Baumann from Treehill Partners warns of fundamental business model challenges facing biotech as clinical markets become crowded, emphasizing the necessity for effective collaboration among stakeholders to navigate these challenges.
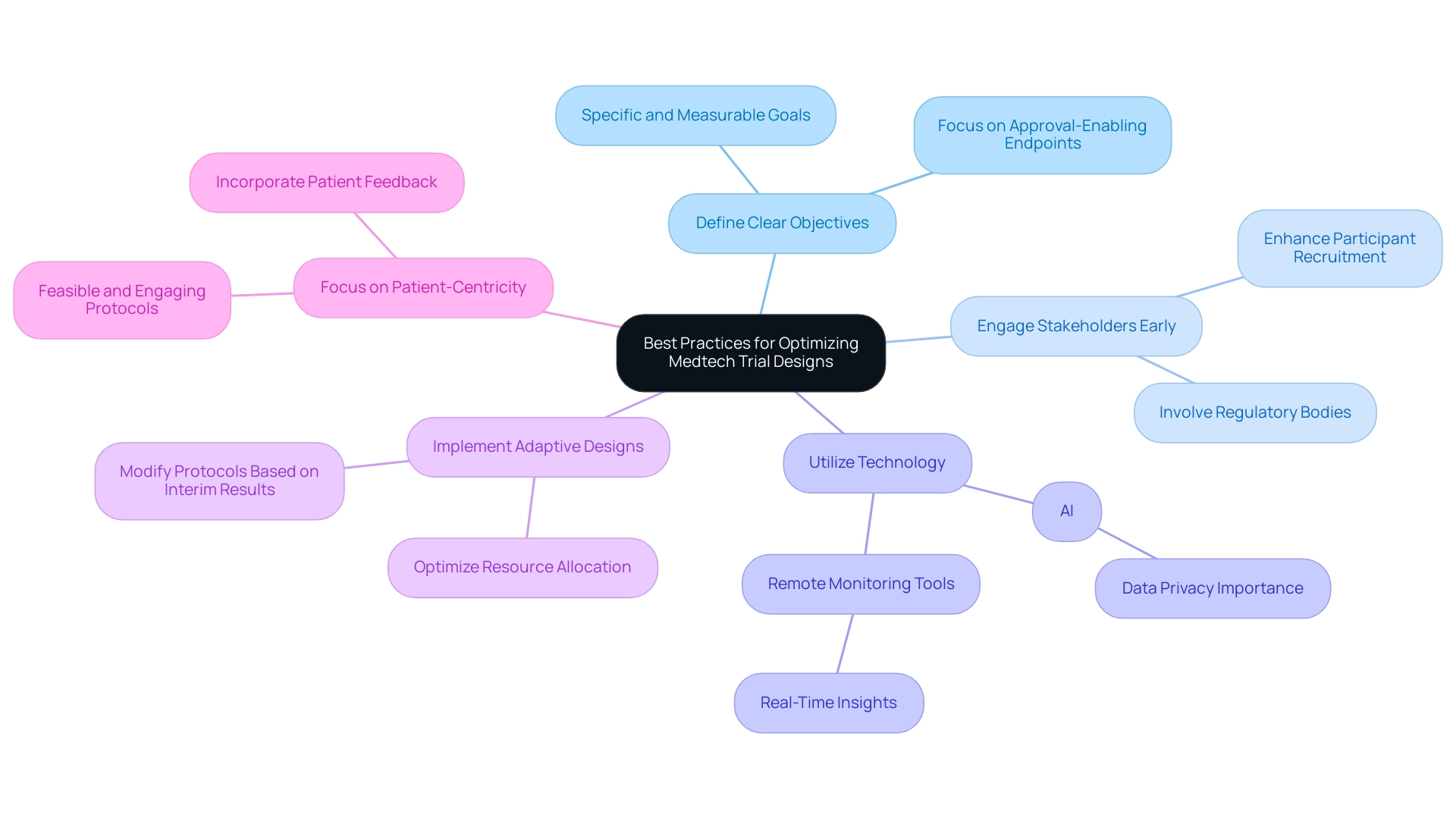
Best Practices for Optimizing Medtech Trial Designs
To optimize Medtech trial designs effectively, it is crucial to implement the following best practices:
- Define Clear Objectives: Establishing specific and measurable goals is essential for guiding both the design and execution of the trial. Clear objectives not only streamline the process but also facilitate the assessment of outcomes, ensuring that the study remains focused on achieving approval-enabling endpoints that qualify for commercial success. As Max Baumann, Head of Execution, states, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
- Engage Stakeholders Early: Involving regulatory bodies, investigators, and patients from the outset fosters alignment and addresses potential concerns. Early engagement can lead to more robust studies that meet regulatory expectations and enhance participant recruitment and retention.
- Utilize Technology: The incorporation of advanced technologies such as AI and analytical methods can significantly improve efficiency and quality of information. For example, remote monitoring tools can offer real-time insights, enabling prompt adjustments and ensuring information integrity throughout the testing process. However, it is essential to prioritize privacy of information to maintain trust, especially as AI is employed to analyze sensitive details.
- Implement Adaptive Designs: Adaptive study designs provide the flexibility to modify protocols based on interim results. This approach not only optimizes resource allocation but also increases the likelihood of achieving meaningful outcomes, as adjustments can be made in response to emerging data.
- Focus on Patient-Centricity: Designing studies with the patient experience in mind is paramount. Ensuring that protocols are feasible and engaging can lead to higher participant satisfaction and retention rates. Examples of patient-centric study designs include simplified consent processes and the incorporation of patient feedback into study protocols.
By adhering to these best practices, Medtech professionals can significantly enhance the likelihood of success through trial design optimization for Medtech, ultimately accelerating the path to market for innovative medical devices. The ongoing advancements in biomarker identification further underscore the importance of these strategies, as they play a critical role in advancing disease diagnosis and therapeutic evaluation, ultimately improving patient outcomes. With more than 20 years of experience in the Medtech sector, bioaccess® is ideally situated to assist these initiatives, providing extensive research management services including feasibility studies, site selection, compliance evaluations, setup, import permits, project management, and reporting.
bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). As a vetted CRO and consulting partner for U.S. medical device companies in Colombia, bioaccess® is dedicated to navigating the complexities of the Latin American Medtech landscape, addressing unique challenges and opportunities to facilitate successful market access.
Future Trends in Medtech Trial Design Optimization
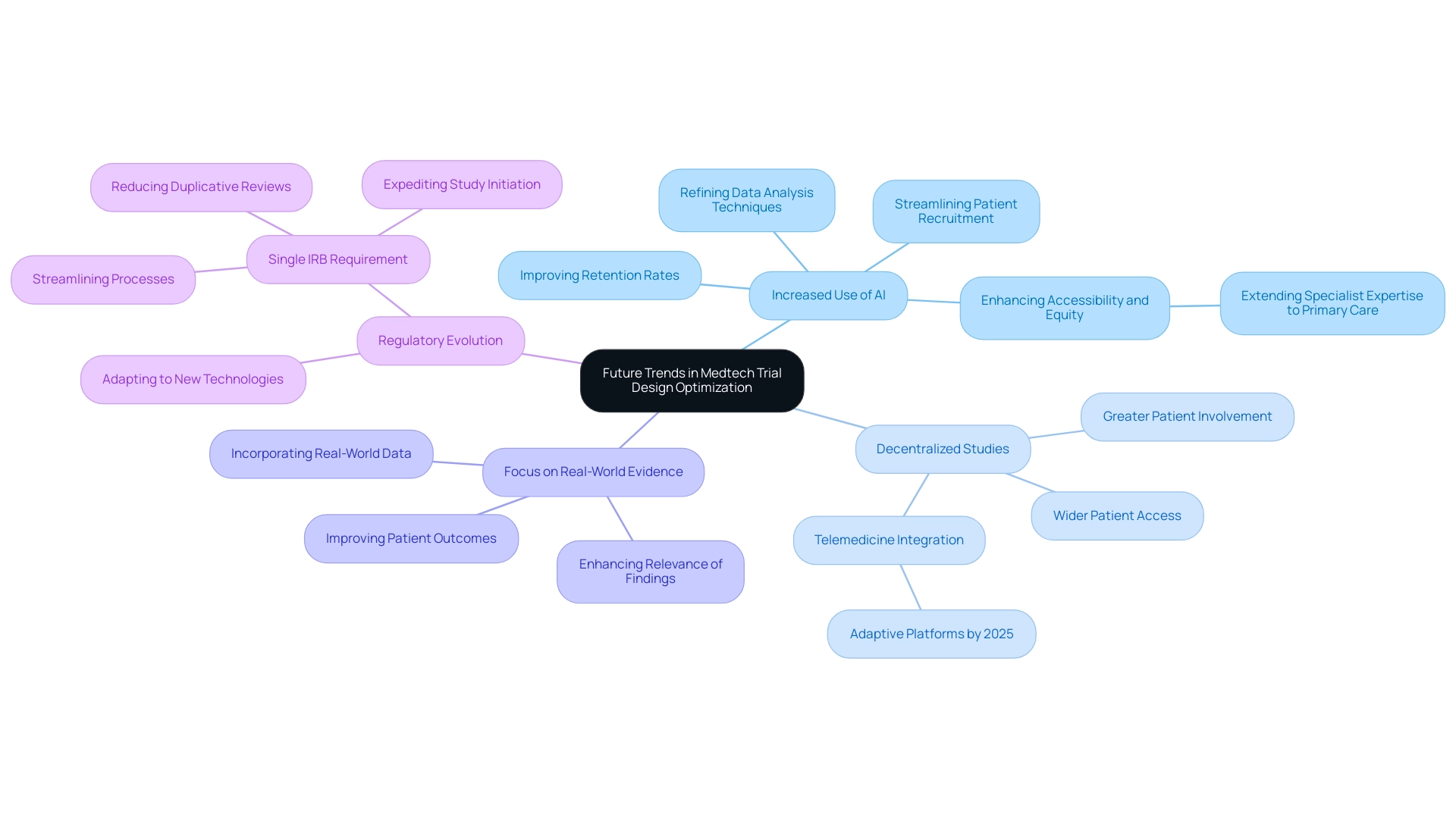
As the Medtech landscape continues to evolve, several pivotal trends are anticipated to significantly influence trial design optimization in the coming years:
-
Increased Use of AI: The integration of artificial intelligence will increasingly enhance trial designs by streamlining patient recruitment processes, improving retention rates, and refining data analysis techniques. As Bob Seminerio noted, "As AI continues to evolve, it has the potential to extend specialist diagnostic expertise to primary care settings, enhancing accessibility and equity in healthcare delivery." This technological progression is anticipated to enable more efficient and effective clinical studies.
-
Decentralized Studies: The shift towards decentralized and hybrid study formats is poised to gain momentum, offering wider patient access and promoting greater levels of involvement. This shift not only accommodates diverse patient populations but also aligns with the growing demand for personalized healthcare solutions. Notably, telemedicine platforms are projected to become more integrated and adaptive by 2025, further enhancing patient accessibility.
-
Focus on Real-World Evidence: There will be a heightened emphasis on incorporating real-world data into study frameworks, enhancing the relevance and applicability of findings. This approach allows for a more comprehensive understanding of how medical technologies perform in everyday settings, ultimately benefiting patient outcomes.
-
Regulatory Evolution: As regulatory frameworks adapt to accommodate new technologies and methodologies, Medtech companies must remain vigilant and agile. The FDA's anticipated implementation of the Single IRB Requirement in 2025 is a significant development that aims to streamline processes and improve research efficiency. The introduction of single IRB (sIRB) review is expected to reduce duplicative reviews in cooperative research, expediting study initiation. Staying informed about these changes will be crucial for maintaining compliance and optimizing efficiency.
By proactively anticipating these trends, Medtech professionals can strategically prepare for the future of healthcare studies, ensuring that their trial design optimization for Medtech is not only competitive but also effective in meeting the evolving needs of the healthcare landscape. This is especially pertinent for organizations like bioaccess®, which has over 20 years of experience in supporting research in Latin America. With a focus on comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—bioaccess® leverages these trends to enhance the development and commercialization of medical technologies, ultimately driving economic growth and healthcare improvement in the region.
Conclusion
Trial design optimization in the Medtech sector transcends mere procedural necessity; it stands as a strategic imperative that can profoundly influence the success of clinical trials. By emphasizing clear objectives, relevant endpoints, and appropriate patient populations, researchers can establish robust frameworks that align with regulatory standards and significantly enhance the likelihood of favorable outcomes. The integration of advanced technologies, such as artificial intelligence and remote monitoring, streamlines trial processes, improves data quality, and fosters patient engagement, ultimately accelerating the path to market for innovative medical devices.
Collaboration among stakeholders—including regulatory bodies, clinical investigators, and patients—remains essential in navigating the complexities of trial design. Engaging these parties early in the process fosters alignment and addresses potential challenges, leading to more effective recruitment strategies and improved trial management. As the Medtech landscape continues to evolve, embracing best practices and staying abreast of emerging trends will be crucial for professionals aiming to conduct successful clinical trials.
Looking ahead, anticipated advancements in AI, decentralized trial designs, and an emphasis on real-world evidence will reshape the future of clinical research. By proactively adapting to these trends, Medtech companies can enhance their trial designs, ensuring they remain competitive and effective in meeting the evolving needs of the healthcare landscape. Ultimately, the commitment to optimizing trial design not only benefits the stakeholders involved but also contributes to the advancement of healthcare solutions that improve patient outcomes and drive innovation in the industry.
Frequently Asked Questions
Why is trial design optimization important for Medtech?
Trial design optimization is essential for improving the structure of Medtech studies, enhancing the efficiency and effectiveness of clinical evaluations, and ensuring adherence to regulatory standards.
What are the key components of trial design optimization?
The key components include clearly defined objectives, relevant and measurable endpoints, and the identification of the appropriate patient population.
How will wearables impact clinical trials in the future?
The anticipated incorporation of wearables for daily information gathering from participants in 2025 is expected to enhance the evaluation of endpoints and provide deeper insights into device performance.
What trend is emerging regarding how sponsors conduct studies?
There is a growing trend of sponsors conducting studies internally, which allows for greater control over information quality and enhances transparency.
How does an algorithm contribute to trial design optimization?
An algorithm can analyze previous studies, learn from the data gathered, and produce applicable insights for new studies, thereby improving the setup and validation of systems.
What implications do the ICH E6 R2 and R3 revisions have on data management?
These revisions highlight the shift towards sponsors pursuing greater ownership and clarity of their information, resulting in insourced models for data management that enhance data quality and control over outcomes.
What impact does a well-optimized study structure have on regulatory approval?
A well-optimized study structure enhances the chances of regulatory approval and accelerates the route to market for innovative medical devices.
What is the significance of the FDA's Single IRB Requirement expected in 2025?
It is vital for Medtech professionals to consider how this regulatory change may influence study design and operational practices.
How can early interaction with regulatory agencies benefit trial design?
Early interaction can provide valuable insights into compliance requirements, facilitating better trial design optimization for Medtech.
What challenges do Medtech clinical studies face?
Key challenges include regulatory complexity, device-specific considerations, patient recruitment difficulties, and information collection and management issues.

