Overview
Trial protocols for medtech innovations are indispensable in steering the development and evaluation of medical devices. They ensure safety and efficacy through structured clinical studies. These protocols are not merely procedural; they streamline the regulatory approval process and enhance research credibility. Ultimately, this leads to improved patient outcomes and significant advancements in medical technology.
Introduction
In the rapidly evolving landscape of Medtech, clinical trials serve as the backbone for innovation, ensuring that new medical devices meet the highest standards of safety and efficacy. As the industry navigates complex regulatory requirements and patient recruitment challenges, the importance of meticulously crafted trial protocols cannot be overstated.
These protocols not only guide researchers through the intricacies of study design and methodology but also facilitate smoother regulatory approvals and bolster the credibility of research findings. With advancements in technology reshaping trial processes and a growing emphasis on patient-centric approaches, understanding the critical components of clinical trials is essential for stakeholders aiming to drive meaningful improvements in healthcare outcomes.
This exploration delves into the best practices, challenges, and future trends that define the clinical trial landscape in Medtech, highlighting the pivotal role of collaboration and innovation in shaping the future of medical device development.
Understanding Trial Protocols in Medtech Innovations
Protocols for clinical studies are essential documents that delineate the objectives, design, methodology, statistical considerations, and operational facets of a clinical study. They function as a comprehensive roadmap for researchers, ensuring that investigations are conducted with consistency and ethical integrity. In the realm of Medtech innovations, trial protocols for these advancements are particularly critical, as they establish the framework for assessing the safety and efficacy of new medical devices.
A meticulously crafted protocol not only streamlines the regulatory approval process but also enhances the credibility of research findings, which is vital for patient safety and treatment outcomes.
With over 20 years of experience in the Medtech sector, bioaccess® acknowledges the pivotal role that study protocols play in propelling medical technologies forward, particularly in Latin America. Our extensive research study management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
This ensures that every aspect of the study is meticulously managed with adaptability and specialized expertise. Recent trends indicate a notable shift in clinical trial design, emphasizing the reduction of patient burden during protocol development.
Clinical information leaders increasingly advocate for streamlined collection processes, prioritizing tangible patient benefits prior to the introduction of new technologies. As Leianne Ebert, Head of Clinical Data Operations, remarked, "This is something we monitor regularly, and last week, our records showed that 45% of our information is entered on the same day as the visit date." This approach aligns with the overarching objective of enhancing patient experience while upholding rigorous scientific standards.
Statistics underscore that adherence to study protocols is paramount, with recent figures indicating that 45% of entries occur on the same day as the visit date, showcasing the efficiency of real-time information capture. This trend highlights the significance of innovative study designs that streamline site experiences and minimize manual tasks, such as data entry, thereby accelerating the overall research process.
Furthermore, case analyses illuminate the constraints and future pathways of experimental protocols in medical device research. For instance, a recent study acknowledged the limitations of focusing solely on five publication years and four high-impact journals, suggesting that future research should encompass a broader range of studies to better understand publication trends and biases. bioaccess® is dedicated to addressing these limitations by broadening its research scope and methodologies, particularly in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
In conclusion, the significance of trial protocols for Medtech innovations cannot be overstated. They not only guide the research process but also play a crucial role in ensuring that new medical devices are developed with the highest standards of safety and efficacy in mind. Expert opinions consistently highlight that well-structured study protocols are foundational to the success of clinical research in the Medtech sector, paving the way for advancements that can significantly improve patient care.
The Critical Role of Clinical Trials in Medical Device Development
Trial protocols for medtech innovations form a crucial foundation in the development of medical devices, serving as the primary method for evaluating the safety and effectiveness of these innovative technologies. These meticulously structured tests collect data that verifies whether a device operates as intended and complies with regulatory standards. For instance, before a new cardiac stent can be marketed, it must successfully undergo a series of tests that assess its performance in real-world situations.
In 2025, the trial environment for medical devices is characterized by a significant emphasis on regulatory innovation, with the FDA modernizing regulations to streamline processes while upholding rigorous standards. The introduction of the single IRB (sIRB) review represents a notable advancement aimed at reducing redundant reviews in cooperative research, thereby expediting study initiation and enhancing research efficiency. This shift is anticipated to necessitate modifications in standard operating procedures and resource allocations for some stakeholders, reflecting the evolving nature of research.
Statistics reveal that the success rates of research studies for medical devices remain a critical focus, with approximately 30% of studies reaching their primary endpoints in 2025. Furthermore, the incorporation of artificial intelligence and machine learning is expected to reduce expenses by up to 20%, highlighting the potential for enhanced effectiveness in research. This underscores the significance of robust experimental studies in assessing new medical technologies, as they not only inform regulatory authorities but also provide healthcare providers and patients with essential information for making informed treatment choices.
Recent medical experiments have showcased the potential of groundbreaking devices, such as a new insulin delivery system that demonstrated improved patient outcomes in a pivotal examination. Perspectives from industry leaders emphasize the importance of trial protocols for medtech innovations in promoting research studies, with experts asserting that these studies are essential for enhancing development pathways and ensuring commercial feasibility. Notably, Dr. Jorge Hernando Ulloa presented one-year first-in-human findings on the VenoValve® at the Charing Cross International Symposium, highlighting advancements in vascular medicine and the critical role of such studies in driving innovation.
As Max Baumann, Head of Execution, notes, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." This statement reflects the ongoing regulatory innovations that bolster these optimization efforts.
Real-world examples further illustrate the impact of medical studies, such as the successful assessment of a new orthopedic implant that significantly reduced recovery times for patients. Additionally, sponsors are increasingly transitioning towards insourced models for data management, seeking greater control and transparency over their data. As the Medtech sector continues to evolve, trial protocols for medtech innovations will remain paramount in driving advancements and ensuring that new technologies meet the highest standards of safety and effectiveness.
bioaccess® plays an essential role in this landscape, overseeing various types of research including Early-Feasibility Assessments, First-In-Human Evaluations, and more, thus contributing to economic development and healthcare enhancement in the regions where these evaluations are conducted.
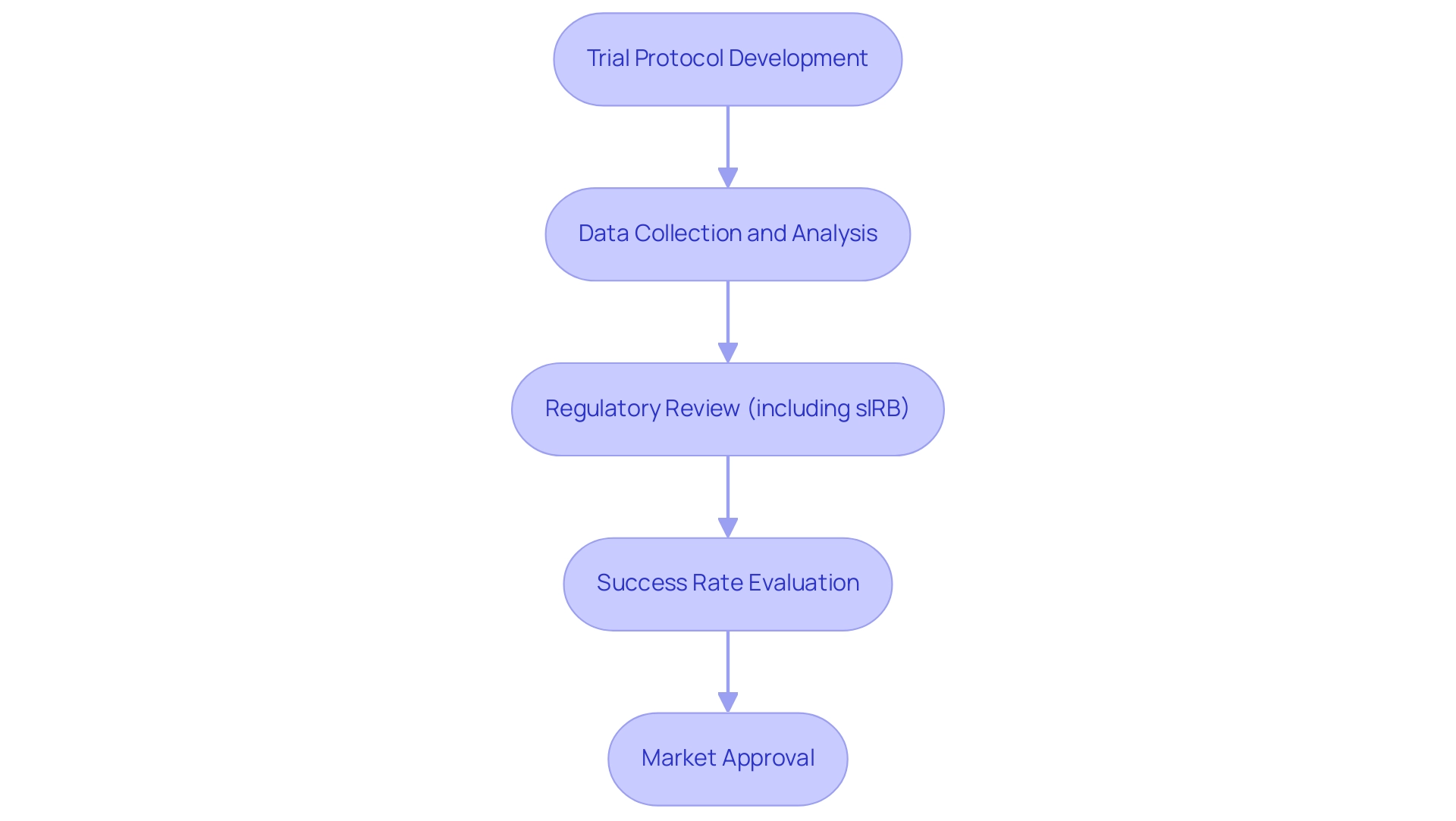
Phases of Clinical Trials: From Concept to Market
Trial protocols for medtech innovations are systematically organized into several essential phases in clinical studies for medical devices, with each phase serving a unique purpose in the development process. At bioaccess®, we leverage over 20 years of expertise in overseeing trial protocols for medtech innovations with a customized approach, ensuring a comprehensive strategy that encompasses feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting, specifically tailored for the Latin American market.
Phase I focuses on safety and dosage, typically involving a small group of 20 to 100 participants. This phase is essential for assessing the device's initial safety profile and determining the appropriate dosage levels. At bioaccess®, we prioritize early-feasibility evaluations (EFS) as part of our trial protocols for medtech innovations to establish a solid foundation for safety assessments.
Phase II expands the participant pool to include 100 to 300 individuals, allowing researchers to evaluate the device's efficacy and identify any side effects. This phase is crucial for understanding how the device performs in a controlled environment, and our team ensures rigorous oversight during this stage.
Phase III trials involve larger populations, often ranging from 300 to several thousand participants. This phase aims to confirm the device's effectiveness in accordance with trial protocols for medtech innovations, monitor adverse reactions, and compare it to standard treatments. The information collected here is essential for regulatory approval, and bioaccess® excels in key research that meets these crucial requirements.
Phase IV, or post-market evaluations, are conducted after a device receives approval. These tests collect further information on the long-term impacts and performance of the device in the general population, ensuring ongoing safety and efficacy. Our post-market evaluations (PMCF) are intended to deliver comprehensive insights into device performance after approval, following the trial protocols for medtech innovations.
Recent trends suggest that interventional studies are expected to dominate the research market, propelled by the rising demand for enhanced diagnostic tests and vaccines. Significantly, the completion rates for research phases have shown notable variability, with Phase I studies completing at a rate of approximately 70%, while Phase III studies can see completion rates as high as 90%.
In addition, GSK is utilizing rule-based automation for data cleaning, which accelerates the time to database lock, showcasing advancements in operational efficiency in research trials. The FDA is also proposing a rule to mandate single IRB review for FDA-regulated research, which aims to reduce duplicative reviews and expedite study initiation, although exceptions will be made for cases requiring local expertise.
Expert opinions underscore the significance of each phase in trial protocols for medtech innovations; for instance, clinical researchers emphasize that Phase I is critical for establishing safety, while Phase III is pivotal for confirming effectiveness before market entry. Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We did a small AI initiative to see if we can generate meaningful test information for setting up and validating our systems." It turns out we can.
This emphasizes the innovative methods being embraced in information management.
Moreover, there is a growing trend for sponsors to insource management processes, moving away from reliance on CROs, which enhances control over quality and operational efficiency.
As the environment of medical research advances, creative study designs and methodologies, including trial protocols for medtech innovations, are being embraced to improve operational efficiency through the use of automation and data management approaches. At bioaccess®, we are dedicated to maneuvering through the intricacies of research in Latin America, ensuring that medical devices are created with the highest level of care and accuracy.
In summary, understanding the phases of research trials—from concept to market—is essential for ensuring that medical devices are safe and effective for patient use. This structured approach not only facilitates regulatory compliance but also fosters trust in the medical technology sector.
Navigating Regulatory Requirements for Medtech Trials
Navigating the regulatory landscape presents significant challenges for conducting clinical studies that adhere to trial protocols for medtech innovations in the medical device sector. Regulatory authorities, such as the FDA in the United States and the EMA in Europe, enforce stringent guidelines that govern every aspect of the research process, from design to result reporting. Adhering to Good Clinical Practice (GCP) standards is crucial, and securing approvals from Institutional Review Boards (IRBs) is a prerequisite prior to commencing studies.
Non-compliance can lead to significant delays, potentially jeopardizing the market entry of innovative devices. In 2025, the impact of regulatory adherence on research timelines remains critical, with organizations facing evolving requirements that necessitate flexibility. For instance, recent statistics indicate that patient retention can be enhanced by 15-25% through improved data management practices, underscoring the importance of effective regulatory navigation.
Key regulatory guidelines for medical device studies encompass adherence to trial protocols for medtech innovations and the integration of adaptable methodologies. Emerging trends, such as decentralized and adaptive study designs, are gaining traction, enhancing the efficiency of trial protocols for medtech innovations and fostering engagement in research. A significant case analysis illustrates how drug development teams effectively prepared for shifting regulatory demands in embedded medical evaluations.
By prioritizing flexible systems and leveraging technology for compliance, these organizations positioned themselves to adeptly navigate regulatory changes, ensuring that evaluations remained safe and effective. Additionally, bioaccess® has established itself as a leader in accelerated medical device research services in Latin America, specializing in Early-Feasibility Assessments (EFA), First-In-Human Evaluations (FIH), Pilot Assessments, Pivotal Evaluations, and Post-Market Follow-Up Assessments (PMCF). Recently, bioaccess® collaborated with Caribbean Health Group to enhance recruitment efforts in Barranquilla, aiming to position it as a premier site for research studies in Latin America. This proactive approach reflects the organization's commitment to effectively navigating the regulatory landscape.
Expert insights reveal that the latest regulatory changes are reshaping the environment for Medtech studies. Regulatory experts emphasize that success in this landscape requires a careful balance of various perspectives, particularly in anticipating future compliance challenges. As the regulatory framework continues to evolve, Medtech firms must remain vigilant and proactive in their approach to compliance, ensuring that their research studies not only meet current standards but are also prepared for future developments.

Challenges in Conducting Clinical Trials for Medtech Innovations
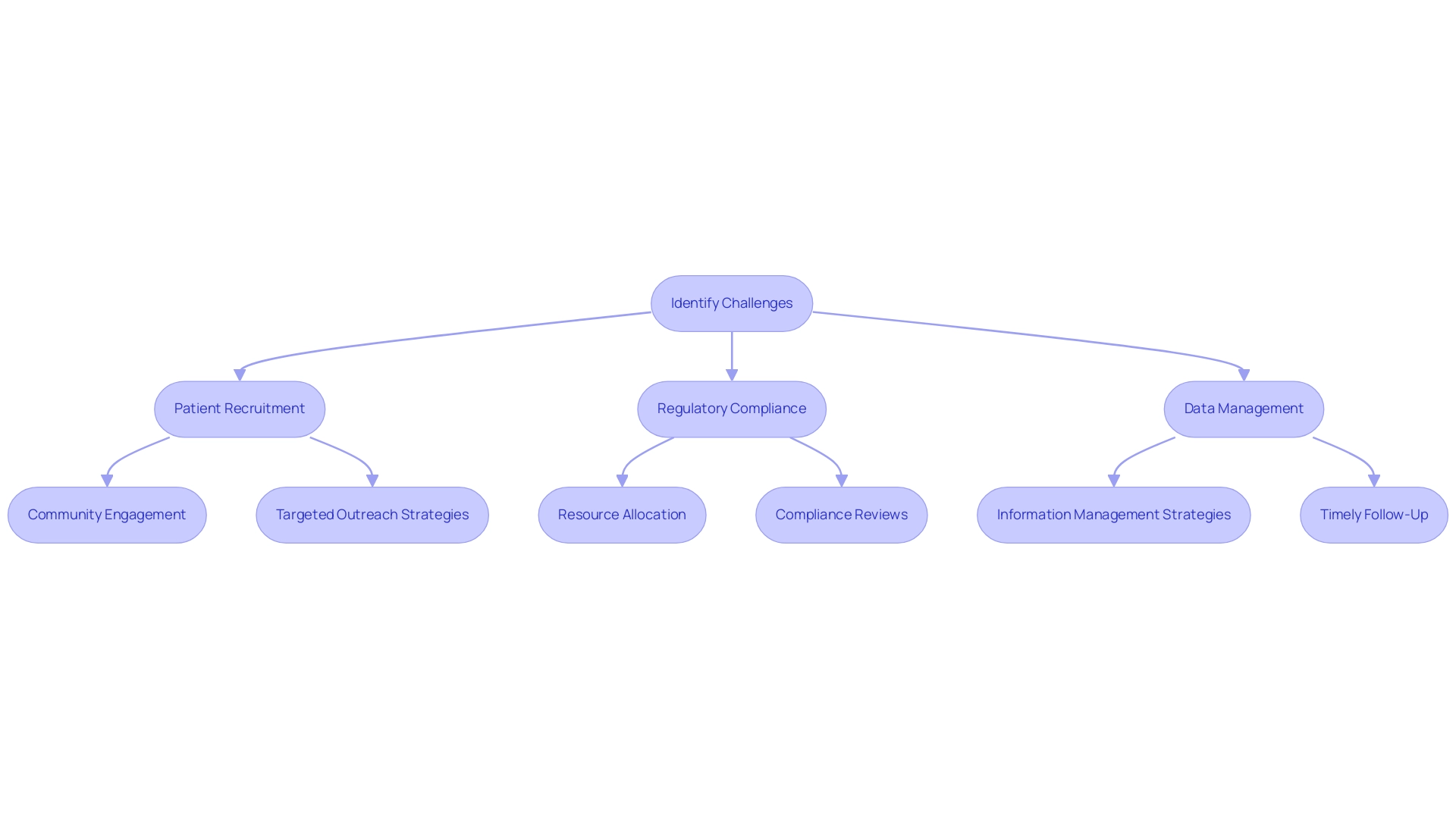
Carrying out trial protocols for medtech innovations requires navigating a complex environment filled with obstacles, including patient recruitment, regulatory adherence, and data management. Recruitment remains a significant hurdle; studies indicate that 28% of potential participants express interest in clinical studies but have reservations that can impede their involvement, particularly in the context of Medtech innovations. This reluctance often stems from stringent eligibility criteria, which can delay study timelines and hinder the overall progress of innovative medical devices.
Regulatory compliance is another critical aspect demanding meticulous attention. Ensuring adherence to evolving regulations throughout the testing process is essential, yet it can be resource-intensive and time-consuming. Organizations must allocate sufficient resources to maintain compliance, which can strain budgets and extend timelines.
In Colombia, the INVIMA plays a vital role as a Level 4 health authority, overseeing medical device regulations and ensuring compliance with international standards, which is crucial for the successful execution of experiments.
Data management further complicates the research landscape. The collection, analysis, and documentation of test information must be executed with precision to uphold accuracy and integrity. In 2025, the sector is anticipated to encounter growing information management challenges, with research suggesting that up to 30% of clinical research timelines could be shortened through efficient information strategies. This necessitates robust strategies to handle the influx of information generated during trials.
To address these challenges, strategic planning and effective communication are paramount. Leveraging advanced technologies, such as artificial intelligence and machine learning, can streamline processes and enhance patient recruitment efforts. For instance, a recent Phase IIb investigation on symptomatic endometriosis successfully recruited 318 patients across 51 sites in ten countries by employing targeted outreach strategies and community engagement. This demonstrates that innovative approaches can yield impressive results even under strict inclusion criteria.
Experts emphasize the importance of building relationships with underserved communities to improve participation. As highlighted by Dipanwita Das, CEO of Sorcero, significant investments in data strategy, patient diversity, and regulatory preparedness are crucial for optimizing study design and enhancing recruitment effectiveness. Furthermore, research from Harvard Business Review has shown that following up with a prospect within 24 hours increases the chance of conversion by 60 times compared to any later follow-up, underscoring the need for timely engagement in recruitment efforts.
By concentrating on these areas, organizations can better navigate the challenges of implementing trial protocols for medtech innovations in 2025. Additionally, bioaccess® offers comprehensive management services for research, including feasibility evaluations, site selection, compliance reviews, setup, import permits, project oversight, and reporting. Their expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies positions them as a leader in the field, particularly in Latin America.
The partnership between bioaccess® and Caribbean Health Group aims to establish Barranquilla as a prominent location for medical studies, backed by Colombia's Minister of Health, which additionally boosts the local economy through job creation and international cooperation.
Best Practices for Developing Effective Clinical Trial Protocols
Creating effective trial protocols for medtech innovations requires a strategic approach grounded in best practices. A fundamental step involves the precise definition of study objectives and endpoints within these protocols, which must align with regulatory expectations to ensure compliance and facilitate approval processes. Engaging stakeholders early—spanning from investigators to regulatory bodies—can significantly enhance collaboration and streamline the approval journey for these trial protocols.
Statistics reveal that studies with early stakeholder engagement experience a notable increase in success rates, with over 90% agreement in Delphi surveys regarded as consensus. This underscores the critical role of such engagement in clinical research.
Incorporating adaptive study designs stands as another best practice, offering flexibility in response to emerging data and thereby enhancing overall study efficiency. This approach permits modifications based on interim results, leading to more informed decision-making and efficient resource allocation.
Moreover, thorough training for all personnel involved in the trial protocols is essential. This training ensures adherence to the protocols and compliance with regulatory requirements, which is vital for preserving research integrity. A case study on cluster randomized experiments (CRTs) illustrates the complexities involved, including the need to account for clustering effects, potential imbalances due to post-randomization recruitment, and the necessity for small sample corrections when the number of clusters is low.
Recognizing these challenges has led to the development of specific guidelines aimed at improving the quality and clarity of reporting, ultimately enhancing the reliability of results from such studies.
As the landscape of medical research evolves, staying informed about trial protocols for medtech innovations is imperative. Industry experts assert that these protocols must be continuously updated to reflect the latest regulatory changes and technological advancements. Dipanwita Das, CEO & co-founder of bioaccess®, emphasizes that "regulatory preparedness is essential, as regulations are becoming increasingly complex and prescriptive."
This highlights the importance of monitoring FDA guidance on innovative study designs and decentralized clinical trial (DCT) approaches to ensure a seamless commercialization process. Furthermore, with over 20 years of experience in Medtech, bioaccess® provides extensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. With a focus on Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® ensures that Medtech startups can progress effectively from concept to commercialization, leveraging its adaptability and expert knowledge to navigate the unique challenges of research in Latin America.
The Importance of Collaboration in Medtech Clinical Trials
Cooperation among stakeholders is crucial for the success of trial protocols for medtech innovations in 2025. Collaborations among medical device firms, research organizations, regulatory bodies, and healthcare providers are essential for developing trial protocols that promote improved communication, resource sharing, and enhanced data quality. A significant example is the partnership between bioaccess® and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by Colombia's Minister of Health. This initiative is pivotal in attracting more research projects to the region, thereby improving the overall environment for trial protocols for medtech innovations.
Statistics indicate that sponsors who provide varied options for onboarding and visit experiences significantly enhance patient satisfaction and expand the participant pool, which is crucial for success. By offering more flexible onboarding processes, sponsors can better accommodate patient needs, ultimately leading to increased participation rates. Engaging healthcare experts in the creation of trial protocols for medtech innovations not only offers valuable perspectives on patient needs and preferences but also leads to more patient-focused investigations. Recent collaborations have demonstrated that integrating feedback from clinicians can enhance study protocols, resulting in higher recruitment rates and improved retention of participants.
Moreover, strong relationships with regulatory agencies can streamline the approval process for trial protocols in medtech innovations and ensure compliance with evolving regulations. The incorporation of artificial intelligence and machine learning in research studies has been shown to shorten study timelines by up to 30% and decrease expenses by as much as 20%. These technologies not only enhance efficiency but also facilitate collaboration among stakeholders by providing real-time data sharing and communication, which is essential for successful execution.
Successful collaborations in medical device research illustrate how teamwork can improve trial protocols for medtech innovations. A recent case study highlighted how a biotech firm partnered with a CRO and a healthcare provider to navigate complex regulatory landscapes, ultimately achieving both regulatory approval and commercial viability. This approach not only optimized the development journey but also ensured that the product met market needs, addressing the fundamental business model challenges faced by the biotech industry.
Industry leaders emphasize the significance of these partnerships. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shares from his experience with bioaccess® during its initial human study in Colombia, collaboration is essential to achieving positive health results. Ryan Jones, CEO of Florence Healthcare, observes, "By emphasizing interoperable, site-focused technology, sites, sponsors, and CROs can enhance relationships, speed up timelines, and pave the way in an increasingly digital research environment." Expert opinions consistently underscore that collaboration is not only advantageous but essential for advancing trial protocols for medtech innovations and achieving positive health outcomes in 2025.
Future Trends in Medtech Clinical Trials: Embracing Innovation
The future of Medtech clinical studies is poised for a significant transformation, primarily fueled by advancements in technology and analytics, alongside enhanced trial protocols for medtech innovations. Innovations such as artificial intelligence (AI), machine learning, and decentralized study models are set to elevate efficiency and patient engagement in trial protocols for medtech innovations. For instance, AI can markedly improve patient recruitment processes by analyzing extensive datasets to identify the most suitable candidates with greater precision.
Moreover, the integration of wearable devices and remote monitoring technologies into trial protocols for medtech innovations facilitates real-time information collection, thereby enhancing the accuracy and timeliness of trial results.
As the medical research landscape evolves, the resurgence of Medical Device Regulation (MDR) highlights the critical necessity for trial protocols for medtech innovations to ensure standardized data practices. This shift not only bolsters the development of medical guidelines but also enables future indirect comparisons among interventions, as noted by industry experts. As Peng Lu, chief medical officer of Dutch biotech Pharvaris, articulated, "Homogenizing the use of specific outcomes and outcome measures for studies will support guidelines development and future indirect comparisons among interventions."
The research study failure rate remains alarmingly high at 90%, underscoring the unpredictability of the process and the pressing need for trial protocols for medtech innovations.
In this context, bioaccess® distinguishes itself with its comprehensive study management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Leveraging over 20 years of experience in Medtech, bioaccess® empowers sponsors to navigate the complexities of trial protocols for medtech innovations effectively, enhancing the quality of information and patient outcomes.
Additionally, a growing trend among sponsors is the insourcing of information management processes, moving away from traditional outsourcing models. This strategic shift enables sponsors to maintain greater control over their information, resulting in improved quality and better patient outcomes. As stakeholders in the Medtech sector embrace these innovations, it is imperative to remain adaptable and proactive in integrating trial protocols for medtech innovations into research processes, ensuring that advancements translate into tangible benefits for patients and the healthcare system alike.
Furthermore, advancements in research studies are streamlining site experiences, such as faster query responses and intuitive data entry systems, further enhancing the efficiency of the research process. The impact of these clinical studies extends beyond individual trials, contributing to local economies through job creation, economic growth, and improved healthcare outcomes, thereby fostering international collaboration in the Medtech field.
Conclusion
The exploration of clinical trials in the Medtech sector underscores their fundamental role in advancing medical device innovation. From the meticulous crafting of trial protocols to the systematic phases of clinical studies, every aspect is designed to ensure safety, efficacy, and regulatory compliance. Effective collaboration among stakeholders—including sponsors, regulatory bodies, and healthcare providers—enhances the clinical trial experience, improving patient recruitment and retention, and ultimately driving successful outcomes.
Moreover, emerging trends such as the integration of artificial intelligence and decentralized trial models are reshaping the landscape of clinical research, promising increased efficiency and better patient engagement. As the industry adapts to evolving regulatory requirements and embraces innovative methodologies, it is evident that the future of Medtech clinical trials will hinge on flexibility, strategic planning, and a commitment to continuous improvement.
In conclusion, the significance of well-structured clinical trials cannot be overstated. They serve as the backbone of the Medtech industry, ensuring that new medical devices not only meet rigorous safety and efficacy standards but also address the needs of patients and healthcare providers. As stakeholders continue to navigate the complexities of clinical research, a focus on innovation, collaboration, and best practices will be essential in driving meaningful advancements in healthcare outcomes. The journey from concept to market is a collaborative effort, and by embracing these principles, the Medtech sector can pave the way for a healthier future for all.
Frequently Asked Questions
What are clinical study protocols and their importance?
Clinical study protocols are essential documents that outline the objectives, design, methodology, statistical considerations, and operational aspects of a clinical study. They serve as a comprehensive roadmap for researchers, ensuring consistency and ethical integrity in investigations.
Why are trial protocols particularly critical in Medtech innovations?
Trial protocols are crucial in Medtech innovations as they establish the framework for evaluating the safety and efficacy of new medical devices. A well-crafted protocol streamlines regulatory approval processes and enhances the credibility of research findings, which is vital for patient safety and treatment outcomes.
What services does bioaccess® provide in relation to study protocols?
bioaccess® offers extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting, ensuring meticulous management of all study aspects.
What recent trends are emerging in clinical trial design?
Recent trends indicate a shift towards reducing patient burden during protocol development. Clinical information leaders advocate for streamlined data collection processes that prioritize tangible patient benefits before introducing new technologies.
How effective is real-time information capture in clinical studies?
Statistics show that adherence to study protocols is paramount, with about 45% of data entries occurring on the same day as the visit date, highlighting the efficiency of real-time information capture and innovative study designs that minimize manual tasks.
What limitations have been identified in experimental protocols for medical device research?
Recent studies have highlighted limitations in focusing solely on a narrow range of publication years and journals, suggesting that future research should encompass a broader scope to better understand publication trends and biases.
What role do trial protocols play in the development of medical devices?
Trial protocols serve as the foundation for evaluating the safety and effectiveness of medical devices, ensuring that they meet regulatory standards before being marketed. They guide the research process and are essential for the advancement of new technologies.
How is the regulatory environment for medical devices evolving?
The regulatory environment is characterized by innovations such as the FDA's modernization of regulations and the introduction of the single IRB (sIRB) review to streamline processes while maintaining rigorous standards.
What is the expected success rate for medical device research studies in 2025?
Approximately 30% of research studies for medical devices are expected to reach their primary endpoints in 2025, indicating a critical focus on research effectiveness.
How is bioaccess® contributing to the Medtech sector?
bioaccess® oversees various types of research, including Early-Feasibility Assessments and First-In-Human Evaluations, contributing to economic development and healthcare enhancement in the regions where these evaluations are conducted.

