Introduction
The FDA's 510(k) premarket notification is a rigorous process that ensures medical devices are safe and effective. It requires devices to be on par with existing devices in terms of safety and efficacy. This article explores the eligibility criteria for 510(k) clearance, the concept of substantial equivalence, the content of a 510(k) submission, preparing and submitting a 510(k) application, the 510(k) review process, post-clearance requirements and inspections, and what to do if a device is not found substantially equivalent.
Navigating the 510(k) process requires a thorough understanding of the device, its users, and the competitive environment. By adhering to FDA guidelines, medical device companies can enhance their submission's likelihood of success and contribute to patient care and innovation in the healthcare industry.
What is a 510(k) Premarket Notification
The FDA's 510(k) premarket notification is a rigorous process that requires medical instruments to be as safe and effective as existing, legally marketed instruments. This submission, crucial for instruments not classified as Class I exempt, ensures that any new apparatus is on par with a predicate device in terms of safety and efficacy. For example, the Impella Connect System, consisting of both software and hardware, needed to showcase these standards, offering essential remote monitoring capabilities for ventricular support equipment in a healthcare environment. The system's ability to deliver real-time alerts and detailed case information is a testament to the stringent requirements of a 510(k) submission. Moreover, with technologies like Masimo SET, which significantly improved pulse oximetry, the 510(k) process has been instrumental in bringing innovative solutions to market that enhance patient care and reduce healthcare costs. These examples highlight the FDA's commitment, as stated in their recent communications, to public health by enforcing standards that ensure devices are both safe and effective for public use. To maneuver through this intricate approval landscape, a deep comprehension of the apparatus, its users, and the competitive environment is crucial, along with a comprehensive comparative analysis of potential predicate devices. By following these procedures, the FDA continues to support the introduction of cutting-edge healthcare technology that ensures improved patient outcomes and fosters further innovation in the industry.
Eligibility Criteria for 510(k) Clearance
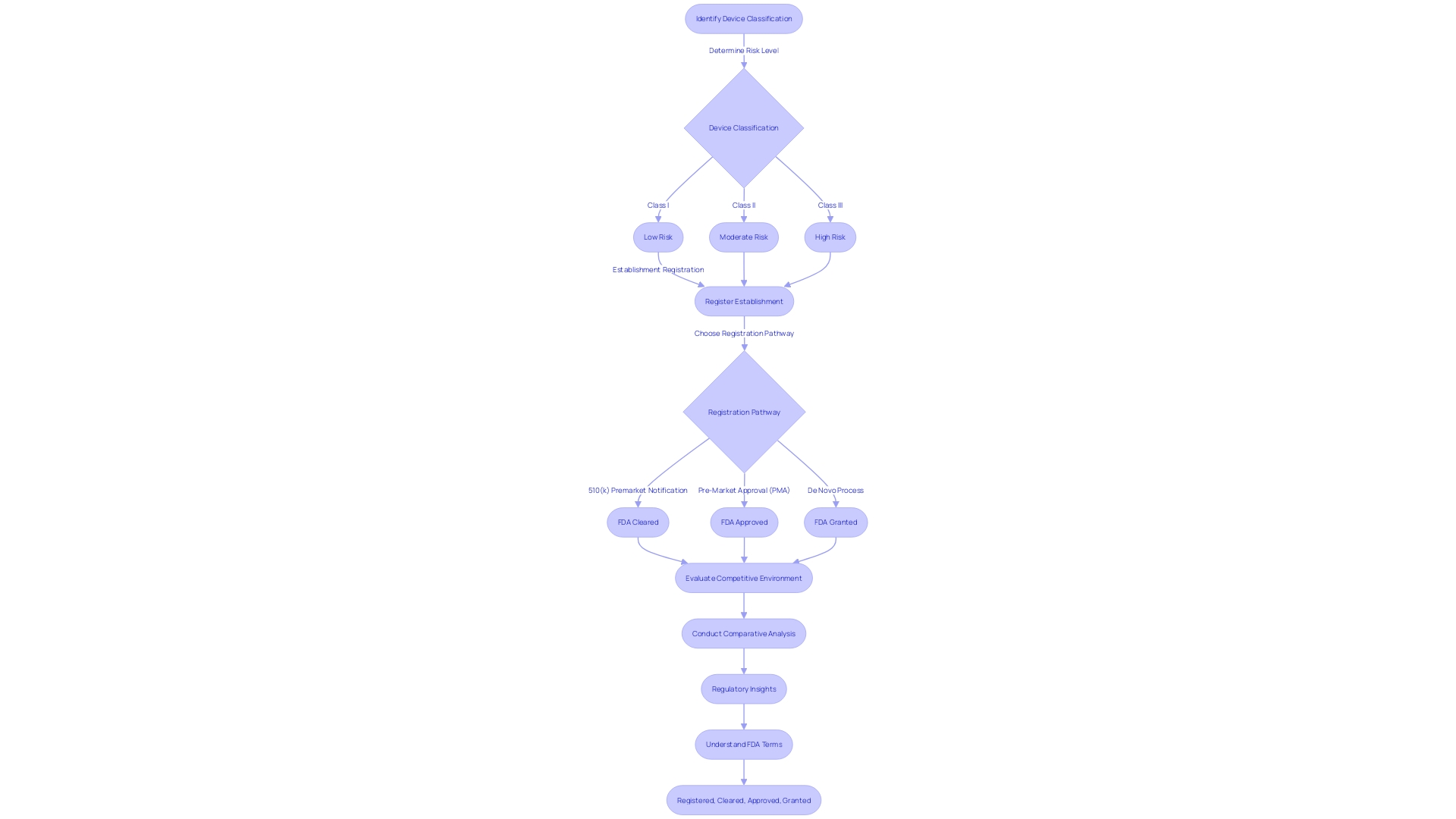
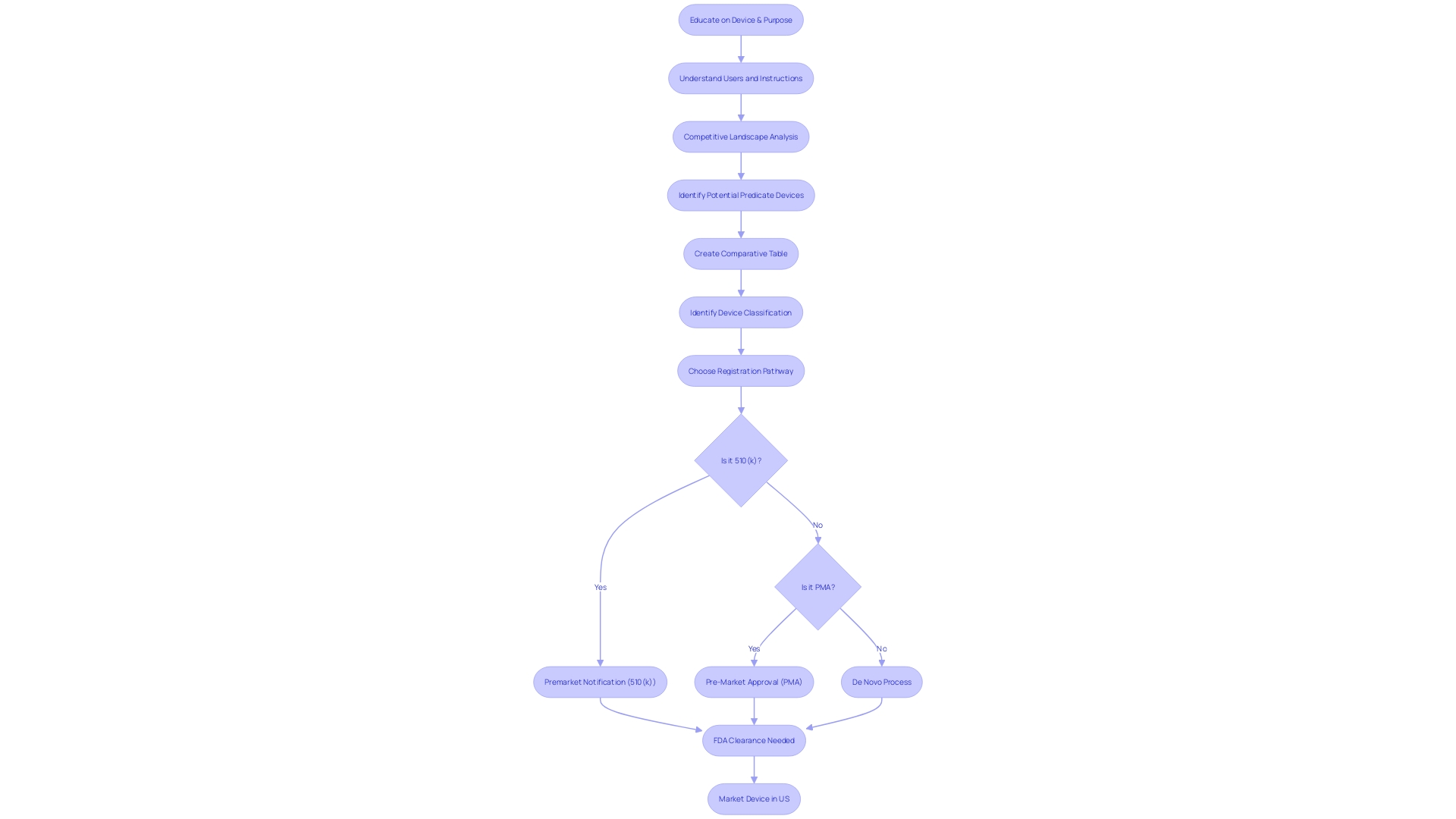
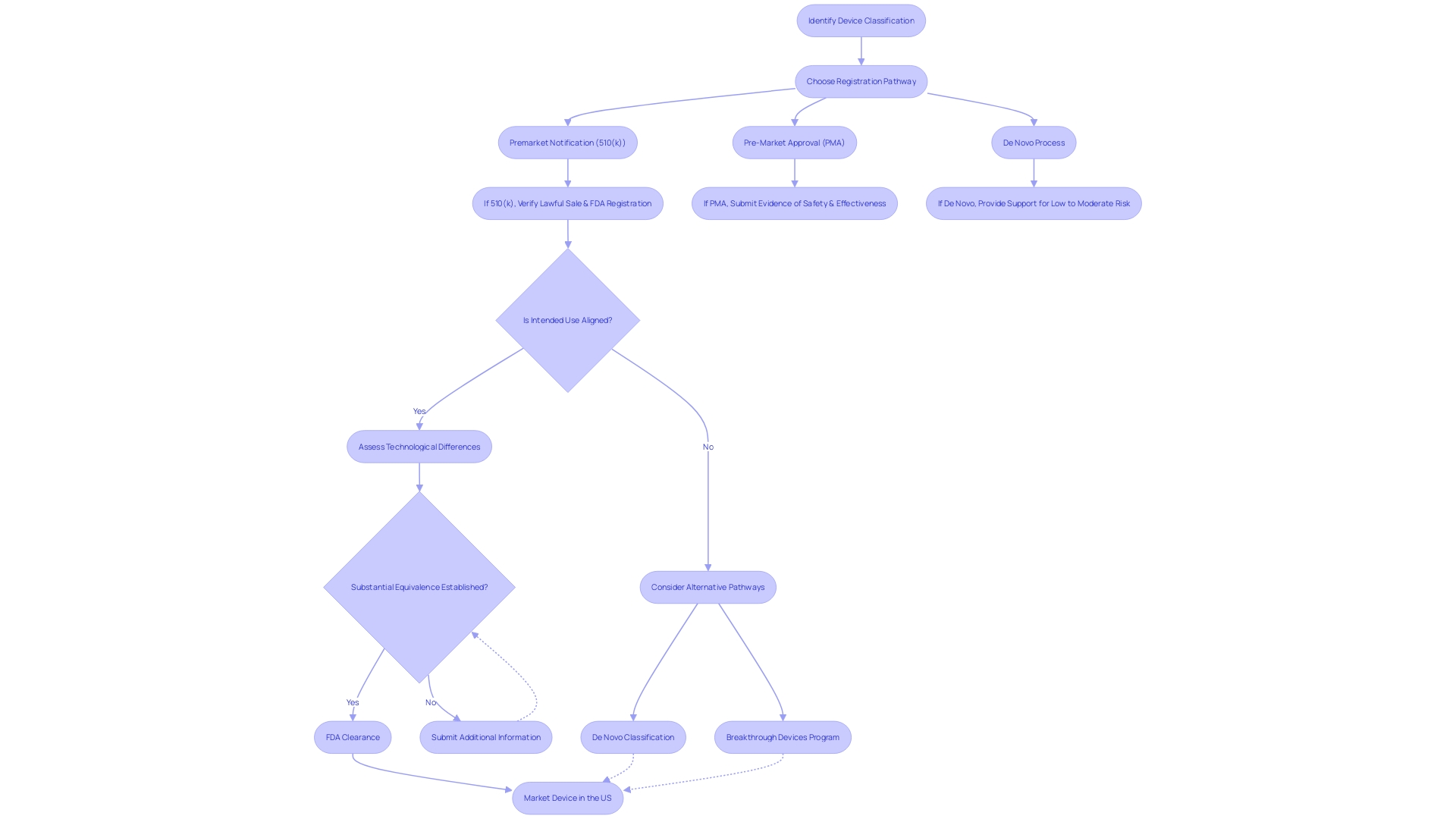
Navigating the FDA's 510(k) clearance process requires meticulous preparation, starting with the accurate classification of the medical equipment in question. Devices are segmented into three distinct categories based on the level of risk they pose to patients. This classification is the foundation for determining the appropriate regulatory pathway, be it a 510(k) Premarket Notification, Pre-Market Approval (PMA), or De Novo process. Attaining a profound comprehension of the apparatus, encompassing its users, instructions for use, and any potential warnings, is crucial. Working together with the Marketing team to evaluate the competitive environment can reveal similar products, which act as references for the 510(k) submission.
Producing a comparative table that carefully contrasts the subject apparatus with these associated devices, emphasizing on intended purpose and technological characteristics, is a strategic measure. This comparative analysis is not only a regulatory requirement but also a crucial tool in elucidating how the new apparatus is at least as safe and effective as its predicate.
Moreover, staying informed about the latest developments and regulatory insights is critical. For example, the FDA's recent webinar on updated final guidance for Breakthrough Device designation emphasizes the intricacies and criteria that products must fulfill to be eligible for expedited review procedures. This knowledge can prove invaluable in preparing a submission that aligns with FDA expectations and ultimately facilitates market entry.
To demonstrate the practical use of these principles, consider the example of VersaWrapâan innovative tool that has been customized to address intricate anatomical injuries and is especially advantageous for pediatric patients. The design and functionality of the product highlight the significance of its specific characteristics in relation to FDA clearance.
Understanding the distinctions between terms such as Registered, Cleared, Approved, and Granted is also crucial. Each term represents a distinct level of FDA review and authorization, reflecting the extent of evaluation a healthcare instrument has undergone. It's essential to comprehend these differences to navigate the regulatory environment successfully.
By following these guidelines, companies in the field of healthcare instruments can improve the chances of their 510(k) submission being successful, guaranteeing that their advancements can reach the market and have a positive impact on patient care.
The Predicate Device: Understanding Substantial Equivalence
The notion of 'significant similarity' is crucial in navigating the 510(k) clearance pathway for healthcare instruments. It entails a thorough evaluation of a new medical apparatus in contrast to an existing instrument already authorized for sale. To be deemed substantially equivalent, the new equipment must not only have the same intended purpose but also demonstrate similar technological characteristics and provide comparable performance results.
When identifying an appropriate statement, it is crucial to have a thorough comprehension of the equipment's users, including clinicians and patients, and to carefully examine its instructions for use, as well as any related cautions and advisories. Working together with the Marketing department can provide valuable insights into the competitive landscape, enabling a comparison with competitor products through a variety of sources such as literature, clinical studies, and marketing materials. This research culminates in the creation of a comparative table that pinpoints both similarities and distinctions in technological characteristics.
It's essential to delve into the 510(k) Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA’s database. This step ensures a strong evaluation of the safety and effectiveness of the primary apparatus, thus laying the foundation for a successful submission.
Moreover, the FDA has outlined scenarios in which clinical data might be necessary to demonstrate substantial equivalence. These include variations in indications for use, technological characteristics, instances where non-clinical testing is inadequate, or when new risks associated with the reference product arise. Understanding these nuances is crucial for compliance with FDA guidelines and for ensuring that submissions meet the stringent criteria set forth by the agency.
Considering the evolving landscape of regulations for healthcare devices, it is noteworthy that the FDA has been refining its 510(k) program. This includes drafting guidance on when clinical data is required for submissions, reaffirming the agency's commitment to safeguarding public health while fostering innovation in medical technology.
In summary, grasping the nuances of substantial equivalence is crucial for a successful 510(k) submission. It necessitates a strategic approach that includes a comprehensive comprehension of the subject machinery, prudent selection of the predicate, and adherence to FDA regulatory frameworks.
Content of a 510(k) Submission
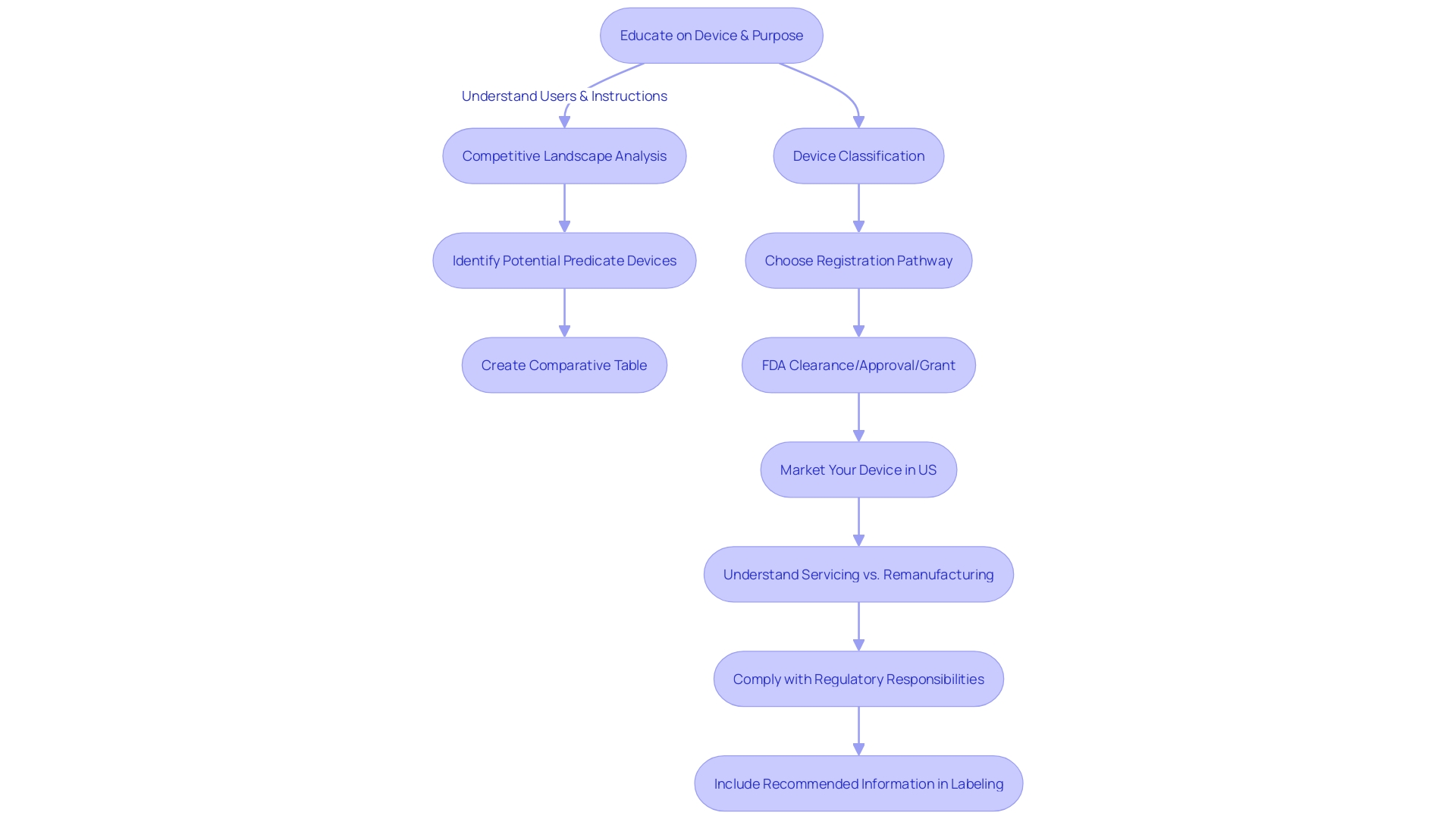
Creating a comprehensive 510(k) submission is crucial, as it needs to include a complete set of documentation that supports the assertion of significant similarity to a previously cleared product. This includes a detailed account of the medical equipment's intended use, precise technical specifications, and valid performance data. The inclusion of labeling, along with results from biocompatibility and safety testing, is also imperative.
It is crucial for those preparing the submission to thoroughly educate themselves about the apparatus's users, which may vary from clinicians and physicians to patients, and to understand the intricacies of its use, including any warnings and cautions. A comparative analysis of the device against similar ones in the market is also recommended, drawing information from an array of sources such as research literature, clinical studies, and marketing materials.
Furthermore, the FDA's continued endeavors to update the 510(k) process have resulted in the creation of a draft guidance document published on September 7, 2023, which outlines recommended methods for choosing a suitable reference. This includes verifying that the potential predicate is legally marketed and registered with the FDA, and that it shares the same intended use without introducing new safety and effectiveness concerns due to differing technological characteristics.
Preparing and Submitting a 510(k) Application
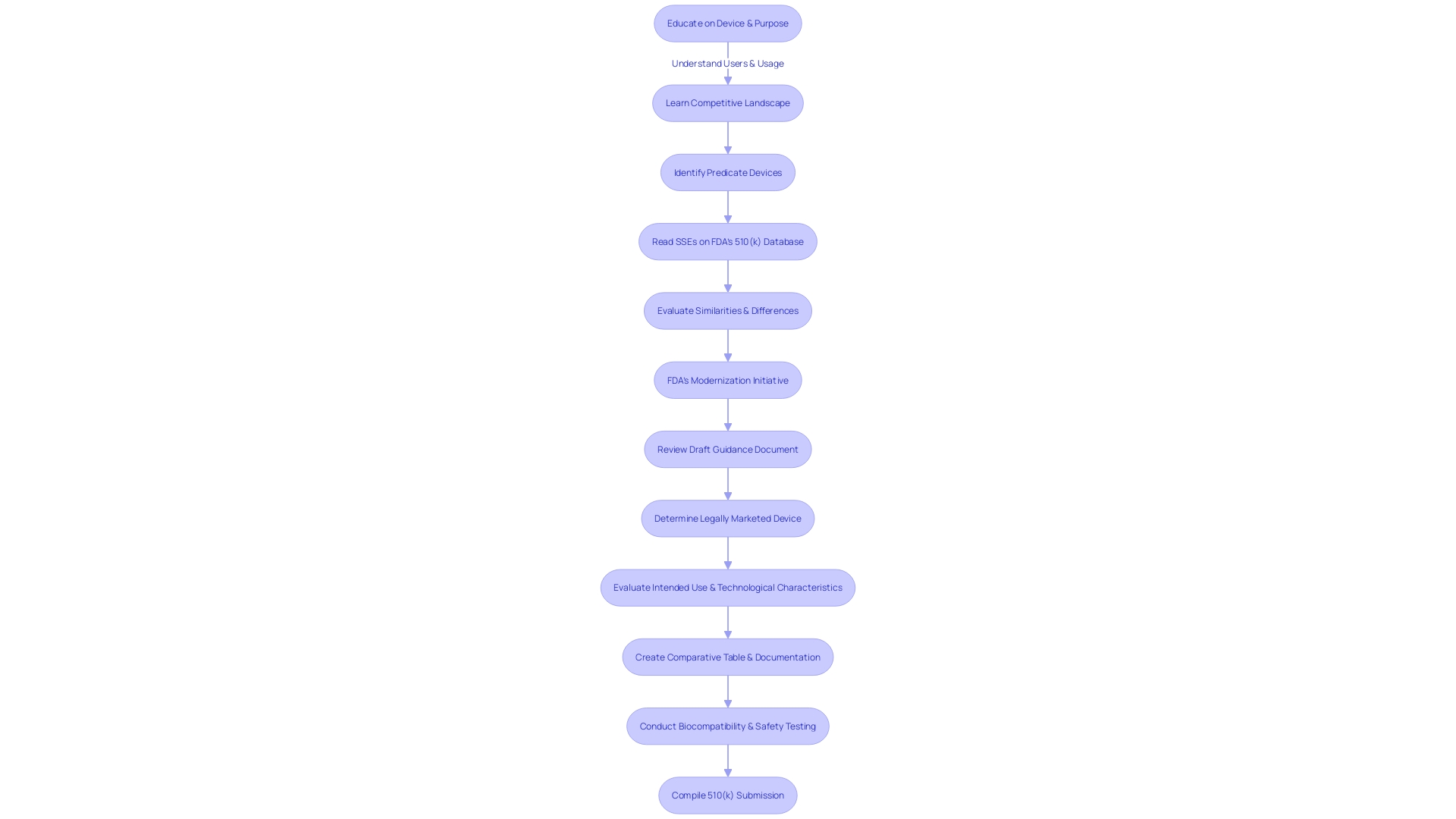
Navigating the 510(k) submission process for medical products is a intricate undertaking that demands a methodical approach. It starts with a comprehensive understanding of the subject equipment, including its users—clinicians, physicians, dentists, patients, etc.—and clearly defined instructions for use, complete with warnings and cautions. In collaboration with the marketing team, it is necessary to examine the competitive landscape and identify a device that has the same intended use and technological characteristics. This involves an intensive review of research literature, clinical studies, and competitor marketing materials to construct a comparative table that can serve as the backbone of the submission.
The FDA's ongoing modernization efforts, including the release of a draft guidance document on September 7, 2023, outline best practices for criteria selection. This guidance emphasizes the significance of selecting a reference product that is not only lawfully marketed but also serves the same purpose without raising new concerns about safety and effectiveness because of varying technological features. Moreover, the use of older predicates can offer substantial benefits, such as the accumulation of long-term safety data.
As per the FDA, the safety, effectiveness, and security of healthcare equipment are of utmost importance. With this in mind, it is crucial to ensure that all aspects of the 510(k) application are meticulously prepared, from the gathering of testing data, labeling, and manufacturing information to the completion of the necessary FDA forms and fee payments. Understanding each step and requirement within the 510(k) submission is critical to navigating the process successfully and achieving a smooth submission that meets all regulatory standards.
As the industry evolves, it's imperative to stay informed about recent FDA publications and standards, such as those pertaining to direct-to-consumer prescription drug advertisements, which demand clarity, conspicuousness, and neutrality in the presentation of major side effects and contraindications. Such standards are indicative of the FDA's commitment to consumer-friendly communication, which also resonates in the context of medical instrument submissions.
Finally, the perspectives provided by experienced professionals underscore the importance of gaining thorough understanding about the apparatus meant for submission. This knowledge foundation, combined with a strategic analysis of the market and competitors, equips companies with the necessary tools to navigate the intricacies of the 510(k) process and paves the way for a successful product introduction.
The 510(k) Review Process: Timeline and Communication
Upon submission of a 510(k) to the FDA, medical equipment companies must navigate a carefully organized review process aimed at assessing whether the product is substantially similar to an existing, legally marketed item known as a reference. This evaluation is crucial for items categorized under the lower-risk categories of class one and two, requiring them to be compared to a predicate instrument for 510(k) clearance. The FDA's three-tier classification system categorizes products based on risk, with class three itemsâlike life-sustaining implantablesâundergoing more stringent scrutiny.
Medical equipment companies should thoroughly educate themselves on their product, including its intended use, user instructions, and potential risks. This understanding, combined with a comprehensive comparative examination of the competitive scenery and reference equipment, establishes the basis of a prosperous submission. A well-prepared comparative table exhibiting similarities in intended use and technological characteristics with the predicate equipment is essential.
The FDA's review timeline is punctuated with specific milestones, allowing for structured communication between the agency and the sponsor of the equipment. It's imperative for companies to understand this timeline and the associated communication protocols to effectively manage expectations and maintain an informed position throughout the regulatory journey. Effective navigation of this procedure necessitates a thorough understanding of the instrument's categorization, the intricacies of the 510(k) authorization, and the wider framework of FDA's responsibility in guaranteeing instrument safety and efficacy, all while being aware of the possibility of delays in coverage or payment subsequent to clearance.
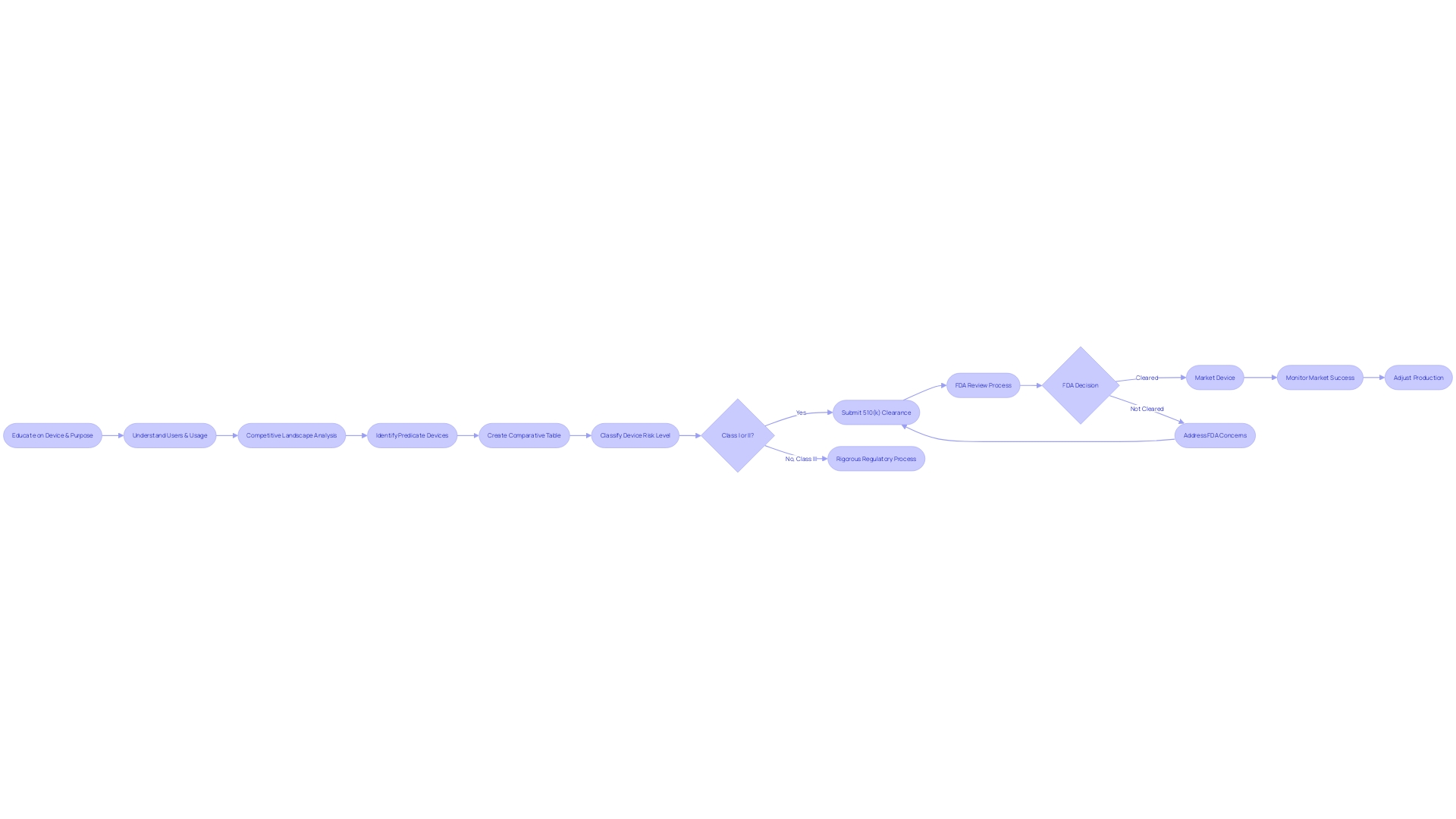
Acceptance Review and Substantive Review
When medical companies submit a 510(k) application to the FDA, the review process is rigorous and thorough, involving two primary stages: acceptance review and substantive review. The initial acceptance review is designed to check for completeness, verifying that all necessary components are included to allow for a more detailed examination. If the submission successfully passes this preliminary phase, it proceeds to the substantive review. In this stage, the FDA examines the scientific and technical data provided to determine whether the new equipment is significantly similar to a legally marketed reference equipment. The outcomes of these reviews can be significantly influenced by the quality of the information submitted. For instance, a company's response that lacks details on the outcomes of corrective actions, such as those seen in the case of a firm addressing complaint coding and quality data analysis issues, may not satisfy the FDA's requirements for a complete response. Moreover, failing to establish and maintain proper design validation procedures, including a comprehensive risk analysis as mandated by 21 CFR 820.30(g), can further complicate the review process. It is, therefore, crucial that companies thoroughly educate themselves on the intended use and technological characteristics of their product, as well as the competitive landscape, to create a robust submission. Recognizing the proper classification and selecting the appropriate regulatory pathway—whether it's a 510(k) notification, PMA, or De Novo process—is fundamental to navigating the FDA's evaluation successfully. While the FDA upholds its mission to ensure the safety and effectiveness of healthcare devices, it is crucial for companies to present their submissions in a clear and organized manner, reflecting the standards set forth by the FDA for transparency and comprehensibility in public communications.
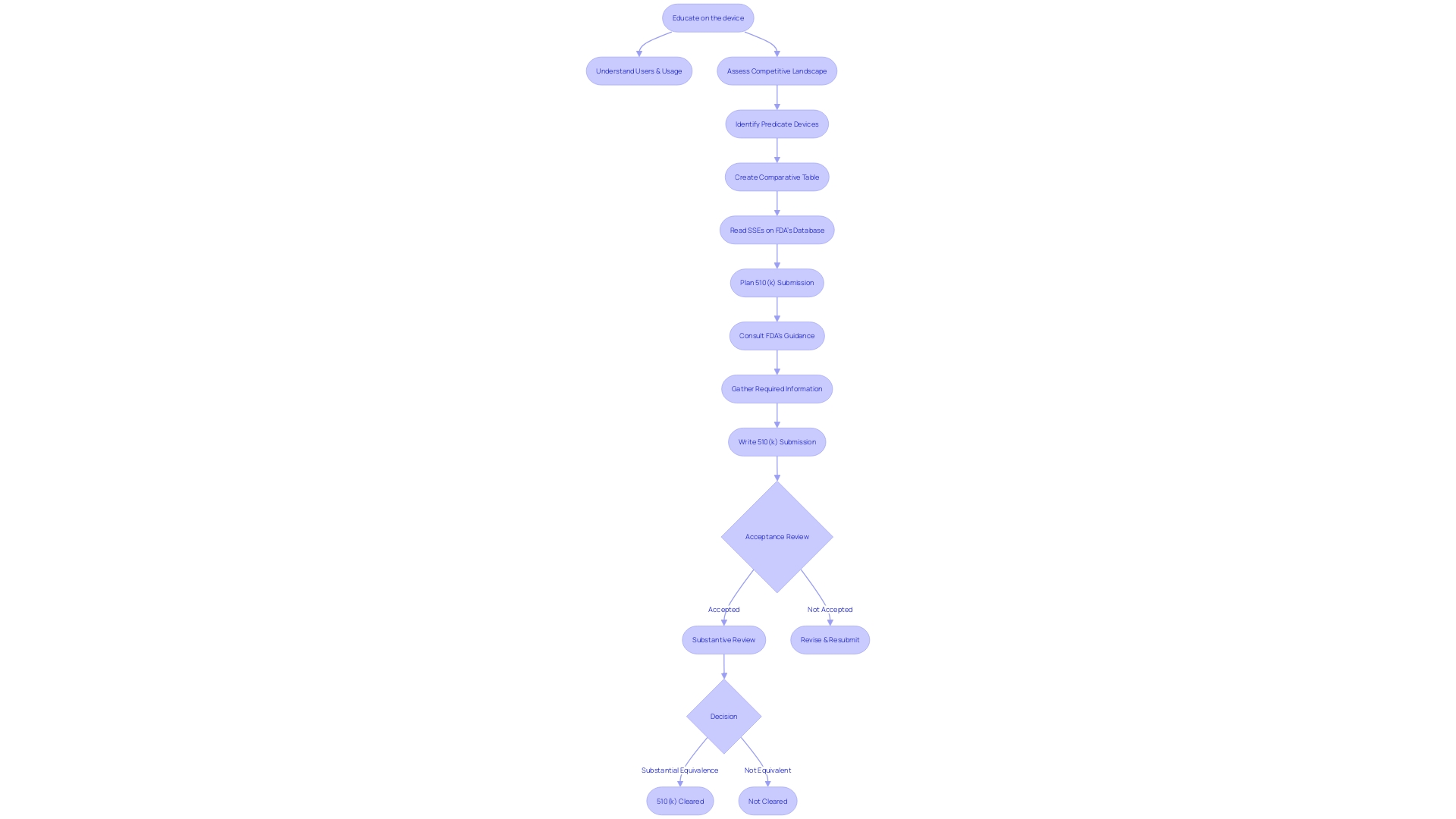
Determining Substantial Equivalence
Obtaining 510(k) clearance from the FDA is dependent on showing that a new medical equipment is essentially the same as a legally marketed reference instrument. This crucial decision involves a comprehensive comparison of the intended use, technological features, and performance data of the new product against the reference's. In certain cases, clinical data becomes a vital component of this review process. The FDA's draft guidance outlines four specific scenarios where clinical data is essential:
- When the intended use of the new tool varies from that of the reference.
- When there are technological differences between the new equipment and the reference which cannot be adequately assessed through non-clinical testing alone.
- If non-clinical testing (analytical, bench, and/or animal studies) is insufficient to determine substantial equivalence.
- If the predicate apparatus presents new or increased risks that require clinical data to ensure safety and effectiveness for the new mechanism.
Clinical studies may include a range of data points such as bench performance testing, sterilization and shelf-life testing, biocompatibility, and safety testing in various domains including electrical, mechanical, thermal, electromagnetic compatibility, software reliability, and cybersecurity. The recent efforts by the FDA to update the 510(k) program have highlighted the importance of clinical data in guaranteeing the safety, purity, and effectiveness of healthcare instruments. In parallel, the agency's mission to safeguard public health underpins the rigorous scrutiny applied during the clearance process. The examples given in the draft guidance, covering both diagnostic and therapeutic instruments, offer practical illustrations for companies in the field of healthcare technology as they prepare their submissions.
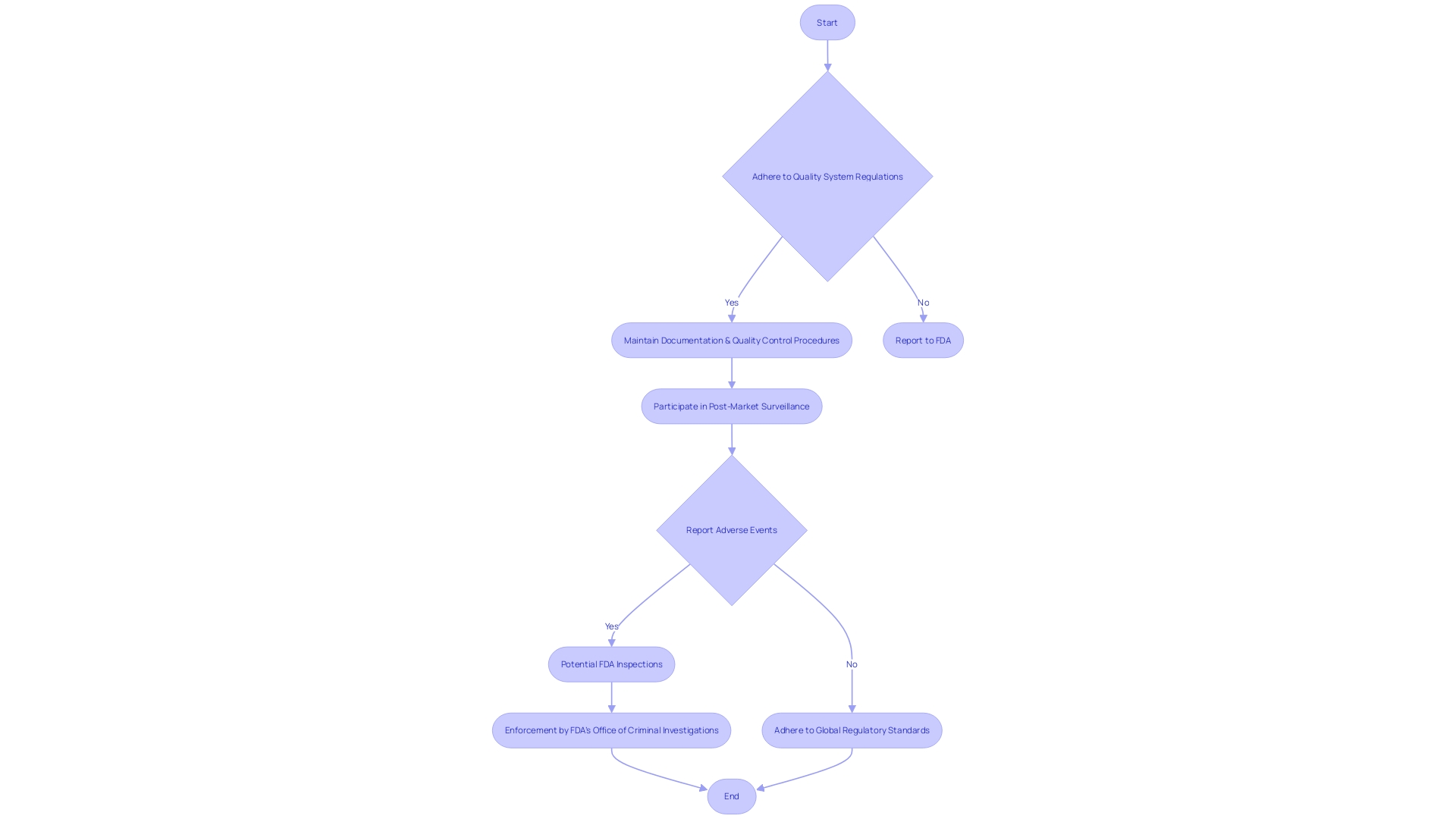
Post-Clearance Requirements and Inspections
Navigating post-clearance obligations is an essential aspect of medical instrument regulation. Once a product obtains 510(k) clearance, manufacturers must adhere to rigorous post-clearance requirements to ensure ongoing compliance with the FDA. These obligations encompass maintaining adherence to quality system regulations, which demand rigorous documentation and quality control procedures. Moreover, businesses are responsible for participating in post-market surveillance to monitor the performance of their products in real-world settings. Adverse event reporting is another critical component, requiring prompt notification to the FDA of any serious incidents associated with the utilization of the equipment.
It's crucial for medical tool manufacturers to understand these post-clearance requirements thoroughly. Furthermore, they must be prepared for potential FDA inspections, which serve to verify compliance and the continued safety and efficacy of the product on the market. The FDA's Office of Criminal Investigations vigilantly enforces these standards, as non-compliance can pose significant risks to patient safety. To remain compliant, companies often opt for global regulatory adherence, which simplifies the complex landscape of varying regional regulations. This method guarantees that their equipment meets the utmost criteria of safety and efficiency, regardless of where they are sold or used. Staying updated on changing regulations and industry best practices is crucial, as the sector for healthcare technology continues to progress and regulatory requirements become more demanding.
What to Do if Your Device is Not Found Substantially Equivalent
Finding an instrument substantially similar to its predecessor can be challenging, especially when the FDA does not consider it as equivalent. However, this scenario opens up opportunities for companies to reassess and pursue different strategies. The initial crucial stage is verifying that the potential product is lawfully sold and presently registered with the FDA. Then, it's essential to ensure the intended use aligns and that any technological differences do not introduce new safety or effectiveness concerns. The FDA's draft guidance document, published on September 7, 2023, provides comprehensive recommendations for choosing a suitable reference, highlighting the reliance on FDA-acknowledged consensus standards, guidance documents, and qualified tools for developing medical devices. Utilizing established methods and having access to comprehensive information about the predicate can provide a solid foundation for submission. In instances where substantial equivalence is not established, companies might consider additional information submission or alternative regulatory pathways, such as the De Novo classification process, to achieve FDA approval. The recent update to the Breakthrough Devices Program also offers a pathway for equipment that offers more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. In light of the FDA's longstanding commitment to assuring public health and safety, as well as the ever-evolving regulatory landscape, medical device companies must stay informed and adaptable in their approach to FDA clearance and approval processes.
Conclusion
In conclusion, navigating the FDA's 510(k) premarket notification process requires a thorough understanding of the device, adherence to FDA guidelines, and meticulous preparation of the submission. By adhering to the eligibility criteria, understanding substantial equivalence, and crafting a comprehensive 510(k) submission, medical device companies can enhance their likelihood of success.
The 510(k) review process involves structured communication between the FDA and the device's sponsor. It is essential for companies to understand the review timeline and associated communication protocols to effectively manage expectations and maintain an informed position throughout the regulatory journey.
Post-clearance requirements and inspections are crucial aspects of medical device regulation. Adhering to quality system regulations, engaging in post-market surveillance, and promptly reporting adverse events are obligations that ensure ongoing compliance and the continued safety and efficacy of the device.
In cases where a device is not found substantially equivalent, companies have the opportunity to reassess and pursue different strategies. Confirming the legality and registration of the potential predicate device is a critical first step, and exploring alternative regulatory pathways, such as the De Novo classification process or the updated Breakthrough Devices Program, can be considered.
In summary, navigating the 510(k) process requires thorough preparation, adherence to FDA guidelines, and staying informed about regulatory updates. By following these steps, medical device companies can enhance their submission's likelihood of success, contribute to patient care and innovation, and ensure the safety and effectiveness of their devices in the healthcare industry.




