Introduction
The landscape of medical device regulation in Brazil is undergoing transformative changes, driven by recent updates from ANVISA that aim to enhance safety and efficacy standards. With the approval of RDC 925/2024, manufacturers are now faced with a comprehensive overhaul of classification criteria and clinical evaluation requirements that align more closely with global benchmarks. This article delves into the implications of these regulatory shifts, offering insights into:
- The new classification systems
- The intricate registration process
- The critical role of post-market surveillance
As the demand for medical devices continues to rise, understanding these regulations becomes paramount for manufacturers seeking to navigate the complexities of compliance and market entry in Brazil's evolving healthcare environment.
Recent Updates to ANVISA Medical Device Regulations
ANVISA has recently enacted significant updates to its health product regulations, especially regarding the approval of RDC 925/2024 in December 2023, which aligns with ANVISA medical device regulations. These changes include a comprehensive revision of the classification criteria for medical devices, now aligning more closely with the ANVISA medical device regulations and international standards. Updated requirements for clinical evaluation reports have been established under the ANVISA medical device regulations, emphasizing the necessity for robust evidence demonstrating product safety and efficacy.
Enhanced guidelines for risk management processes now require producers to adopt more rigorous assessment strategies in accordance with ANVISA medical device regulations. Notably, the updated Product Classification Form (NPRA/413/01) is now available for download, facilitating the classification process for therapeutic products. Our service capabilities—such as feasibility studies, site selection, compliance reviews, and project management—are designed to help navigate these regulatory updates effectively.
Specifically, our expertise in trial setup and approval processes ensures that producers can align with the ANVISA medical device regulations, thus avoiding potential delays in the approval process. The recent release of a list of approved companies producing or importing custom healthcare products highlights the significance of compliance with ANVISA medical device regulations, demonstrating ANVISA's commitment to maintaining high safety and efficacy standards in the healthcare product market, which directly impacts market entry for producers.
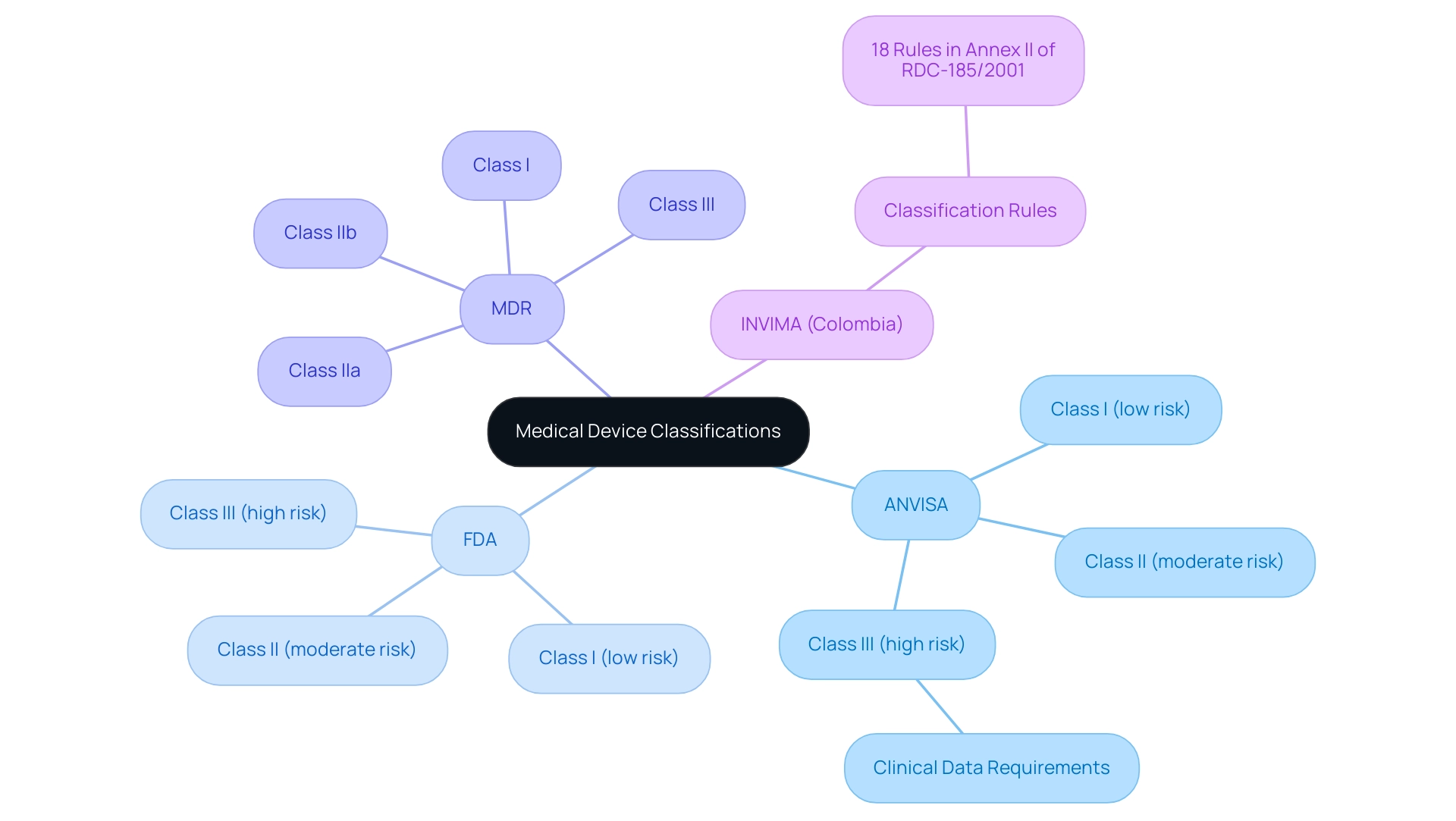
Comparative Analysis of ANVISA, FDA, and MDR Classifications
The ANVISA medical device regulations categorize medical equipment into three main classifications:
1. Class I (low risk)
2. Class II (moderate risk)
3. Class III (high risk)
This classification mirrors the FDA's classification system. In contrast, the EU's Medical Device Regulation (MDR) provides a more detailed classification framework, delineating four classes:
1. Class I
2. Class IIa
3. Class IIb
4. Class III
Along with additional subcategories. This distinction is essential for manufacturers to grasp, as it directly impacts the product registration and compliance processes in relation to ANVISA medical device regulations across various markets.
For instance, while Class III products in Brazil may necessitate extensive clinical data under ANVISA medical device regulations, FDA counterparts might have different requirements, reflecting the varying rigor of regulatory scrutiny. In Colombia, INVIMA supervises the classification and regulation of health equipment through its Directorate for Health Products and other Technologies. This directorate is tasked with overseeing and regulating healthcare equipment, ensuring adherence to established standards, and proposing technical guidelines for production and promotion.
INVIMA is acknowledged as a Level 4 health authority by PAHO/WHO, guaranteeing that the safety, efficacy, and quality of healthcare products meet rigorous standards. According to Annex II of RDC-185/2001, there are 18 established rules for classifying health products in Colombia, emphasizing the structured approach INVIMA employs. Additionally, it is crucial to note that the FDA's medical equipment database and the EU's EUDAMED are separate and independent, further complicating the regulatory landscape.
Understanding these differences is not only crucial for compliance with ANVISA medical device regulations but also essential for strategic planning in product development and market entry. As BRISA ADVISORS aptly states,
At BRISA ADVISORS, we offer a free classification service and determination of the regulatory pathway upon receiving product usage instructions at contactBR@brisa.com.br.
This service is especially pertinent for producers navigating the complexities of classifications and regulatory pathways, particularly concerning INVIMA's processes.
Navigating the Medical Device Registration Process in Brazil
The healthcare equipment registration process in Brazil is vital for manufacturers seeking to introduce their products to market effectively, especially in an environment where thorough clinical trial management services are indispensable. Engaging expert services like those offered by bioaccess®, which specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), can significantly streamline this process. With over 20 years of experience in Medtech, bioaccess® provides a customized approach that is vital for navigating complex regulatory landscapes.
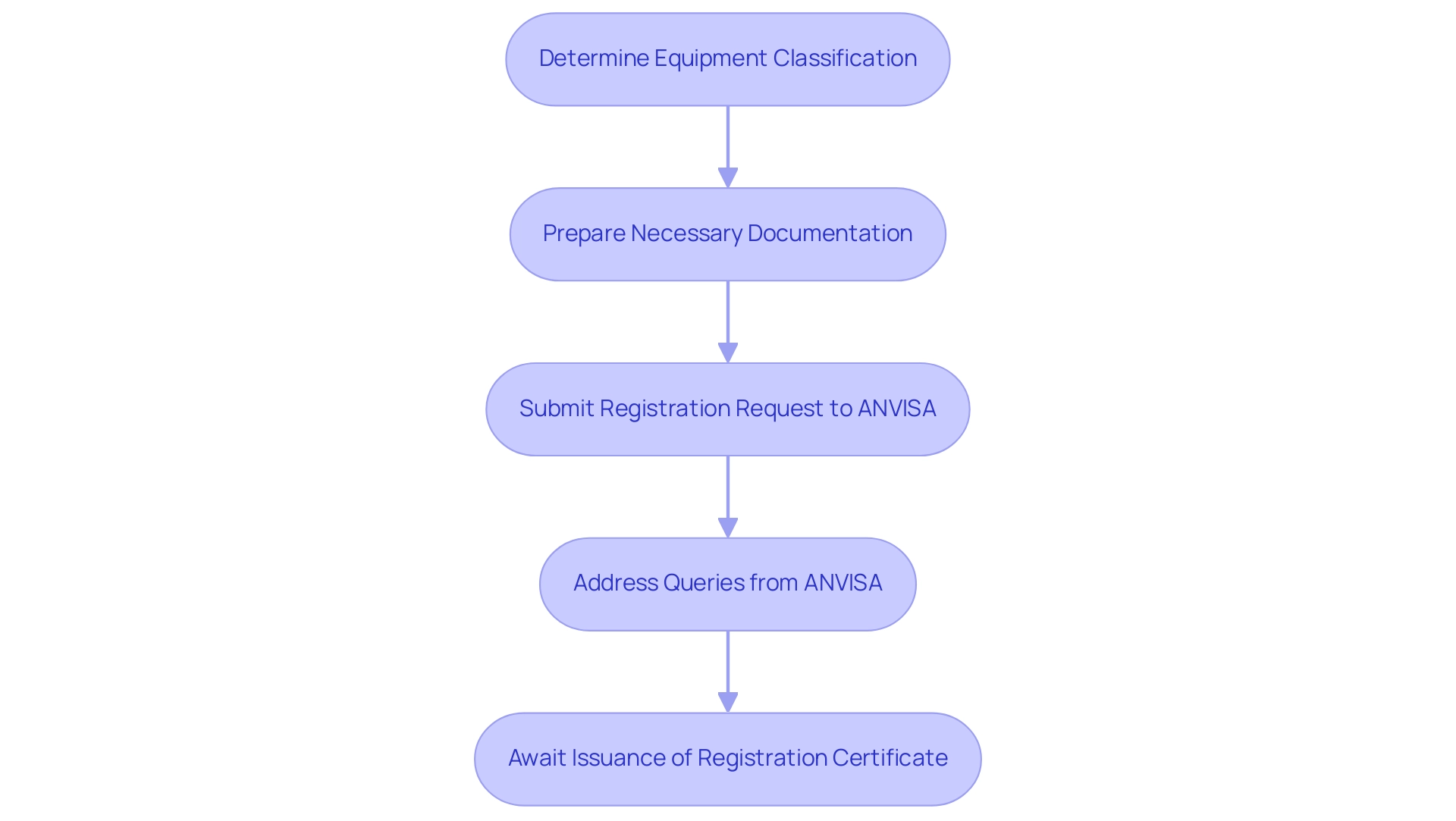
The registration includes several crucial steps:
1. Determine the Classification of the Equipment: Understanding the classification is essential, as it influences the regulatory pathway.
2. Prepare the Necessary Documentation: This includes technical files and clinical evaluations that demonstrate the equipment's safety and efficacy.
3. Submit the Registration Request to ANVISA following the anvisa medical device regulations: A comprehensive submission can facilitate a smoother review process.
4. Address Queries from ANVISA: Manufacturers must be prepared to respond promptly to any requests for additional information or clarification.
5. Await the Issuance of the Registration Certificate: The review period typically involves a thorough assessment, with ANVISA conducting inspections based on Brazilian GMP guidelines to ensure adherence.
Notably, the average time for medical equipment registration in Brazil can vary, but according to ANVISA medical device regulations, there is generally a 180-day period for reviewing local Phase I and II clinical trials. The expertise of Regulatory Affairs professionals, such as Katherine Ruiz, is critical in preparing for consultation meetings with ANVISA health authorities concerning anvisa medical device regulations before initiating key milestones in product development.
Additionally, as the Brazilian Institute of Geography and Statistics predicts a significant increase in the senior population over the next two decades, the demand for affordable medicines for age-related diseases will rise, making the registration process even more vital. Furthermore, compliance with the anvisa medical device regulations and Brazilian GMP regulations, as illustrated in the case study on the Quality Management System for Medical Devices in Brazil, is essential for successful device registration. The execution of the MDSAP program can also lessen the load on producers.
By understanding and anticipating these steps and potential challenges, producers can streamline their submissions, ultimately leading to faster approvals and successful market entry.
Understanding ANVISA's New Labeling and Artwork Standards
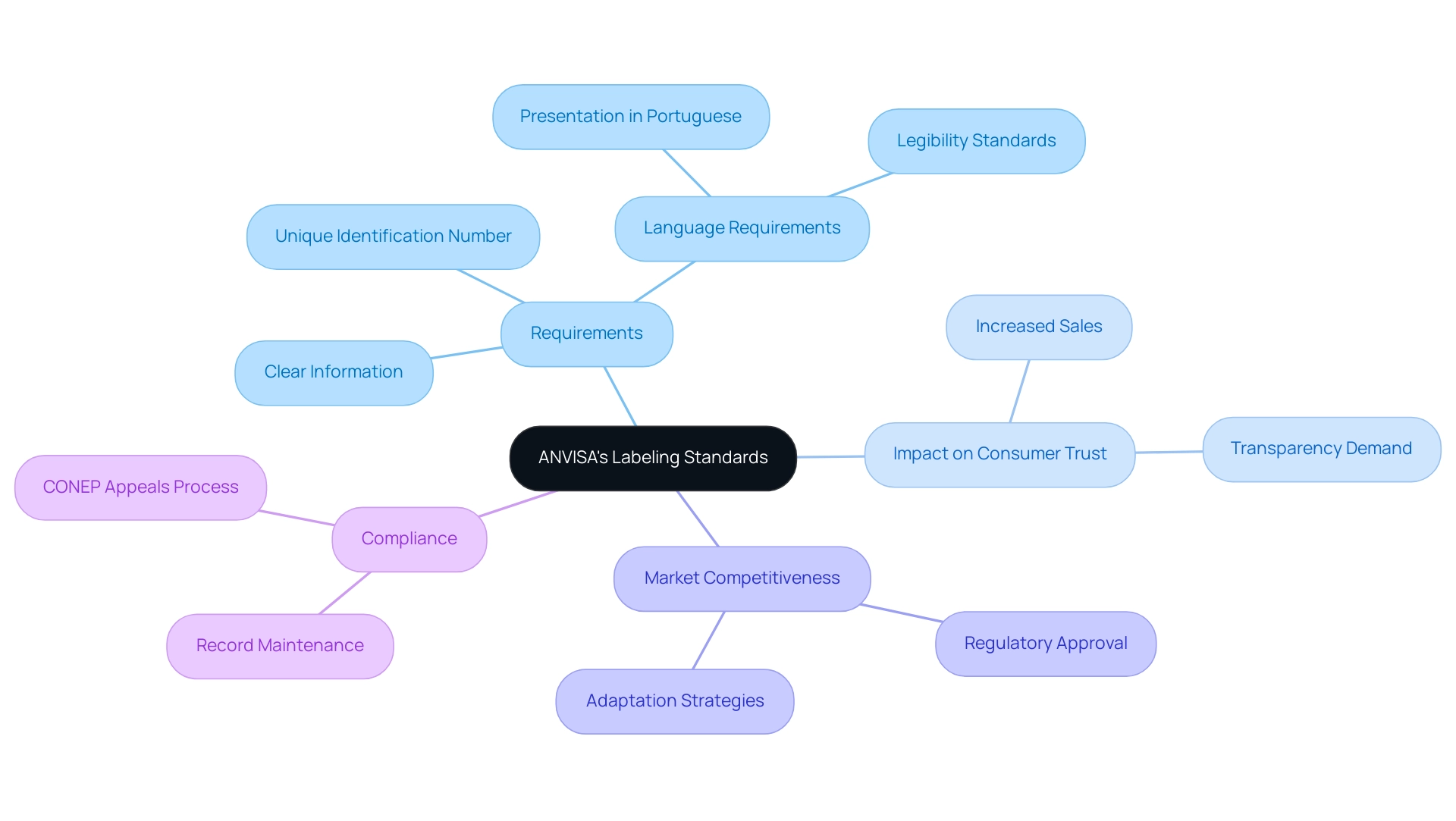
ANVISA has implemented updated labeling standards that mandate manufacturers provide clear and concise information regarding the intended use of medical devices, associated safety warnings, and details about the manufacturer. These standards became effective on September 1, 2022, and are shaped by specialists in Regulatory Affairs like Ana Criado, Director of Regulatory Affairs at Mahu Pharma, who highlights the significance of adherence in building consumer trust. Each label must conform to specific artwork formats and incorporate a unique identification number, which aids in traceability and adherence.
It is crucial that all labeling is presented in Portuguese and remains easily legible to ensure accessibility for all consumers. This stringent adherence to labeling standards is vital for obtaining regulatory approval in accordance with ANVISA medical device regulations and plays a significant role in enhancing market competitiveness. As of 2024, the emphasis on clear labeling is anticipated to influence the market significantly, with adherence connected to improved sales performance in Brazil’s competitive medical device landscape.
For instance, a recent case study on the impact of new nutritional labeling regulations demonstrated that manufacturers who adapted their packaging saw a notable increase in consumer trust and sales. Manufacturers must adapt their strategies to meet the ANVISA medical device regulations, which may include modifying their labeling practices to comply with the new requirements and addressing consumer demand for transparency. As stated by the National Research Ethics Commission (CONEP), 'The cancellation decision may be appealed to CONEP within 30 days,' reflecting the importance of adhering to these standards for maintaining compliance.
The Role of Post-Market Surveillance in Medical Device Compliance
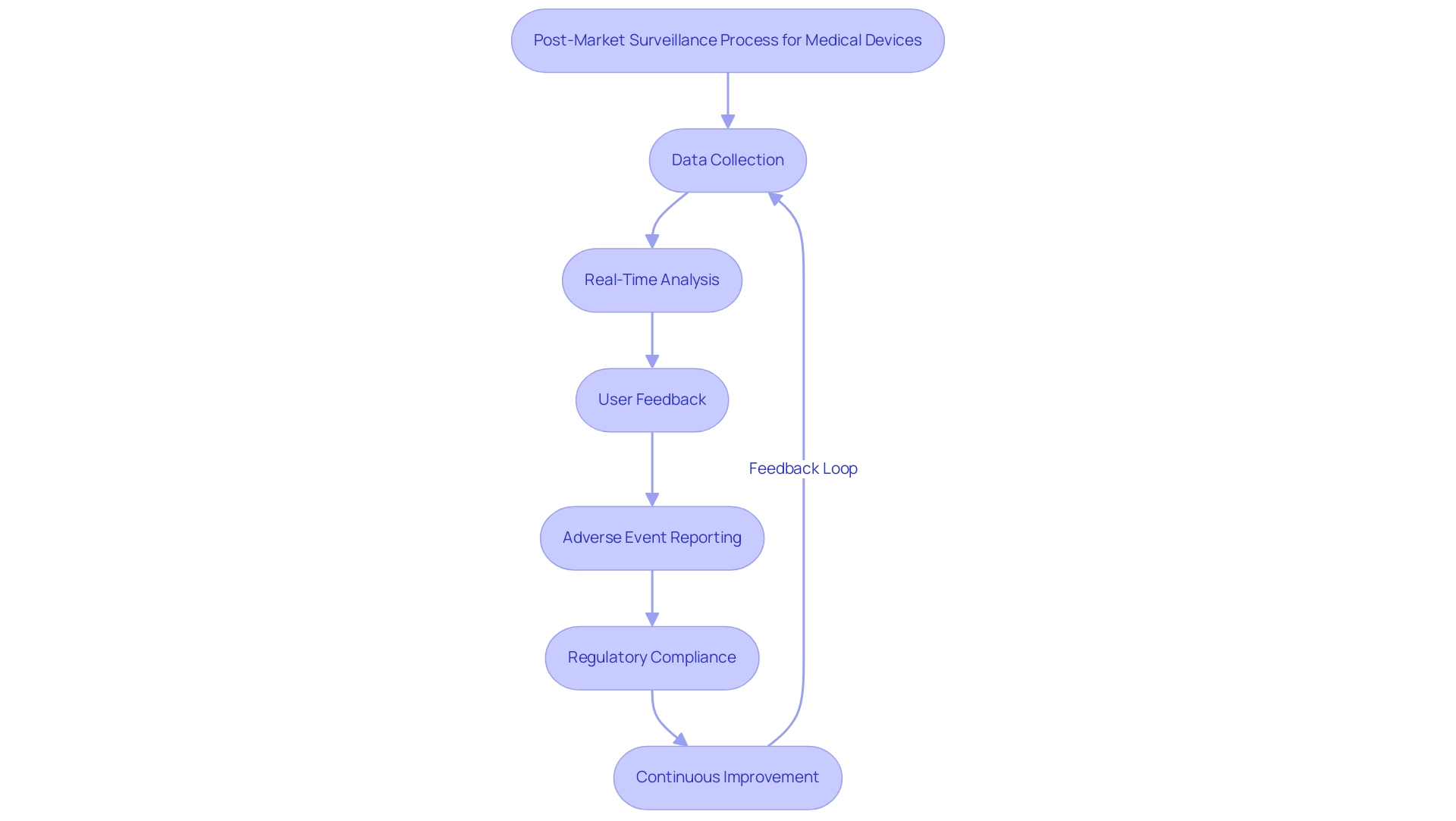
Post-market monitoring is crucial for guaranteeing the safety and effectiveness of health products after they enter the market. At bioaccess®, we utilize over 20 years of experience in managing medical equipment clinical trials across Latin America to assist manufacturers in creating comprehensive post-market surveillance plans. These plans systematically gather data related to performance, adverse events, and user experiences, which are crucial for proactive identification of safety issues and timely reporting in accordance with ANVISA medical device regulations.
We employ methodologies such as real-time data analysis and user feedback mechanisms to enhance the effectiveness of our surveillance efforts. In Brazil, where regulatory structures for pharmaceutical products are still evolving, adherence statistics reveal that many instruments struggle to meet post-market surveillance requirements, indicating significant areas for enhancement. Our tailored strategies directly address these regulatory challenges by implementing robust monitoring systems and regular audits.
Adverse event reporting rates are crucial in evaluating safety; therefore, an effective post-market strategy not only meets regulatory requirements but also safeguards patients and boosts the credibility of manufacturers. As illustrated by successful case studies, such as general surveillance for GM crops, robust monitoring mechanisms can mitigate risks and improve compliance outcomes. However, the current system often relies on data from applicants, which presents challenges that can be addressed through improved post-market strategies.
Therefore, a well-structured post-market surveillance plan is indispensable for maintaining high standards of medical device safety and compliance with ANVISA medical device regulations in the rapidly evolving landscape of Latin American healthcare.
Conclusion
The recent updates to ANVISA's medical device regulations signify a crucial transformation in Brazil's healthcare sector, mandating that manufacturers adapt to new classification criteria, clinical evaluation requirements, and enhanced post-market surveillance standards. With the approval of RDC 925/2024, Brazil is aligning more closely with international benchmarks, emphasizing safety and efficacy while improving market access.
Understanding the differences between ANVISA's classification system and those of the FDA and the EU's MDR is essential for manufacturers. These distinctions highlight the need for a strategic approach to product registration and development.
Moreover, the updated labeling and artwork standards emphasize the importance of transparency and consumer trust in the medical device industry. Compliance with these labeling requirements is critical for maintaining competitiveness and meeting consumer expectations.
Post-market surveillance remains vital in ensuring the ongoing safety and effectiveness of medical devices. By implementing robust monitoring systems and proactive data collection, manufacturers can comply with regulatory obligations and enhance patient safety.
In conclusion, as Brazil's medical device regulatory landscape evolves, manufacturers must prioritize compliance with these new standards. This proactive adaptation will help navigate challenges and capitalize on opportunities in a growing market, ultimately advancing healthcare and ensuring that patients have access to safe and effective medical devices.




