Introduction
The Corrective and Preventive Action (CAPA) process is a crucial component in the lifecycle of medical devices, ensuring their effectiveness and adherence to regulatory standards. With over 10,000 different types of medical devices in existence, each with unique functionalities and compliance requirements, a robust CAPA system is necessary to manage potential risks effectively. This article explores the importance of CAPA in the medical device industry and delves into topics such as sources of CAPA issues, roles and responsibilities in CAPA, the step-by-step guide to the CAPA process, initiating a CAPA, CAPA investigation and root cause analysis, developing corrective and preventive action plans, implementing and verifying CAPA actions, reviewing and approving CAPA, documenting CAPA, best practices for effective CAPA management, common challenges and solutions in CAPA, and the regulatory focus on CAPA and compliance.
By embracing these concepts, the medical device industry can ensure the highest standards of safety, efficacy, and quality in their products.
What is CAPA and Its Importance
The Corrective and Preventive Action (CAPA) process is an integral component in the lifecycle of medical devices, addressing various challenges from development through to post-market activities. Essential for safeguarding the effectiveness and quality of medical devices, CAPA also ensures adherence to stringent regulatory standards. The medical device realm is vast, comprising an array of products ranging from simple spectacles and bandages to sophisticated MRI machines and pacemakers. The complexity and diversity of these devices necessitate a robust CAPA system to manage potential risks effectively.
The World Health Organization cites over 10,000 different types of medical devices, each with unique functionalities and regulatory compliance requirements. For instance, Class III medical devices, which include life-sustaining implants like pacemakers, are subject to more rigorous regulatory scrutiny, given their high-risk nature. These devices, representing around 10% of FDA-regulated items, must undergo lengthy approval processes to ensure utmost safety and reliability.
Regulatory education, as discussed by industry experts, often aims to address a broad spectrum of devices, touching upon universal considerations such as biocompatibility, sterility, and software integrity. However, specialized areas like radiological health present unique challenges, including radiation dosage and the use of radiopharmaceuticals.
Further illustrating the industry's commitment to excellence and safety, UL Solutions recently opened a medical device testing laboratory in Michigan to support the increasing demand for such services. This facility exemplifies the rigorous testing and adaptation capabilities necessary to meet evolving manufacturer needs and reduce environmental contaminants.
Moreover, the industry's focus on compliance and operational efficiency has been exemplified by companies like US Med-Equip, where tools like Vanta are employed to monitor compliance metrics, leading to immediate corrective actions when discrepancies are identified. Likewise, companies are leveraging technological advancements to optimize device lifecycle management, as seen with Insight's integration center, which has significantly reduced labor requirements and enabled a more sustainable solution.
In conclusion, the CAPA process remains a cornerstone of the medical device industry, ensuring that products meet the highest standards of safety, efficacy, and quality. By embracing diversity in devices and human factors, and through strategic regulatory education and cutting-edge testing facilities, the industry continues to adapt and excel in a landscape characterized by constant change and regulatory evolution.
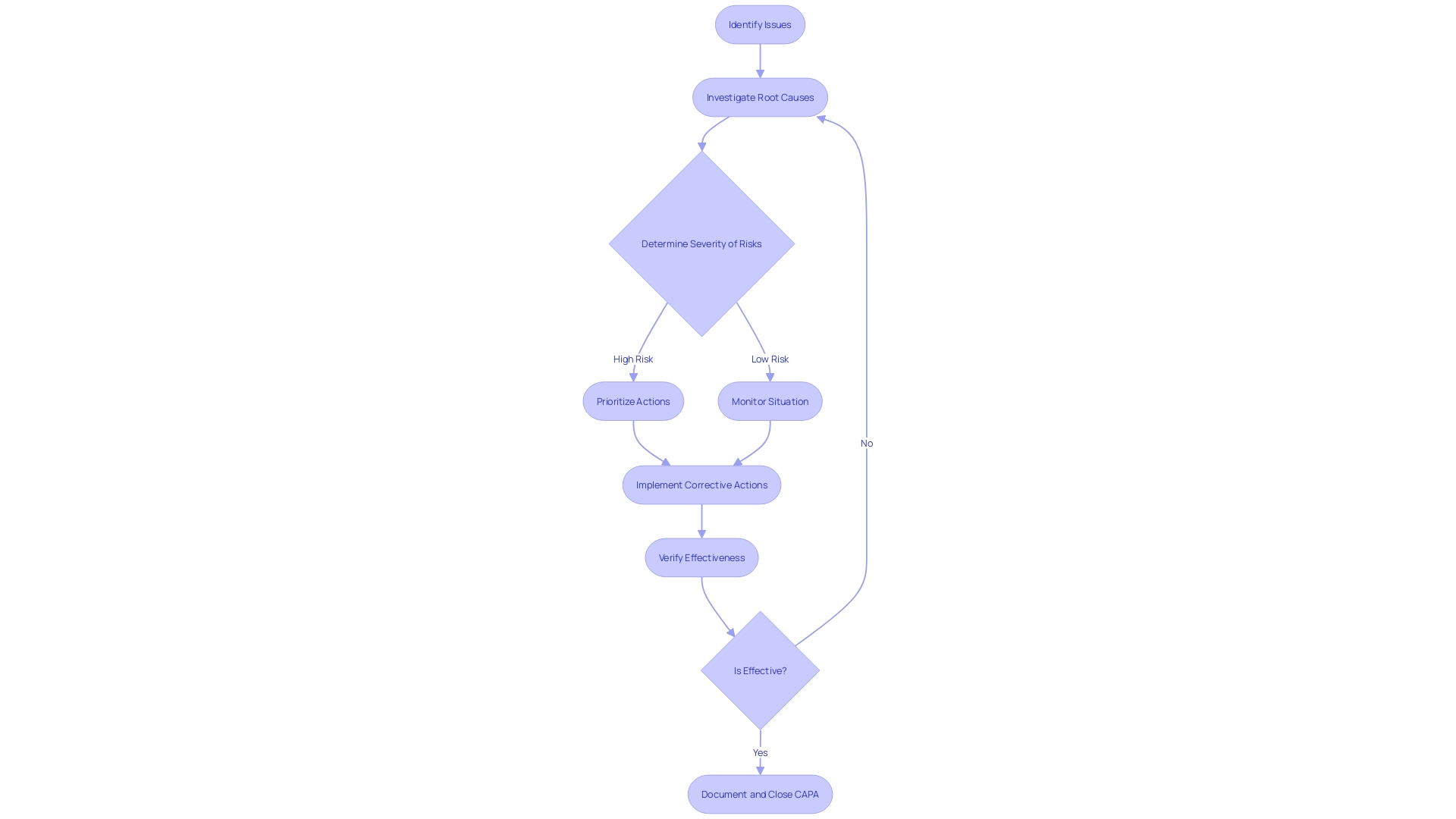
Sources of CAPA Issues
Within the complex realm of medical device systems and processes, Corrective and Preventive Action (CAPA) issues can emerge from a multitude of sources. These sources may include, but are not limited to, non-conforming products or components, equipment or process failures, deviations from standard procedures, customer feedback or complaints, and adverse events or incidents. Each of these elements requires diligent attention to ensure that medical devices, which range from simple consumer products like spectacles to advanced systems such as MRI machines and pacemakers, maintain their integrity and efficacy.
Given the vast array of over 10,000 medical devices identified by the World Health Organization, each with unique human and device factor diversity, the task of identifying CAPA issues becomes even more critical. This complexity is compounded when considering the broad technological span of these devices, which includes knowledge and applications from materials science, bioengineering, electronics, and software, among others.
To manage these challenges effectively, it is essential to have a robust understanding of the regulations that apply to medical devices. This includes familiarizing oneself with consensus standards developed by Standards Development Organizations (SDOs) that uphold principles of transparency, stakeholder participation, and due process. These standards not only foster innovation and standardization, but also ensure that devices meet the highest quality benchmarks for patient access.
Moreover, in a landscape where healthcare is rapidly evolving and private practices are integrating digital solutions to compete, the role of CAPA in medical device systems and processes becomes an indispensable part of maintaining high standards of patient care. Industry experts like Bijan Elahi have emphasized the importance of comprehensive safety risk management in medical devices, highlighting the need for clarity and confidence in the practice.
In summary, by identifying the sources of CAPA issues and adhering to rigorous standards and regulations, professionals in the medical device field can implement effective corrective and preventive measures, thereby safeguarding patient health and advancing medical technology.
Roles and Responsibilities in CAPA
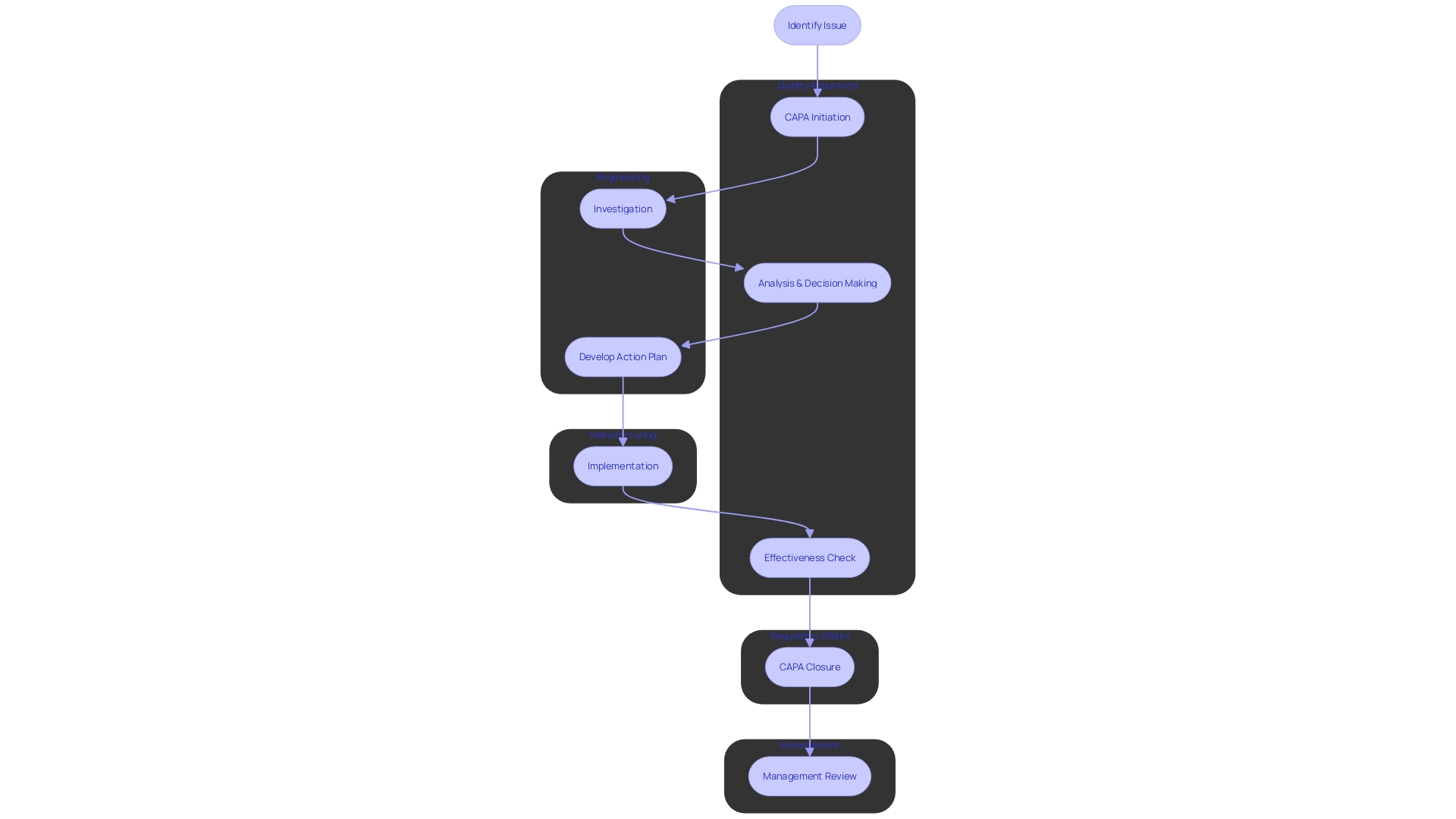
For an effective Corrective and Preventive Action (CAPA) process within medical device systems, it is imperative that each role is clearly defined and aligned with the responsibilities necessary to support compliance and quality assurance. The following delineates the specific roles that are crucial for a robust CAPA process:
-
Management: Management’s commitment is fundamental, as they are tasked with establishing the CAPA system and ensuring the availability of adequate resources. Their leadership is pivotal in fostering a culture of continuous improvement.
-
Quality Assurance: This team is the guardian of compliance, ensuring that all CAPA activities are conducted in accordance with regulatory standards. They play a critical role in monitoring the effectiveness of CAPA implementations and verifying that outcomes meet quality objectives.
-
Engineering: Engineers are the problem solvers who perform thorough investigations to determine root causes. Their analytical skills are essential in identifying issues and developing solutions that prevent recurrence.
-
Manufacturing: The manufacturing team has a hands-on role in executing the corrective and preventive actions. Their involvement is crucial as they are often closest to the processes and can provide practical insights into the implementation of solutions.
-
Regulatory Affairs: They ensure that the CAPA processes are aligned with current regulatory expectations and that documentation meets the standards required for audits and inspections.
With these roles clearly defined, the CAPA process can be effectively managed, promoting accountability and ensuring each department contributes to the mitigation of risks and enhancement of product quality. It is also essential to ensure there is no redundancy of roles, as streamlining responsibilities can prevent inefficiencies and confusion during the CAPA process. Additionally, as the healthcare landscape rapidly evolves with an increasing reliance on digital technology, integration smart digitalization practices within CAPA can enhance data management and process efficiency. By understanding what data is important and how to utilize it effectively, organizations can ensure that their CAPA systems not only meet current regulatory and quality standards but are also positioned to adapt to future advancements in medical device technology.
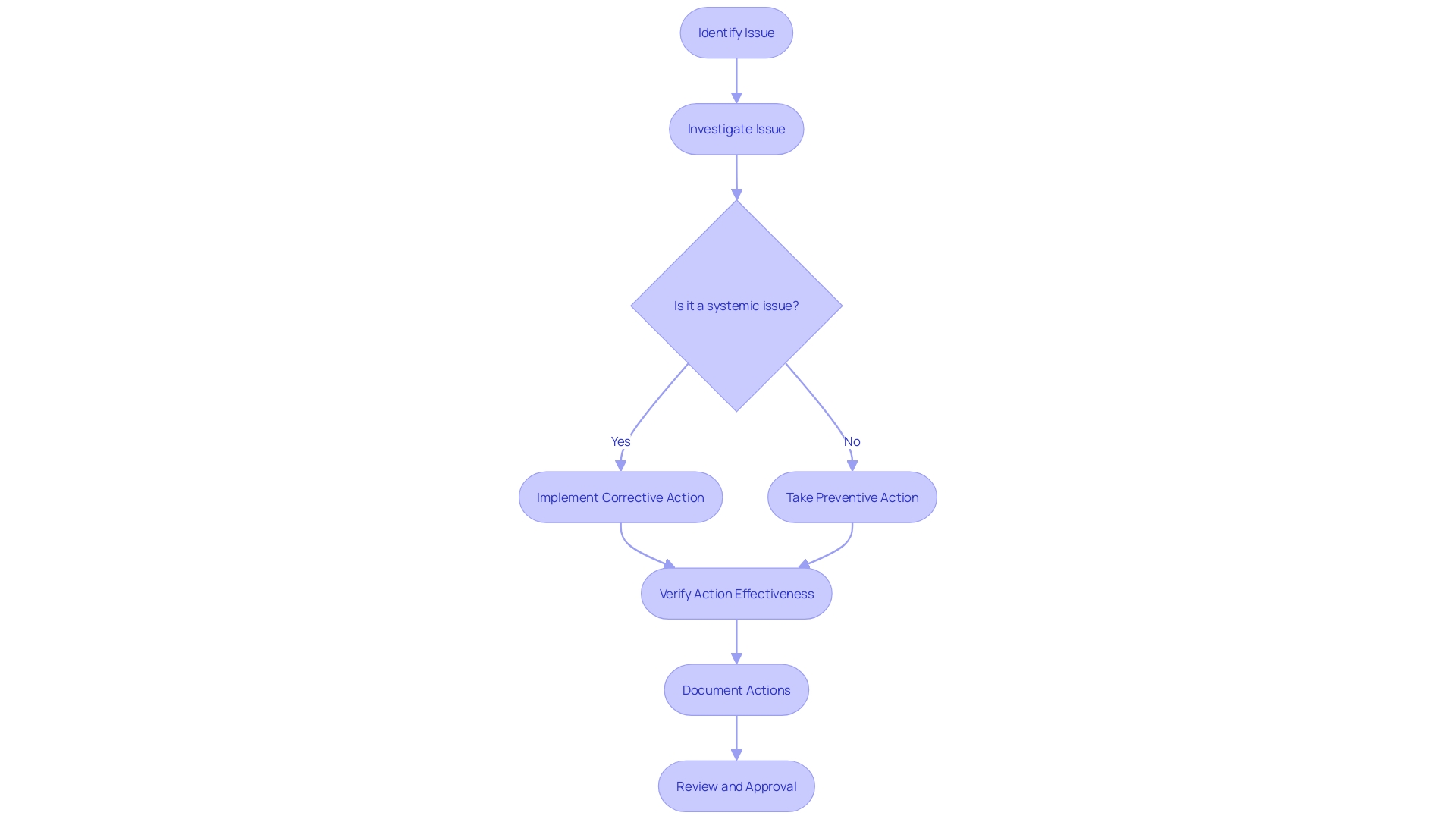
CAPA Process: Step-by-Step Guide
The Corrective and Preventive Action (CAPA) process is a fundamental mechanism in medical device quality management, ensuring that non-conformities are effectively addressed and recurrences are prevented. Each step of the CAPA process plays a critical role in maintaining high standards for medical device systems.
-
Issue Identification: It's crucial to initially recognize and document any discrepancy or non-conformity. This step aligns with practices seen in healthcare settings, such as the NHS, where the identification of a need for new digital technology begins with a formal request submission to assess compliance and appropriateness.
-
Issue Investigation: A rigorous investigation follows to pinpoint the root cause of the problem. This involves a comprehensive evaluation, often starting with questionnaires to gather detailed information, mirroring the process used by the Digital Service Team in healthcare organizations when assessing new technology requests.
-
Corrective Action: Solutions are then developed and implemented to tackle the root cause, preventing future occurrences. This echoes the sentiments expressed by UL Solutions when launching medical device testing services in Michigan, highlighting the importance of advancing safety and interoperability in medical devices.
-
Preventive Action: Concurrently, preventive measures are identified to avert similar incidents, informed by standards such as those recommended by the CAPSEAH guide for collective action to address Sexual Exploitation, Abuse, and Harassment.
-
Action Effectiveness: The effectiveness of these actions is monitored and evaluated to ensure they produce the desired results. This step is reminiscent of Dr. Atul Gawande's emphasis on the use of checklists to guarantee consistent adherence to critical steps.
-
Documentation: All CAPA activities are meticulously documented, capturing the issue, investigation, actions, and outcomes. This reflects the process of regulatory pathway assessments, where strategic decisions are based on well-documented research and analysis.
-
Review and Approval: Finally, the CAPA activities undergo a rigorous review and approval process to ensure they meet the necessary compliance and effectiveness standards. This mirrors the due process and transparency required in the development of voluntary consensus standards, as outlined by organizations like ANSI and in OMB Circular A-119.
By adhering to these structured steps, organizations can maintain a robust CAPA process, contributing to the overall quality and safety of medical device systems and processes.
Initiating a CAPA
Launching a Corrective and Preventive Action (CAPA) in the medical device sector is a meticulous process that begins with the detection of a non-conformity or issue that demands investigation and resolution. This identification might stem from a variety of sources, such as customer feedback, internal audits, or regulatory body inspections. A vivid example is the Class 1 Device Recall of the HeartMate 3 Left Ventricular Assist System, which illuminates the gravity of non-conformities and the imperative nature of CAPA systems in safeguarding patient health.
When an issue is pinpointed, its precise documentation is essential. This includes the specifics of the problem, its potential impact, and any associated risks. For instance, the Manufacturer and User Facility Device Experience (MAUDE) database provides a repository of complaints and malfunctions, highlighting the importance of comprehensive documentation. In a case involving Philips medical devices, over 100,000 reports were filed, detailing a range of patient ailments linked to device use, underscoring the integral role that thorough documentation plays in the CAPA process.
The development of corrective and preventive measures hinges on this foundational documentation, enabling medical device manufacturers to undertake robust investigations and devise effective solutions. As the industry continues to flourish in regions like Michigan, where UL Solutions has recently expanded its testing facilities to meet the burgeoning demand for medical device evaluations, the adherence to CAPA processes remains critical for maintaining the highest standards of device safety and performance.
Medical device companies must remain vigilant and proactive in their CAPA processes to prevent incidents and ensure compliance with stringent regulations. Leveraging case studies and data, such as those provided by independent laboratories, can facilitate informed decision-making and foster successful outcomes in the ever-evolving medical device landscape.
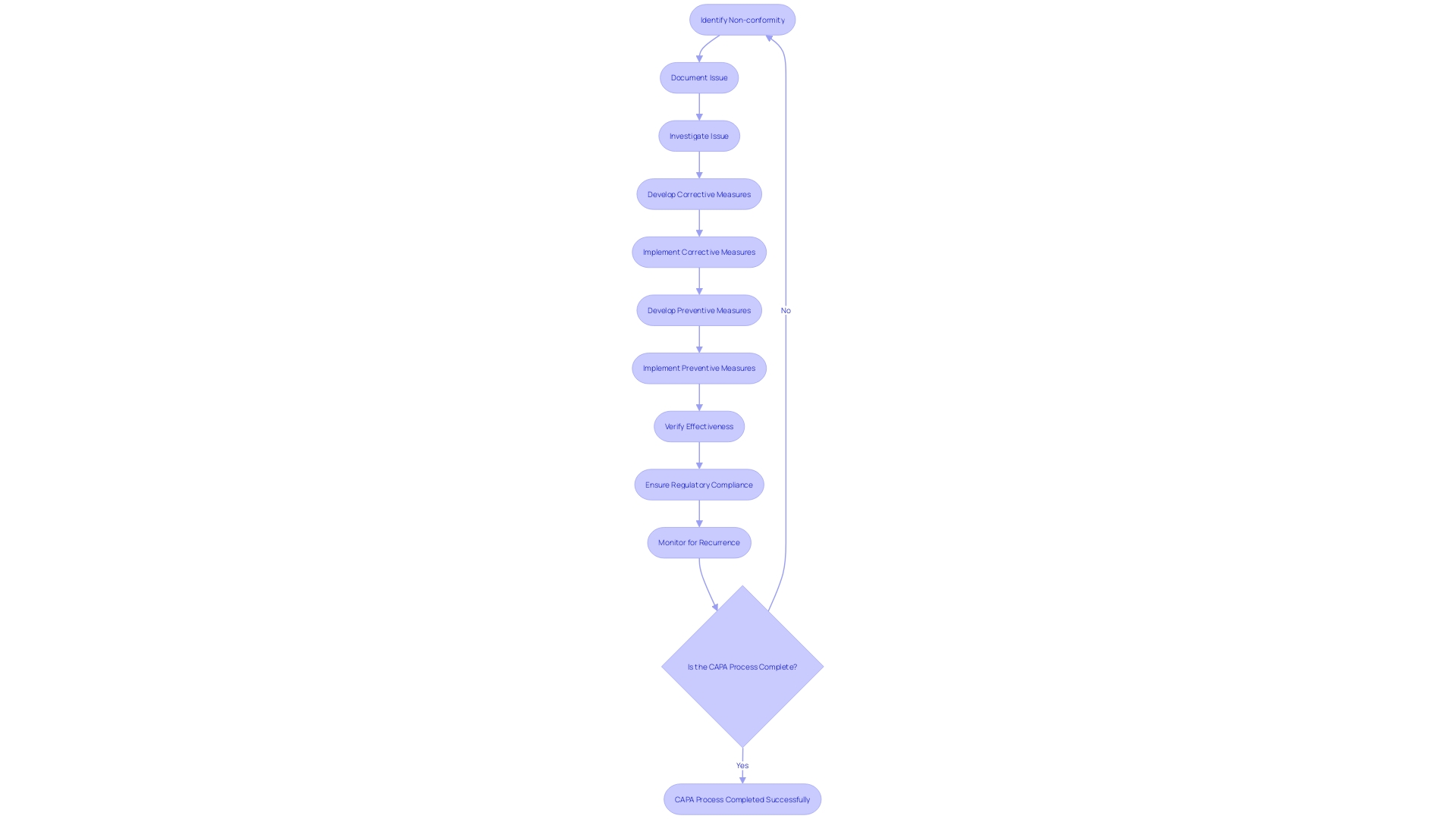
CAPA Investigation and Root Cause Analysis
A Corrective and Preventive Action (CAPA) process is an essential component of medical device quality management systems. Its purpose is to investigate and resolve non-conformities, thus ensuring patient safety and product effectiveness. The CAPA investigation and root cause analysis are critical, comprising data collection, analysis, and identification of root causes.
-
Data Collection: Collecting comprehensive information is the first step, involving the scrutiny of all pertinent data. For instance, Philips Respironics' CAPA process unearthed over 100,000 reports since 2010, submitted by a diverse group including patients and healthcare professionals.
-
Analysis: This involves using analytical tools like fishbone diagrams and the 5 Whys method. For example, Philips, while investigating complaints about 'black particles' in their devices, would have employed such techniques to trace the issues back to their source.
-
Root Cause Identification: Identifying the true cause(s) requires a meticulous review of all contributing factors. Reports of adverse events such as cancer or respiratory issues in the case of Philips' devices, can help pinpoint underlying issues.
The outcome of a thorough CAPA process is the development of measures that correct and prevent recurrence of the non-conformities, ensuring the safety and reliability of medical devices. For example, UL Solutions' new testing facilities in Michigan underscore the industry’s commitment to advancing device safety through rigorous testing and standards compliance, aligning with the increasing digitalization and data significance in medical device manufacturing. Such initiatives exemplify the industry's efforts to enhance product safety and efficacy through the strategic application of data and technology.
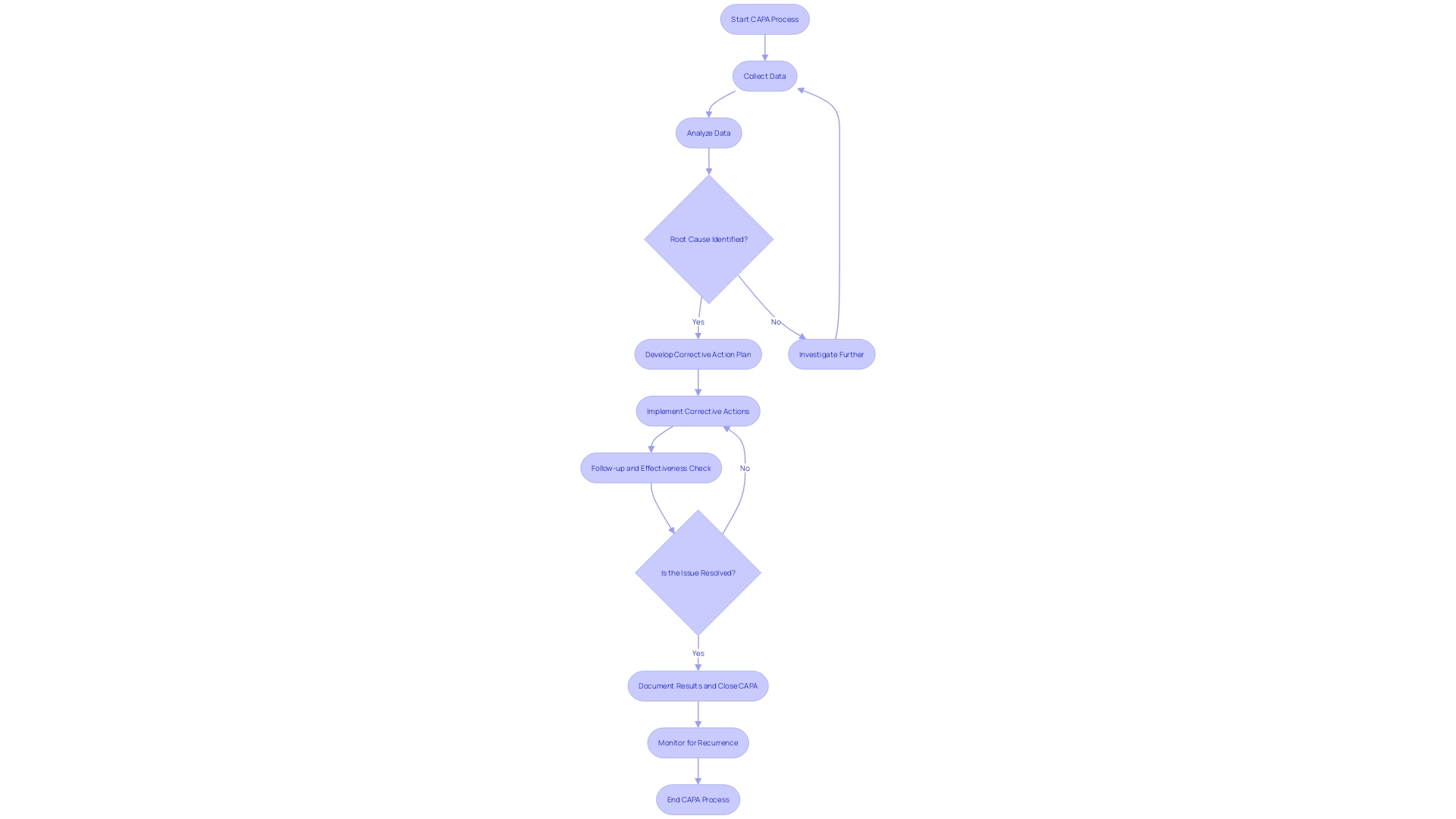
Developing Corrective and Preventive Action Plans
Developing corrective and preventive action plans is a fundamental aspect of CAPA medical device systems and processes. To create effective plans, it's essential to delineate the measures to be implemented, designate responsible parties along with specific deadlines, assess the viability and practicality of proposed actions, and anticipate potential risks and unintended outcomes. For instance, when faced with significant hiring challenges, San Diego County successfully implemented a three-prong strategy, underscoring the importance of a structured approach to problem resolution. The county's experience is a testament to the effectiveness of well-defined action plans in achieving desired outcomes, as evidenced by reaching the milestone of 20,000 employees.
Furthermore, a study on workplace incidents, including those relating to occupational health and safety, highlights the need for plans that address actual and potential non-conformities. As noted in the World Health Organization and Occupational Health and Safety Act documents, it's crucial to have preventive measures in place to mitigate risks that could lead to physical injury. Similarly, the city of Hamilton's proactive leak detection program, which boasted a 95.6% accuracy rate, exemplifies the benefits of preemptive action in managing and improving municipal services.
In the dynamic field of medical device manufacturing, nonconformance issues pose significant risks, potentially affecting patient safety and leading to costly recalls. It is well-documented that human error or deviation from work instructions can be a primary cause of shop floor nonconformances. Utilizing systematic action plans can help manage these risks effectively, thereby maintaining the integrity of the quality management system.
To encapsulate, embedding structured, well-considered action plans within the CAPA process is crucial for the successful resolution of issues and the prevention of their recurrence, ultimately safeguarding patient well-being and organizational efficiency.
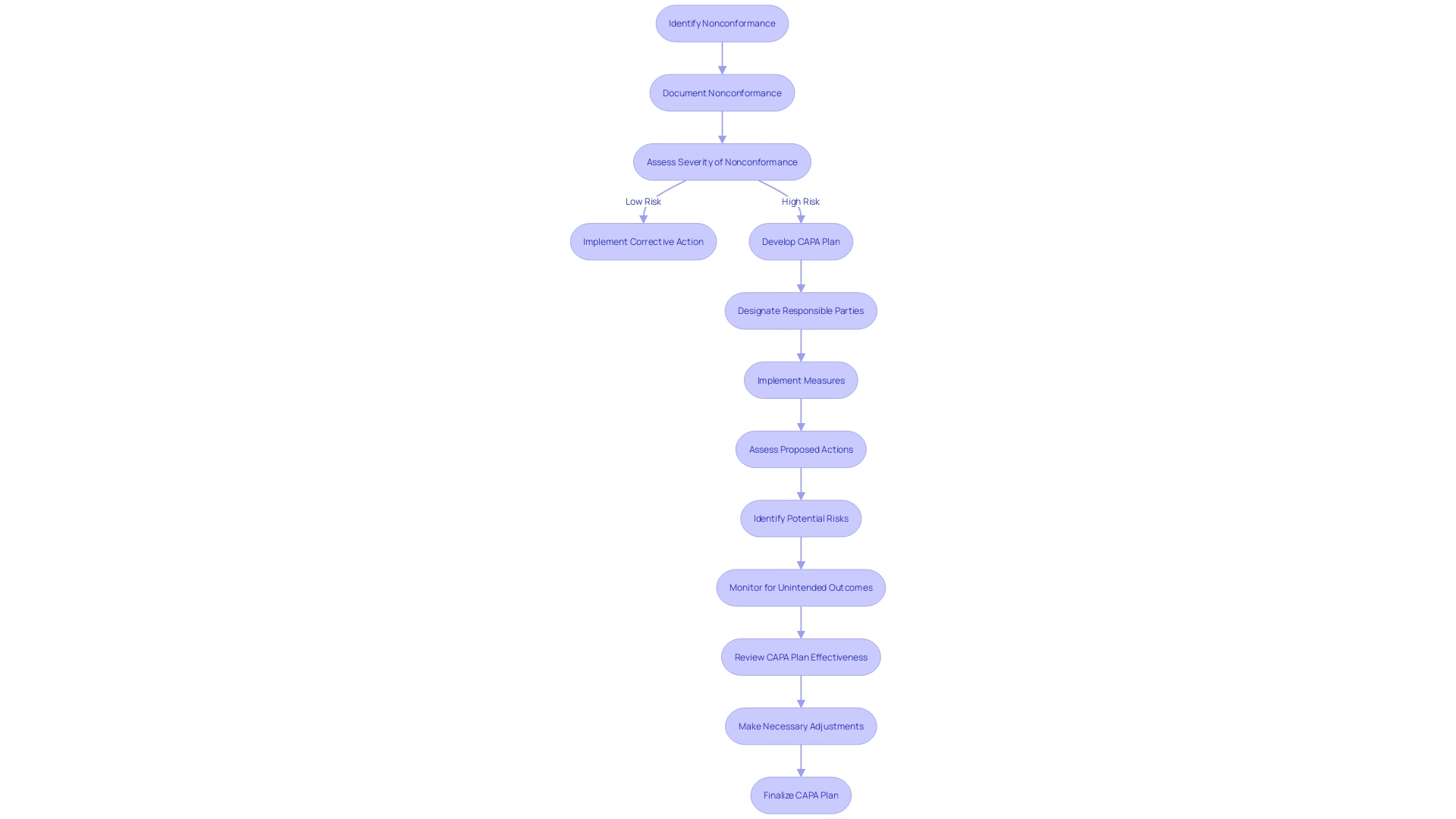
Implementing and Verifying CAPA Actions
When implementing and verifying CAPA actions within medical device systems, it is crucial to execute these actions with precision and to evaluate their effectiveness thoroughly. This process begins with adhering to the defined action plans and timelines, which requires meticulous coordination and monitoring. Documenting each step of the implementation is vital for transparency and for future reference, should any issues arise. The effectiveness of the implemented actions is then evaluated to ensure they have indeed addressed the identified issues. This assessment can involve various methods such as questionnaires, which probe deeper into the needs and expectations of the actions taken. Adjustments and improvements are made as necessary to fine-tune the process.
Verification is more than a procedural formality; it is a safeguard that ensures a significant reduction in the risk of recurrence of the issue. For instance, the Digital Service Team in healthcare settings conducts initial assessments on new digital technologies to ensure security, appropriateness, and compliance. The rigor of such an assessment can be seen in their thoroughness to check for redundancies and existing capabilities within the organization. As one NHS trust discovered, '... when we start digging, asking questions, we find people have already got it and have had it for years.'
Consensus standards play a fundamental role in the verification process. They are developed by Standards Development Organizations (SDOs) and are based on principles of transparency and stakeholder participation. The standards ensure that medical devices meet the necessary performance and safety requirements, as validated by independent laboratories. For instance, UL Solutions' Rochester Hills laboratory conducts a variety of tests on medical devices to verify their compliance with consensus standards, thus supporting the safety, security, usability, and interoperability of these critical products.
Medical devices, ranging from simple consumer products like bandages to complex systems like MRI machines, are subject to diverse regulations and standards. The World Health Organization notes that there are over 10,000 types of medical devices available, underscoring the importance of a thorough CAPA process. The multifaceted nature of these devices necessitates a CAPA system that is as adaptable and comprehensive as the devices themselves, ensuring that actions taken are not only effective but also sustainable in the long term.
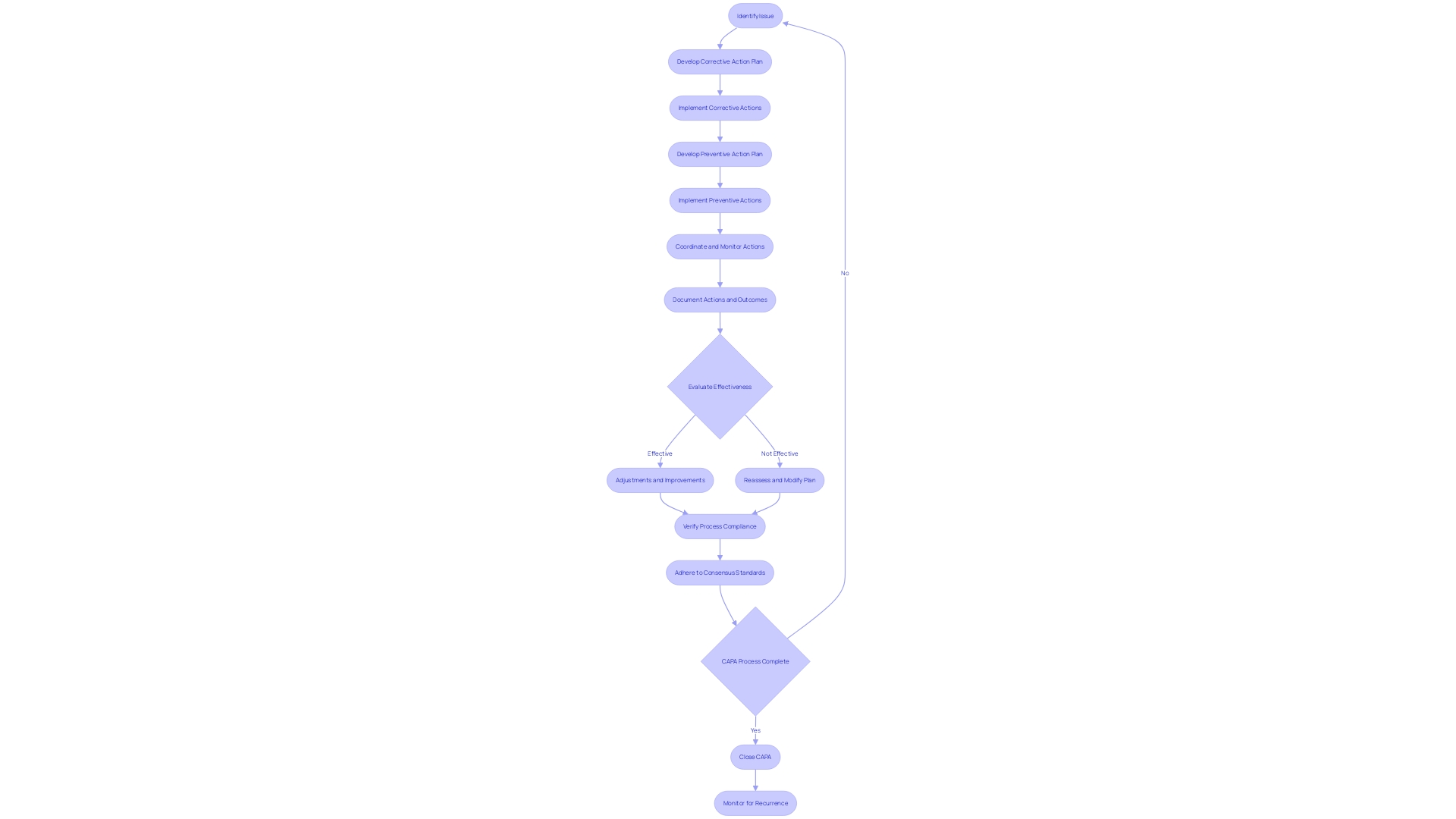
Reviewing and Approving CAPA
The Corrective and Preventive Action (CAPA) process is a pivotal aspect of medical device quality management systems. Meticulous review and approval of CAPA activities not only ensure regulatory compliance but also fortify the quality and effectiveness of the actions taken. This critical step includes a detailed examination of the documented CAPA activities and their results, ensuring they align with regulatory mandates and internal policies. Additionally, it involves the evaluation of the effectiveness of the actions implemented, culminating in the decision to either close the CAPA or recommend further action.
In the context of medical device coverage, the FDA's role is central to assessing safety and efficacy before devices enter the U.S. market. Post-approval, coverage and reimbursement decisions by payors like CMS and private health plans hinge on data that may differ from FDA requirements, potentially delaying patient access to crucial medical devices. This highlights the importance of a robust CAPA process that not only meets FDA standards but also considers the broader implications for device coverage and patient care.
The application of voluntary consensus standards, developed through a transparent process involving all stakeholders, is integral to maintaining regulatory quality. This comprehensive approach ensures devices meet the highest standards of safety and performance, as defined by organizations such as the American National Standards Institute (ANSI) and outlined in OMB Circular A-119.
Furthermore, medical devices, as defined by the World Health Organization (WHO), span a wide range of applications, from simple tools like tongue depressors to complex machinery for medical testing. This diversity underscores the significance of a well-executed CAPA process that can adapt to various levels of technological complexity and ensure devices fulfill their intended medical purposes effectively and safely.
Staying abreast of the latest developments, such as the Right to Try Act, which allows for the use of investigational drugs outside clinical trials, is crucial for regulatory professionals. This act further emphasizes the need for a thorough CAPA process, as the FDA mandates annual summaries from manufacturers regarding the safety and use of investigational drugs.
In summary, reviewing and approving CAPA activities is a multifaceted task that ensures medical devices meet stringent regulatory standards while addressing the nuances of coverage, reimbursement, and patient access. It requires a careful balance of compliance, quality assurance, and foresight into the device's life cycle post-market approval.
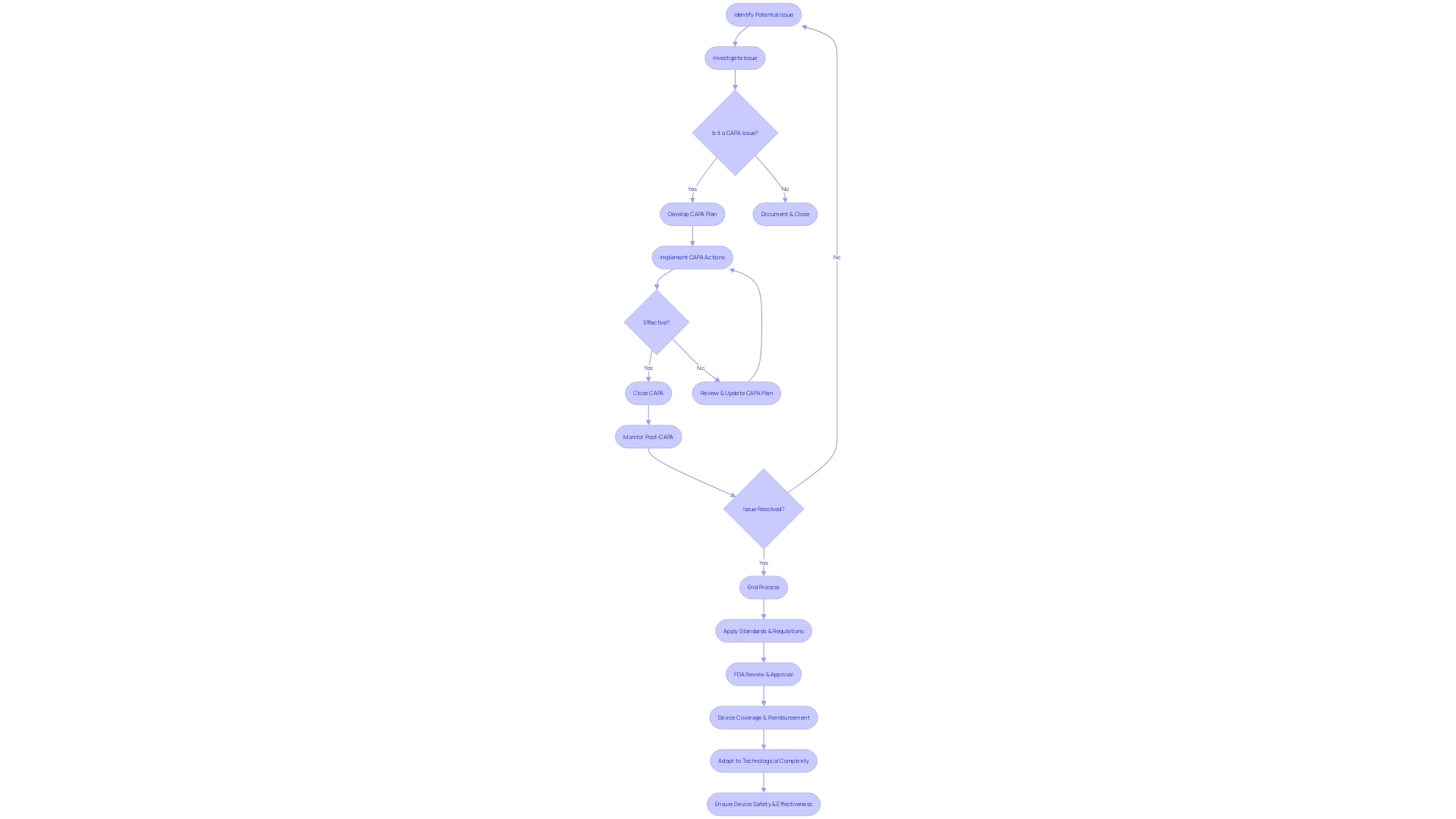
Documenting CAPA: Report Requirements
Maintaining meticulous documentation is an essential component of the Corrective and Preventive Action (CAPA) system for medical devices. It is a regulatory requirement to record all CAPA-related activities, serving as evidence of compliance with governing bodies. To ensure thoroughness, the following elements should be documented:
- A detailed description of the identified issue, including any specifics that would aid in understanding the scope and impact of the problem.
- Findings from the investigation, including root cause analysis, to pinpoint the source of the issue and inform the development of effective corrective and preventive measures.
- Detailed descriptions of the corrective and preventive actions undertaken to address and prevent recurrence of the issue.
- Progress updates on the implementation of the actions, assessing their effectiveness over time to ensure the issue is resolved and prevented from recurring.
- Records of reviews and approvals throughout the CAPA process, which provide a transparent audit trail for internal and external assessments.
By systematically documenting these aspects, organizations underscore their dedication to quality management and adherence to regulatory standards. This is exemplified by the case of Cape Cod Hospital, which, after self-disclosure and cooperation regarding Medicare claims violations, implemented remedial measures and entered into a Corporate Integrity Agreement, demonstrating the value of proactive documentation and corrective action in regulatory compliance.
Further emphasizing the importance of regulatory documentation, the World Health Organization defines medical devices as instruments with diverse uses in diagnosis, prevention, and treatment of diseases, highlighting the necessity of strict documentation practices to ensure the safety and efficacy of these devices. Comprehensive documentation not only supports regulatory compliance but also facilitates knowledge transfer, enabling leaders in the field to disseminate insights across disciplines and enhance collaborative efforts.
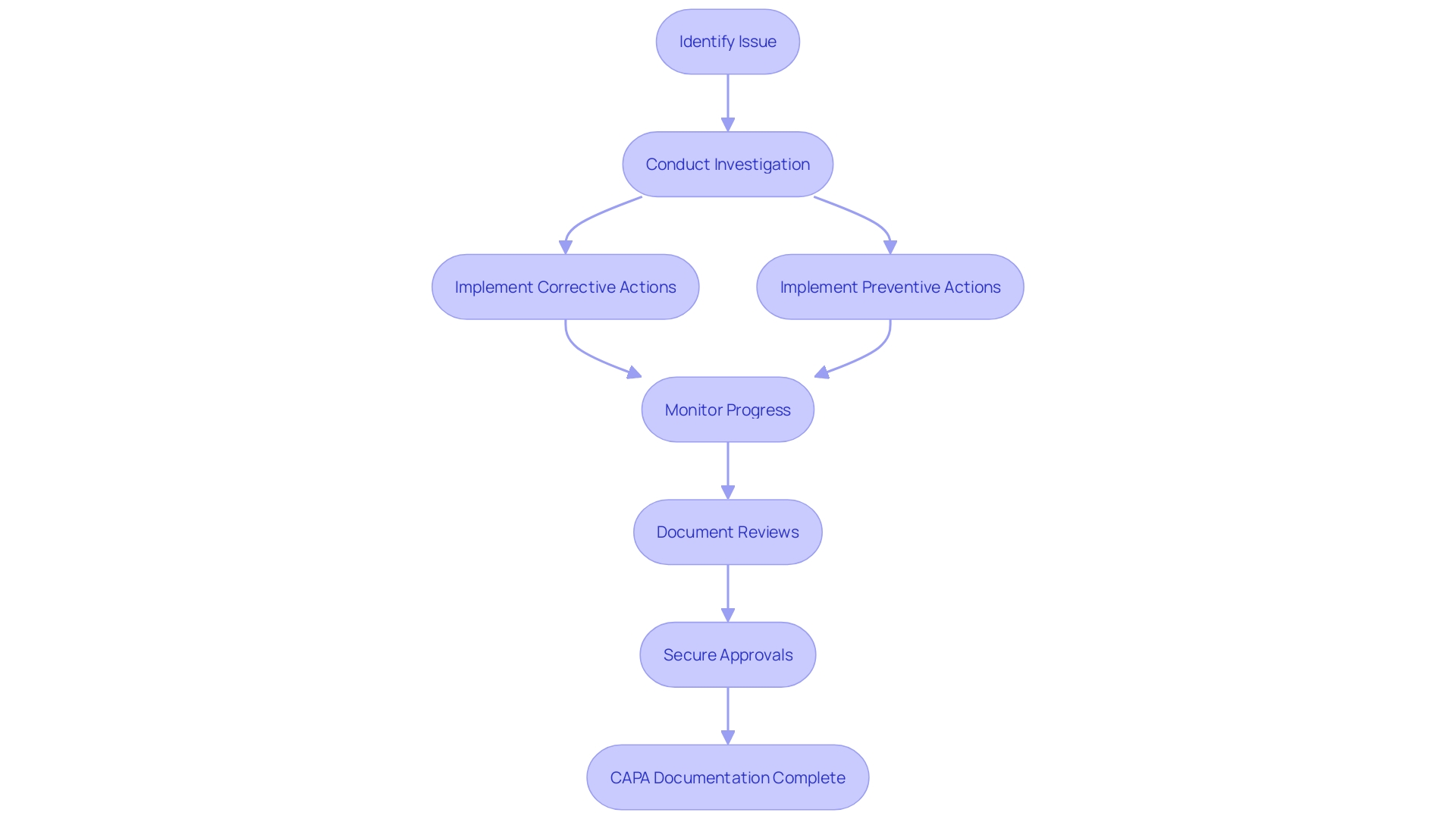
Best Practices for Effective CAPA Management
Enhancing CAPA management is not just about adhering to regulations; it's about embracing a holistic approach that interweaves with the organizational fabric, leading to more resilient and adaptable systems. To transform CAPA systems, it is vital to establish clear procedures and guidelines—akin to the precision and integration seen in Fortinet's security solutions, where intercommunication between applications streamlines operations and minimizes the need for additional personnel.
Training is another cornerstone of effective CAPA management, as it empowers personnel to competently navigate the CAPA process, much like the GRIP workshop's collaborative approach in strategizing technology transitions for a global commercial vehicle manufacturer.
A culture of transparency and accountability, coupled with a commitment to continuous improvement, can elevate CAPA management to new heights, reflecting the psychological safety within workplaces that fosters open expression and idea sharing, leading to enhanced outcomes.
Regular monitoring and evaluation are crucial for maintaining the CAPA system's effectiveness, ensuring it can respond rapidly to needs and changes within the organization—mirroring the operational excellence that enables software engineering managers to deliver high-quality products and services consistently.
Finally, cross-functional collaboration and communication are the lifeblood of effective CAPA management, promoting an environment where different departments can unite towards a common goal, much like the seamless asset transition between the newly formed entities of a luxury and commercial vehicle manufacturer.
By integrating these best practices, organizations can not only refine their CAPA management processes but also harness efficiency gains, akin to those reported in the 'Essentials of Access Control' study by ASIS International, which underscores the importance of cohesive technology deployment and policy application in access management.
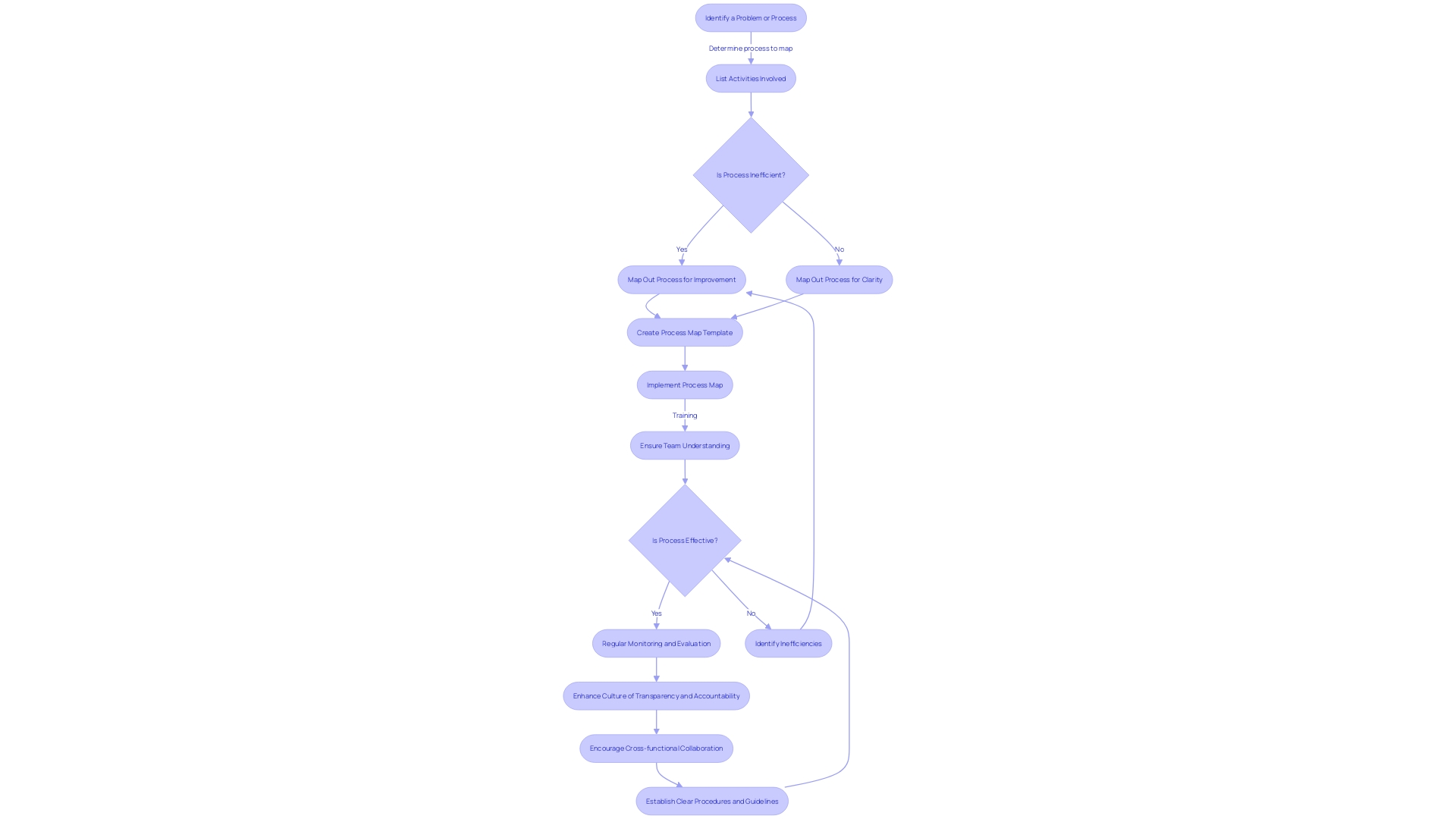
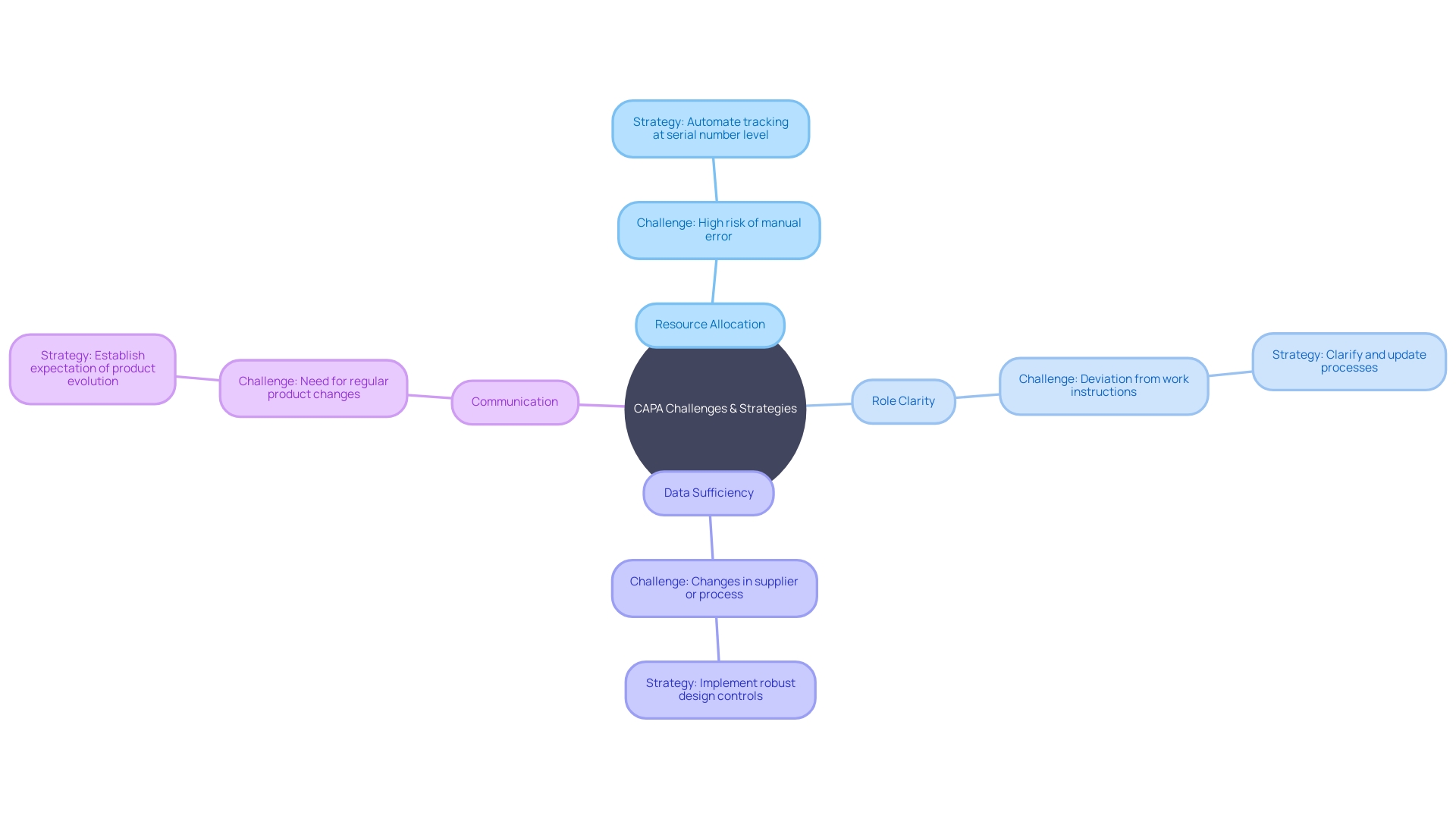
Common Challenges and Solutions in CAPA
In the intricate landscape of Corrective and Preventive Action (CAPA) for medical device systems, challenges such as resource allocation, role clarity, data sufficiency, and communication are not uncommon. To ensure effectiveness, it is vital to adopt structured strategies.
Allocating adequate resources is a foundational step. A case in point is the Digital Service Team in the NHS, which scrutinizes new digital technology requests to ensure compliance and appropriateness, revealing that often solutions are already in place but not universally known—a clear indicator of the need for resource awareness.
Clarifying roles and responsibilities is equally crucial. For example, water utility companies have developed 'history books' detailing asset operation and maintenance, which provide a clear framework for staff responsibilities, thereby streamlining processes and enhancing role transparency.
The importance of robust data collection cannot be overstated. The case of Wallace-Woodworth, which, after receiving a grant, developed an asset management policy and registry, underscores the value of formalized data management in capturing and retaining critical knowledge, essential for thorough root cause analysis in CAPA.
Communication and coordination are the linchpins of successful CAPA management. UL Solutions' launch of medical device testing in Michigan is testament to the benefits of enhanced communication channels. Here, the capacity to reconfigure testing based on manufacturers' needs demonstrates how effective coordination can support innovation while managing risks.
These solutions, when applied, can significantly optimize CAPA management processes, creating a more resilient and efficient system for handling the multifaceted world of medical devices, which, as the World Health Organization states, includes over 10,000 types. The diverse nature of these devices, from simple bandages to complex MRI machines, requires a CAPA system that is equally adaptable and rigorous.
Addressing these challenges is not just about improving processes but also about adhering to stringent regulatory requirements. For instance, the FDA's classification of medical devices into three risk-based categories dictates the level of regulatory control needed, with class three devices such as pacemakers, which are crucial for sustaining life, undergoing the most rigorous processes.
As the medical device industry continues to evolve with digitalization and integration of software, companies that can navigate these challenges effectively will stand out. The track record of successful product launches is a testament to a company's ability to manage the CAPA process adeptly, a quality that buyers should prioritize in an increasingly competitive market.
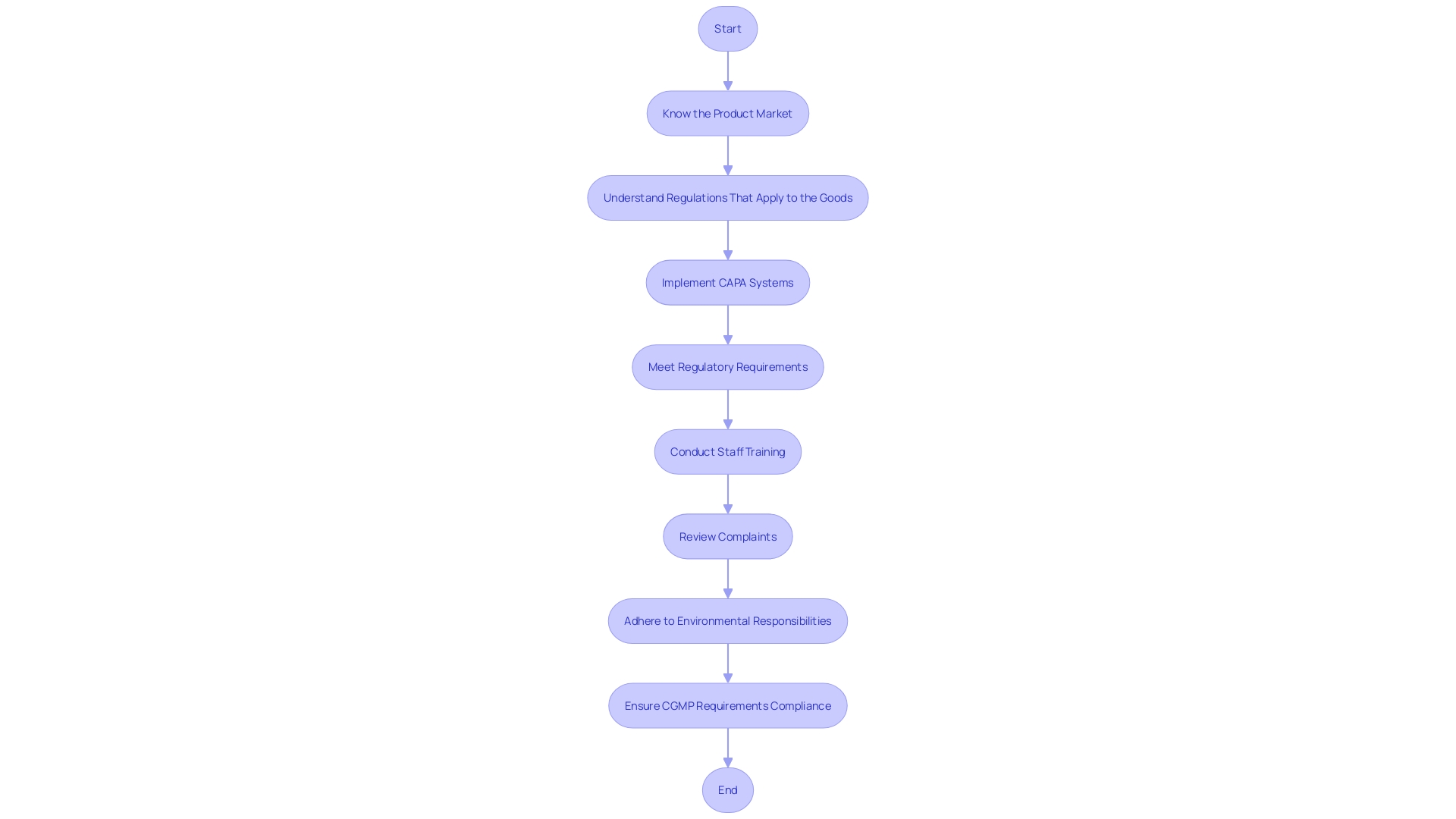
Regulatory Focus on CAPA and Compliance
The medical device industry is under strict scrutiny from regulatory bodies to ensure that Corrective and Preventive Action (CAPA) systems and compliance measures are effectively implemented. For instance, the FDA's Quality System Regulation (QSR) and ISO 13485 standards set forth rigorous requirements for medical devices to be allowed into the market and to safeguard patient health. Organizations are compelled to prioritize CAPA and uphold compliance, not only to meet regulatory expectations but to guarantee the integrity and safety of their medical devices.
Real-world examples underscore the importance of these systems. The FDA's examination of the Impella Connect System highlights the necessity for premarket authorization for software functions that deliver critical patient-specific information. In response to this, firms are taking systemic corrective actions, like the review of complaints and staff training, to address issues such as complaint coding and quality data analysis. This demonstrates the industry's commitment to continuous improvement and adherence to regulatory frameworks.
Even as guidelines evolve, companies are advised to adopt a global perspective on regulatory compliance, as suggested by the OECD's 'Conflict Minerals' policy, which supports responsible supply chain practices. The medical device sector is also adapting to increased regulatory demands, with nearly two-thirds of companies involved in e-labeling initiatives that facilitate real-time information access for healthcare providers, as per the proposed FHIR standard.
The WEEE and RoHS Directives further illustrate the industry's obligation to manage electronic waste and restrict hazardous substances, underscoring the intersection of regulatory compliance and environmental responsibility. As medical devices become increasingly integrated with technology, such as PCBs, the relevance of these directives grows.
Statistics reveal the extent of regulatory influence, with the FDA categorizing medical devices into three classes based on risk, and only 10% of devices falling into the high-risk class three category requiring extensive regulatory processes. Surveys reflect the industry's preparedness to tackle these regulations, emphasizing the rise of regulatory requirements and the impact of various initiatives on market entry and development.
In summary, compliance with medical device regulations is not just a legal mandate but a fundamental aspect of ensuring patient safety and device efficacy. It requires a proactive and comprehensive approach, integrating advanced technology, rigorous assessment, and global awareness into every stage of the device lifecycle.
Conclusion
The Corrective and Preventive Action (CAPA) process is crucial in the medical device industry for ensuring compliance and maintaining the highest standards of safety, efficacy, and quality. With over 10,000 different types of medical devices, a robust CAPA system is necessary to effectively manage potential risks.
By addressing sources of issues, such as non-conforming products, equipment failures, deviations from procedures, customer feedback, and adverse events, professionals in the industry can implement effective corrective and preventive measures. Clear roles and responsibilities, including management, quality assurance, engineering, manufacturing, and regulatory affairs, are essential for a successful CAPA process.
Following a step-by-step guide, including issue identification, investigation, corrective and preventive action, action effectiveness evaluation, documentation, and review and approval, ensures a structured approach to CAPA. Initiating a CAPA involves detecting and documenting non-conformities, while investigation and root cause analysis help identify underlying issues.
Implementing and verifying CAPA actions with precision and thorough evaluation is crucial for reducing the risk of recurrence and ensuring the effectiveness of the measures taken. Compliance with regulations and leveraging consensus standards and independent laboratories support the highest standards of safety and performance.
Documenting CAPA activities is a regulatory requirement that promotes transparency and adherence to standards, enhancing quality management and facilitating knowledge transfer. Establishing clear procedures, providing comprehensive training, fostering transparency and accountability, and promoting cross-functional collaboration and communication optimize CAPA management.
The medical device industry faces strict regulatory scrutiny to ensure CAPA systems and compliance measures are effectively implemented. Adhering to regulations, such as the FDA's Quality System Regulation and ISO 13485 standards, is essential for meeting regulatory expectations and ensuring the integrity and safety of medical devices.
In conclusion, the CAPA process is integral to the medical device industry, ensuring compliance, and upholding the highest standards of safety, efficacy, and quality. By addressing issues, defining roles, following a structured approach, initiating effective actions, and documenting activities, organizations can optimize CAPA management and contribute to the advancement of medical technology while meeting regulatory requirements.




