Introduction
Brazil's recent overhaul of its clinical research law marks a pivotal moment for the landscape of medical device trials within the country. Enacted in 2022, this legislation not only streamlines the approval processes but also enhances ethical standards and transparency, fostering an environment conducive to innovation. As Medtech startups navigate the complexities of bringing their products to market, the law's provisions for ethical approval and compliance are crucial.
With approval times reportedly decreasing by 30%, researchers now have a clearer path to conducting trials, ultimately benefiting patient care through more efficient and ethical practices. This article delves into the key requirements, regulatory challenges, and compliance strategies essential for successfully conducting clinical research in Brazil, positioning stakeholders to thrive in this evolving landscape.
Overview of the New Clinical Research Law in Brazil
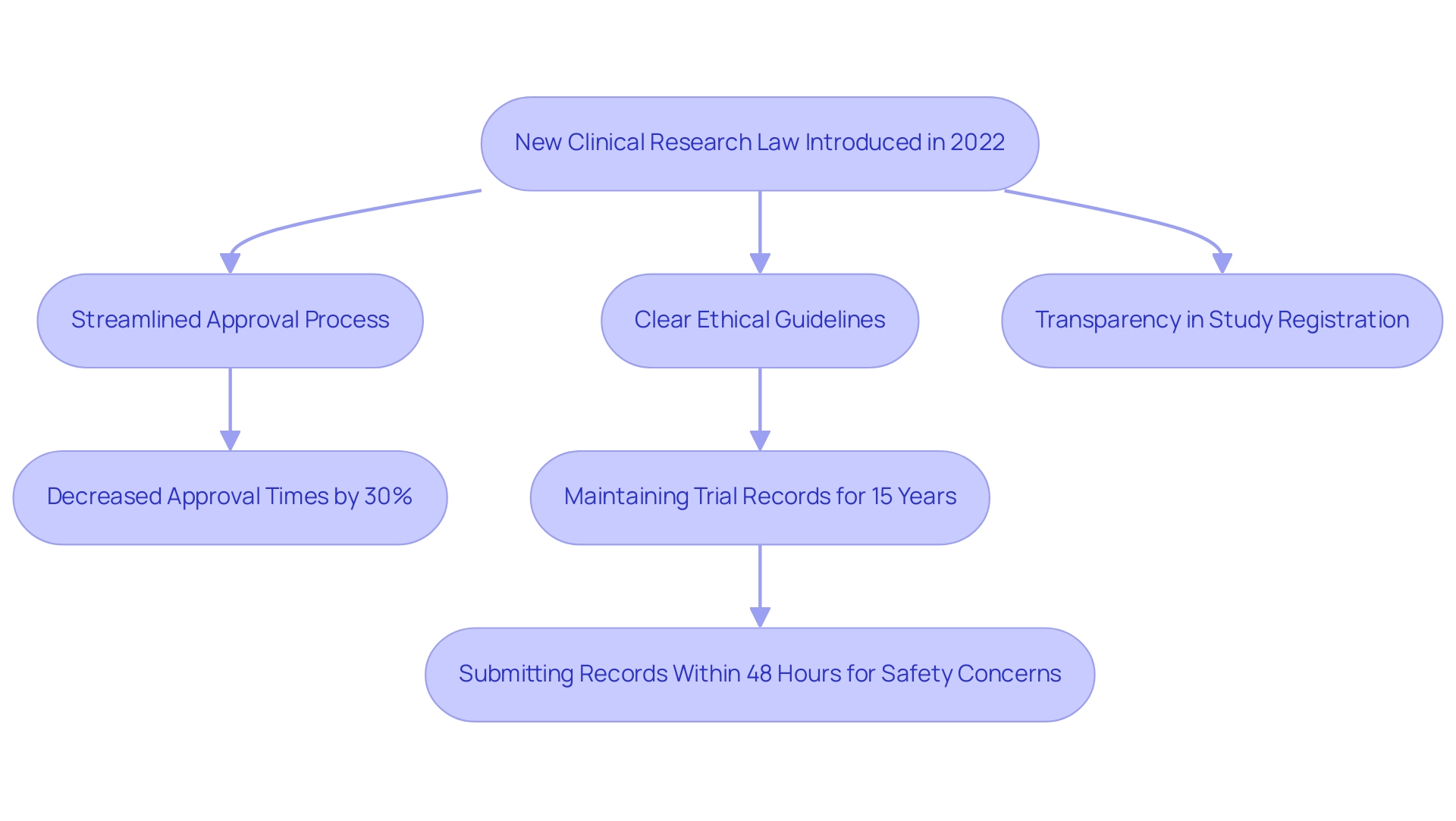
In 2022, Brazil introduced a transformative medical research law that redefined the legal framework for studies, significantly streamlining the approval process for medical device research. This legislation reduces bureaucratic obstacles and accelerates the pace of innovation, a key aspect for Medtech startups seeking rapid deployment of their products. One key provision mandates clearer guidelines for the ethical approval process, ensuring a robust framework for research integrity. Furthermore, the law improves transparency in research study registration, promoting a more accountable research environment.
Legal experts have emphasized the importance of these changes. According to CAN-12, 'if the pediatric participant has the capacity for assent, then affirmative assent is required to participate in a research according to the participant’s level of development and capacities.' This strengthens the law's dedication to ethical standards, a principle that bioaccess® maintains in its thorough research management services, including:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
Recent statistics reveal a notable impact of the legislative changes, with approval times for trials decreasing by approximately 30%, as reported by [insert source]. This reflects the law's efficiency and its positive effects on the research landscape. Updated regulations in 2023 continue to support this streamlined approach, further facilitating clinical research operations.
A case study titled 'Record Keeping for Clinical Trials' highlights the practical benefits of these changes. Under the new law, sponsors must maintain all trial-related records for 15 years, ensuring thorough documentation and accurate data interpretation. In instances of safety concerns, sponsors are required to submit records to relevant authorities within 48 hours, enhancing compliance and oversight. This aligns with the expertise of Katherine Ruiz, a Regulatory Affairs expert at bioaccess®, who has advised many foreign manufacturers on obtaining market clearance for their innovations in Colombia. Her work exemplifies the commitment of bioaccess® to not only adhere to these regulations but also to guide clients through the complexities of compliance.
Overall, Brazil's new research law fosters a more supportive environment for researchers and ultimately benefits patients through more efficient and ethical trials. This legislative framework strengthens bioaccess®'s role as a prominent CRO in Latin America, committed to enhancing medical device research services.
Key Requirements and Processes for Clinical Research in Brazil
Conducting clinical research in Brazil necessitates adherence to several critical processes and requirements, which bioaccess® expertly navigates to ensure successful outcomes for your clinical trials:
-
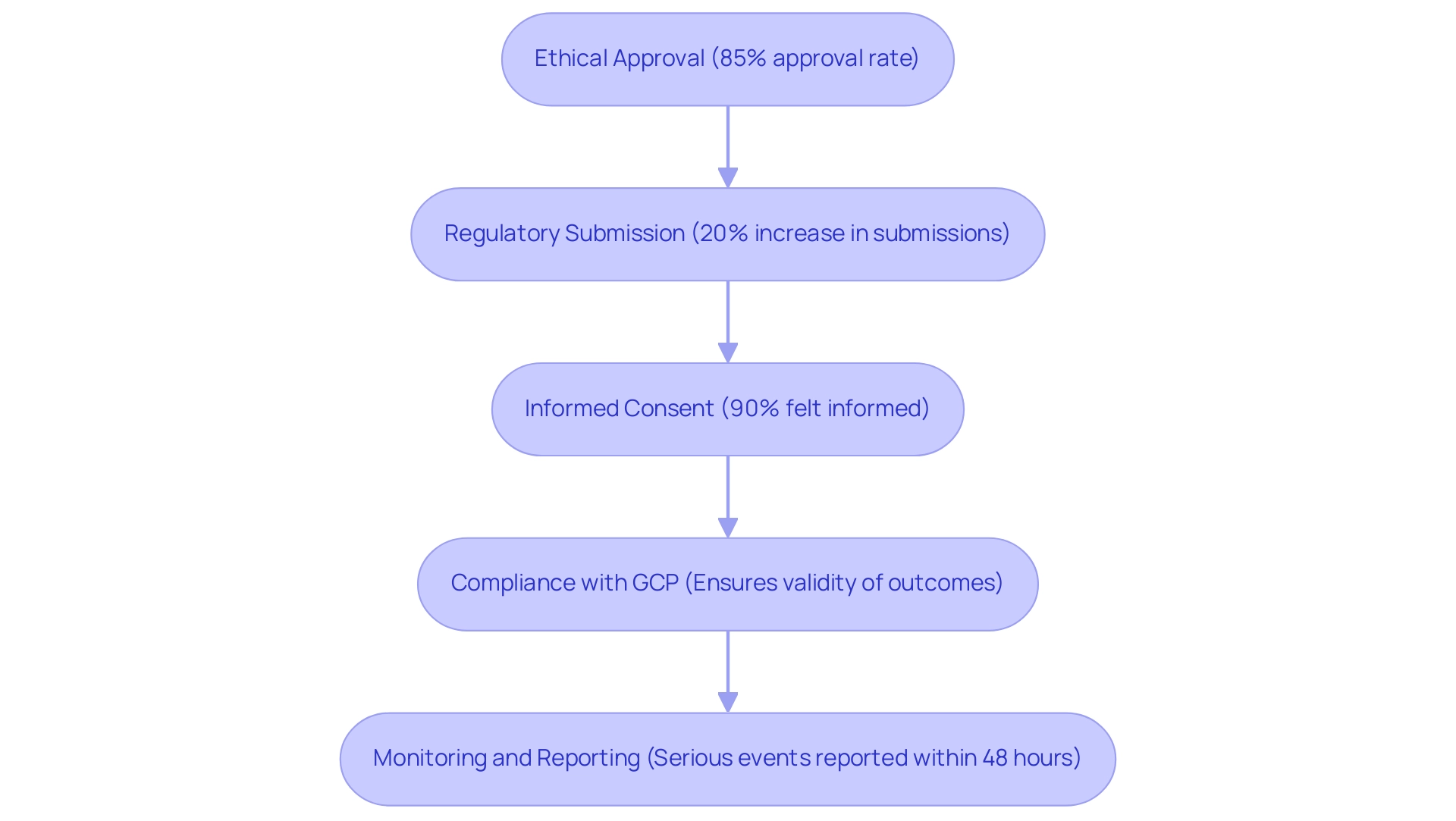
Ethical Approval: Before commencing any research, scholars must obtain permission from an ethics committee, referred to as Comitê de Etica em Pesquisa (CEP). The CEP meticulously reviews the research protocol to safeguard participants’ rights and welfare, ensuring ethical standards are met. In 2023, the process for obtaining ethical approval has been streamlined, emphasizing the need for detailed participant protection measures. Approximately 85% of research received ethical approval within the revised timeframes, reflecting improvements in the process.
-
Regulatory Submission: A formal submission to the Brazilian Health Oversight Agency (ANVISA) is mandatory for obtaining approval. This process entails submitting comprehensive documentation on the medical device, its intended use, and the proposed research design. In 2024, ANVISA reported a 20% increase in regulatory submissions, reflecting enhanced scrutiny and the need for thorough compliance. Bioaccess® assists clients in preparing clear and complete documentation, which has become paramount in light of recent changes in submission requirements.
-
Informed Consent: Researchers must prepare an informed consent form that articulates the project's purpose, procedures, risks, and benefits in clear terms to potential participants. This ensures participants can make well-informed decisions regarding their involvement. The focus on transparency and participant understanding remains a cornerstone of ethical research in Brazil. Recent surveys indicate that 90% of participants felt adequately informed about the research they were involved in, illustrating the effectiveness of current consent practices.
-
Compliance with Good Clinical Practice (GCP): All clinical research must adhere to GCP guidelines, which delineate the responsibilities of researchers, the rights of participants, and the integrity of data collection. The latest updates reinforce the importance of maintaining high ethical and scientific standards throughout the research process. Complying with GCP is not merely a compliance necessity; it's crucial for guaranteeing the validity of research outcomes, a principle that bioaccess® maintains in all its projects.
-
Monitoring and Reporting: Continuous monitoring of the study is vital, with regular reporting of adverse events to both the ethics committee and ANVISA. This practice ensures transparency and accountability, allowing for timely interventions and adjustments to safeguard participant safety. Recent policy updates have mandated more frequent reporting intervals, enhancing oversight and compliance. For instance, the requirement to report serious adverse events within 48 hours has significantly improved response times during trials.
Navigating these requirements requires a comprehensive grasp of the compliance environment, particularly for carrying out expedited medical device trials in Latin America. Specialists such as Katherine Ruiz, an affairs expert at bioaccess®, stress that careful preparation and compliance with guidelines are crucial for successful research. By following these steps, researchers can ensure that their studies are ethically sound, scientifically valid, and compliant with all standards, ultimately contributing to the advancement of healthcare research in the region.
Navigating Regulatory Challenges in Clinical Research
Conducting clinical trials in Brazil presents several regulatory challenges that researchers must navigate effectively:
-
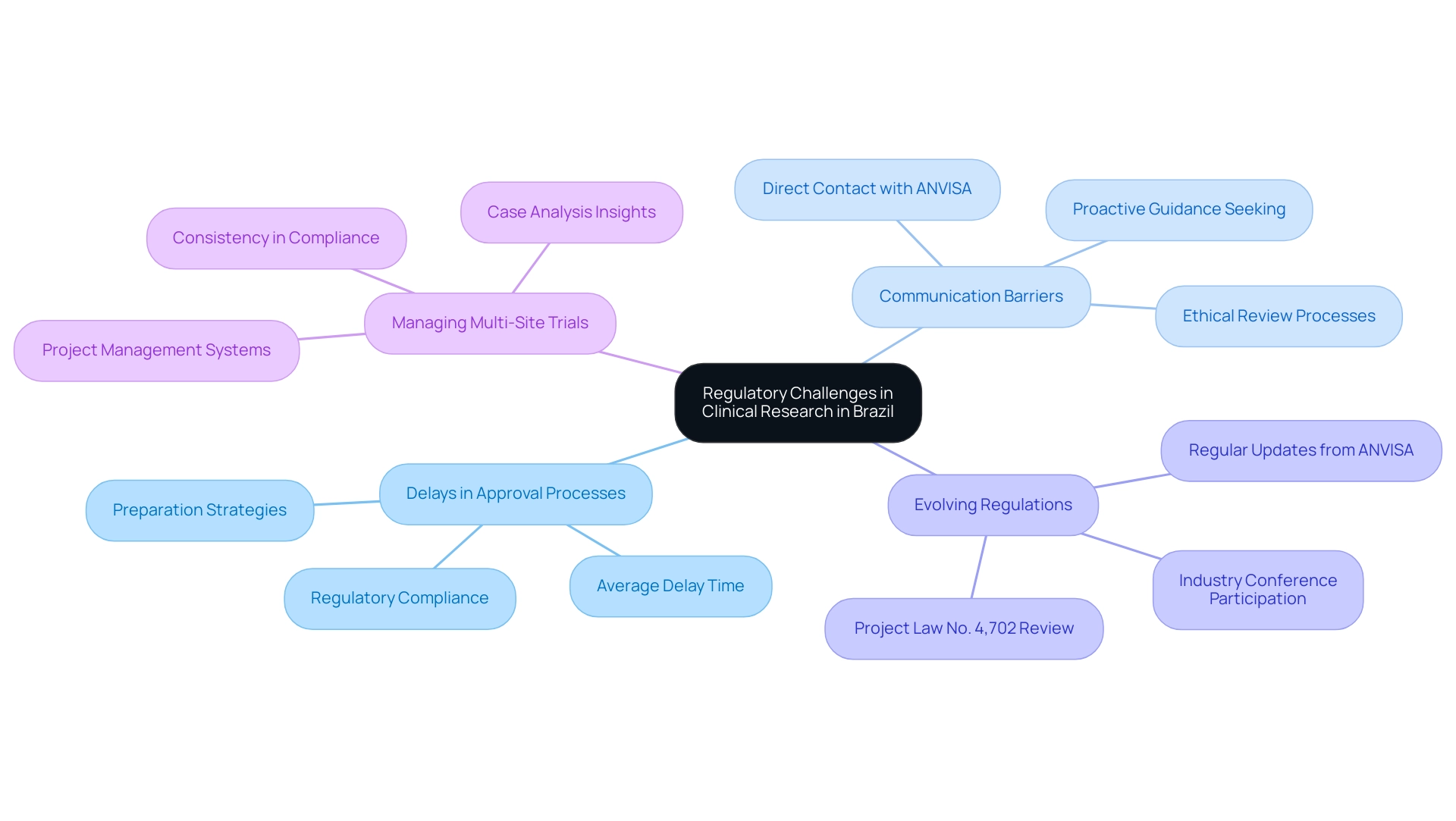
Delays in Approval Processes: Regulatory approvals can frequently exceed anticipated timelines, presenting substantial difficulties for prompt study initiation. For instance, the average delay in clinical trial approval in Brazil has been a critical concern, with recent data indicating waiting periods that could extend up to six months or more into 2024. To mitigate these delays, it is essential for researchers to meticulously prepare and ensure that all submitted documents are thorough and in strict compliance with the latest regulations.
-
Communication Barriers: Effective communication with oversight authorities, such as ANVISA, is crucial for smooth approval processes. Researchers should establish direct and consistent contact channels with ANVISA representatives. Proactively seeking guidance and clarification can prevent misunderstandings and expedite the approval process. As highlighted by Thayse Mayra Chaves Ramos, who noted, 'This study received approval from the Human Research Ethics Committee of the Universidade Federal de Minas Gerais. The participants signed a statement of informed consent,' it underscores the necessity of clear communication and compliance in facilitating ethical review processes, particularly in ensuring participant rights are upheld.
-
Evolving Regulations: The regulatory landscape in Brazil is continually evolving, with new guidelines and requirements emerging frequently. For example, Brazil's Chamber of Deputies is currently reviewing Project Law No. 4,702 from November 2012, which could introduce significant changes to existing regulations regarding clinical studies. Understanding the implications of this law is vital for researchers, as it may affect approval processes and compliance requirements. Regularly reviewing updates from ANVISA and actively participating in industry conferences are essential practices to remain compliant and informed. In fact, researchers should consider how these changes could impact timelines and project planning.
-
Managing Multi-Site Trials: Conducting research across multiple sites can introduce complexities in maintaining consistency in compliance and documentation. Implementing a robust project management system is essential to streamline processes and ensure uniformity across all research locations. A case analysis on the illicit tobacco trade in Colombia underscores the necessity of comprehensive strategies to address regional regulatory challenges. Likewise, researchers in Brazil can gain from adopting organized methods to manage multi-site studies effectively.
Additionally, leveraging the comprehensive clinical trial management services provided by bioaccess®, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, can significantly enhance the efficiency of clinical trials. With over 20 years of expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® is well-equipped to navigate the complexities of the compliance environment. Katherine Ruiz, a compliance affairs expert, brings invaluable experience in facilitating market clearance for medical devices and in vitro diagnostics in Colombia, further supporting the drive for global health improvement through international collaboration and innovation in Medtech. By tackling these regulatory challenges with strategic planning and proactive communication, researchers can enhance the efficiency and success of their trials in Brazil.
Maintaining Compliance Throughout the Study Lifecycle
To maintain compliance throughout a clinical study in Latin America, particularly in Colombia, researchers should prioritize the following key practices:
-
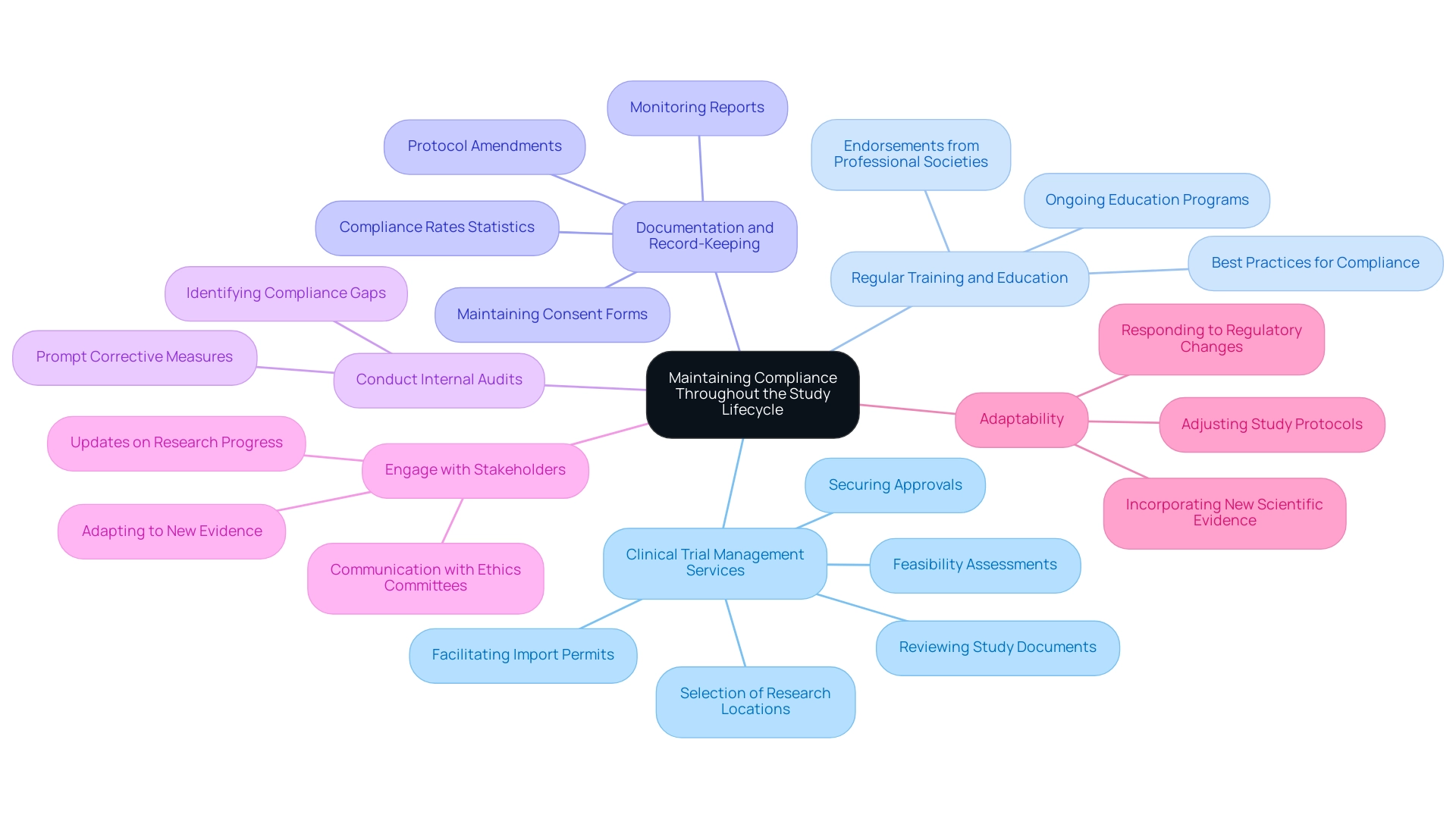
Comprehensive Clinical Trial Management Services:
Participate in feasibility assessments and the selection of suitable research locations and principal investigators (PIs). Create a strong procedure for reviewing and giving feedback on study documents to ensure they meet both local and international compliance standards. Set up and start trials efficiently, securing approvals from ethics committees and health ministries. It is also critical to facilitate import permits and the nationalization of investigational devices to streamline the research process. -
Regular Training and Education:
It's essential that all team members are thoroughly educated in compliance requirements and sound medical practices. Ongoing education is essential, as demonstrated by the latest best practices for compliance in research for 2023. Effective training programs, such as those endorsed by the Pediatric Infectious Diseases Society, are essential for ensuring that staff remain current with evolving guidelines and compliance changes. Expert views confirm the importance of continuous training to adjust to new advancements in medical studies. -
Documentation and Record-Keeping:
Accurate and detailed records of all study-related activities are crucial. This includes maintaining consent forms, protocol amendments, and monitoring reports. Statistics indicate that organizations with compliance rates exceeding 90% in documentation significantly lower the risk of rule violations, illustrating the importance of meticulous record-keeping in clinical trials. -
Conduct Internal Audits:
Regular internal audits are essential for identifying compliance gaps early. Proactive auditing enables prompt corrective measures, ensuring that research complies with the latest compliance standards and best practices. -
Engage with Stakeholders:
Open and regular communication with stakeholders, including ethics committees and sponsors, is vital. Keeping all parties informed of research progress and any emerging issues fosters transparency and trust. The release of Version 2.0.0, which included updated recommendations on treatments like hydroxychloroquine and remdesivir, highlights the importance of stakeholder engagement in adapting to new evidence and protocols. -
Adaptability:
Researchers must be prepared to adjust study protocols and practices in response to changes in regulations or findings from ongoing studies. For example, recent suggestions against the regular use of convalescent plasma for immunocompromised individuals emphasize the necessity for adaptability in medical studies, ensuring that practices conform to the most recent scientific evidence.
Katherine Ruiz, a regulatory affairs specialist with experience at Colombia's INVIMA, highlights the significance of understanding the regulatory environment and ensuring compliance throughout the trial process. Her expertise in obtaining market clearance for medical devices and in vitro diagnostics highlights the critical nature of these practices in ensuring successful clinical research outcomes.
Conclusion
Brazil's recent overhaul of its clinical research law represents a significant advancement in the realm of medical device trials, fostering an environment more conducive to innovation and ethical practices. The introduction of streamlined approval processes has led to a reported 30% decrease in approval times, allowing researchers to conduct trials more efficiently while maintaining high ethical standards through improved transparency and regulatory compliance.
The key requirements established under this new legislation, including ethical approval, regulatory submission, and informed consent, underscore the commitment to participant safety and research integrity. As stakeholders navigate the complexities of these requirements, it is evident that thorough preparation and adherence to Good Clinical Practice (GCP) are essential for successful outcomes. The emphasis on meticulous documentation and proactive communication with regulatory authorities further enhances the integrity of the clinical research process.
However, challenges remain, particularly concerning delays in approval processes and the evolving regulatory landscape. By adopting structured approaches and leveraging the expertise of organizations like bioaccess®, researchers can effectively address these challenges and ensure compliance throughout the study lifecycle. Ultimately, the new clinical research law not only supports the growth of Medtech startups but also promises to improve patient care through more efficient and ethically sound clinical trials.
As Brazil continues to refine its regulatory framework, the potential for advancing healthcare research in the region becomes increasingly promising.




