Overview
The article focuses on understanding clinical research site networks, emphasizing their evolution, operational models, and the critical role they play in enhancing patient recruitment and data quality in medical studies. It highlights how these networks foster collaboration among stakeholders, leverage technology for efficiency, and address challenges like regulatory compliance and patient engagement, ultimately contributing to improved healthcare outcomes in Latin America.
Introduction
The landscape of clinical research is undergoing a transformative evolution, driven by the increasing complexity of trials and the demand for stringent regulatory compliance. As organizations strive to enhance the efficiency and effectiveness of clinical studies, the emergence of clinical research site networks has become essential.
These networks not only facilitate collaboration among key stakeholders—including sponsors, investigators, and regulatory bodies—but also significantly improve patient recruitment and retention. Highlighting successful partnerships, such as that between bioaccess™ and Caribbean Health Group, this article delves into the operational models, future trends, and technological advancements that are shaping the future of clinical research in Latin America.
By examining the critical role of these networks, the discussion underscores their potential to enhance patient access, drive economic growth, and ultimately contribute to improved healthcare outcomes.
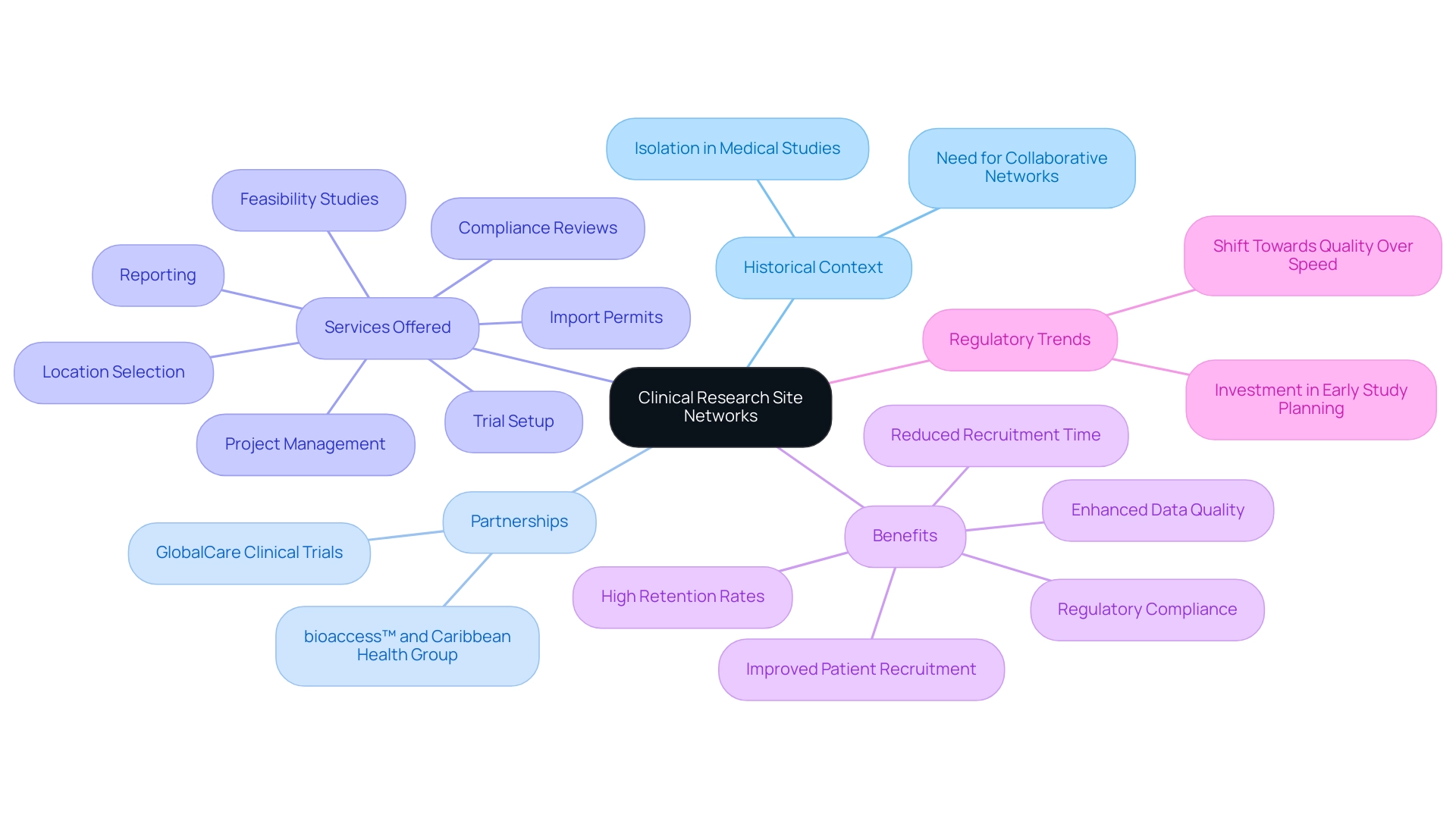
The Evolution and Importance of Clinical Research Site Networks
The development of healthcare study site networks has been characterized by major improvements designed to address the growing intricacy of medical investigations and the rigorous requirements of regulatory adherence. Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for medical studies in Latin America, with the backing of Colombia's Minister of Health. This partnership was officially revealed during a meeting in Miami, FL, on March 29, 2019, emphasizing the dedication of both organizations to improve research in the region.
Historically, medical studies were frequently conducted in isolated environments, leading to inefficiencies and disconnected methods for patient recruitment. However, as the necessity for strong medical evidence has intensified, the value of collaborative networks has become paramount. Currently, these networks, known as clinical research site networks, serve as essential infrastructures supporting multi-site studies that enhance patient recruitment, improve data quality, and ensure adherence to regulatory standards.
bioaccess™ and Caribbean Health Group offer extensive trial management services, including:
- Feasibility studies
- Location selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Furthermore, the partnership with GlobalCare Clinical Trials has proven effective, achieving over a 50% reduction in recruitment time and a remarkable 95% retention rate. Based on the 2022 State of Trial Operations survey, over 72 data points from more than 90 locations, sponsors, and CROs highlight the increasing significance of the clinical research site network in conducting medical studies.
They connect various stakeholders—including sponsors, investigators, and regulatory bodies—through a clinical research site network, creating an environment conducive to advancing medical knowledge and improving patient outcomes. As emphasized in the case study titled 'Risk Sharing in Clinical Trials,' high location failure rates necessitate innovative approaches to recruitment and retention, with location networks offering potential risk-sharing relationships. This dedication to innovation is further shown by Florence's emphasis on attaining a 95% adoption rate for eBinders, highlighting continuous efforts to improve collaboration and efficiency within the health study environment.
Moreover, the recent trend among regulators promoting greater investment in early study planning indicates a wider industry shift towards quality over speed, emphasizing the essential role of a clinical research site network in achieving successful research outcomes. Furthermore, the growth of the clinical research site network through both organic and inorganic expansion has resulted in a greater share of clinical trial participants involved in studies conducted by this clinical research site network, thereby strengthening their crucial role in the clinical ecosystem.
Operational Models of Clinical Research Site Networks
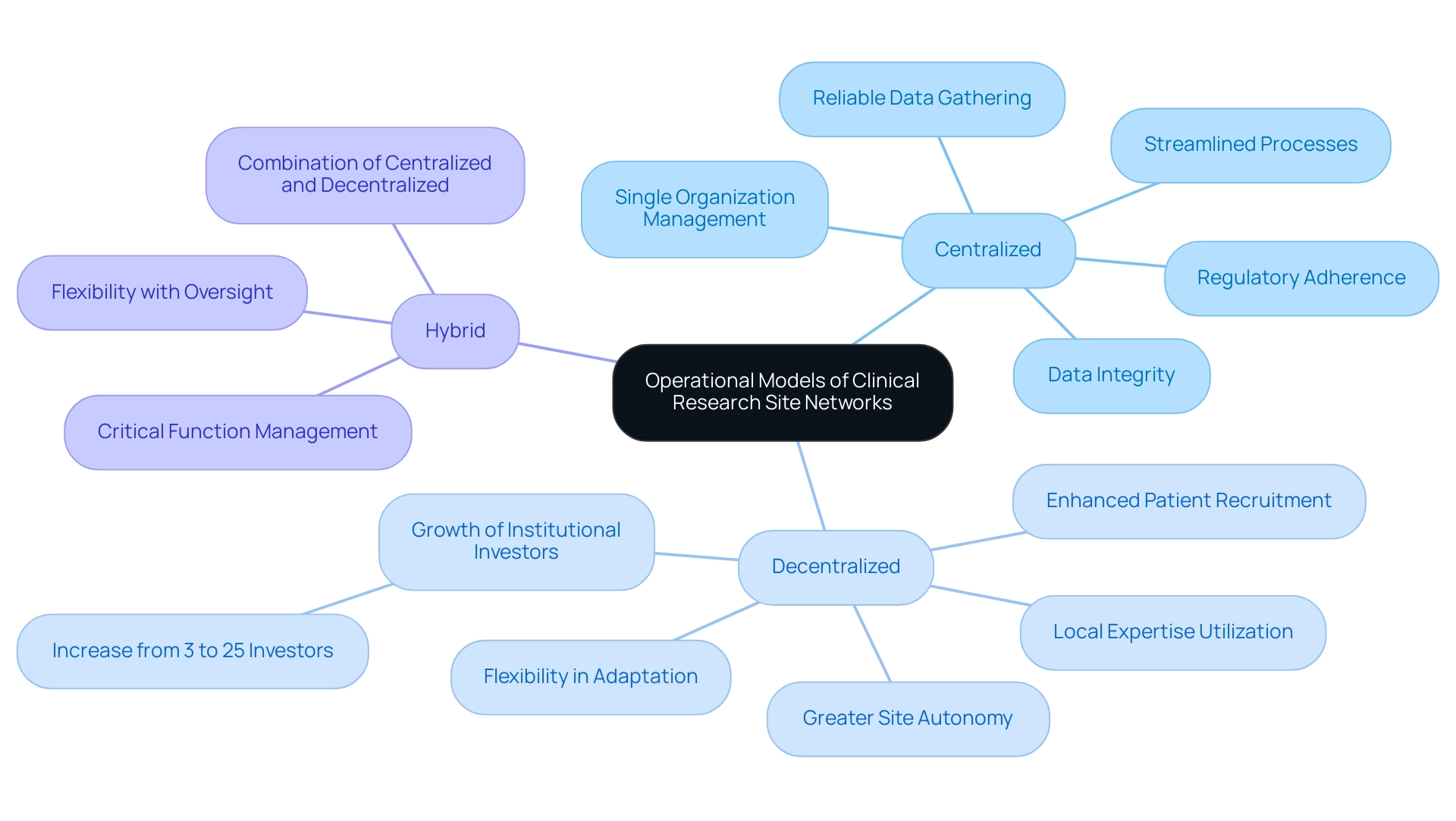
Clinical research site networks can be classified into various operational models, primarily centralized, decentralized, and hybrid structures. Centralized networks typically involve a singular organization managing multiple locations, which streamlines processes and ensures adherence to protocols. This model frequently results in reliable data gathering and regulatory adherence, essential for the integrity of medical studies.
In fact, 83% of sponsors indicated a willingness to distribute electronic Investigator Files (eISFs) to locations lacking them in 2022, highlighting the growing importance of technology in management. Our extensive research management services include:
- Feasibility studies
- Location selection
- Compliance assessments
- Study setup
- Import permits
- Project management
- Reporting
All of which are essential for successful execution and supervision. Specifically, our project management includes monitoring study progress, managing resources, and ensuring timely reporting on study status, inventory, and serious and non-serious adverse events.
Conversely, a clinical research site network allows individual sites greater autonomy, enabling them to leverage local expertise and adapt to specific patient populations. This flexibility can enhance patient recruitment and retention, as evidenced by the increasing interest from institutional investors in decentralized models, which has surged from 3 to 25 since 2015. Hybrid models incorporate elements of both approaches, offering the flexibility of decentralized networks while maintaining centralized oversight for critical functions in a clinical research site network.
Each operational framework in a clinical research site network carries its own set of advantages and challenges, particularly in terms of resource management and data integration. The case study titled 'Optimal Characteristics of Site Networks' emphasizes that strong leadership and a collaborative culture are essential for maximizing the efficiency of medical studies, aligning well with the distinct advantages of these operational models. Moreover, the influence of medtech research extends beyond study results, aiding local economies through job creation, economic development, healthcare enhancement, and promoting international cooperation.
As the saying 'Garbage in, garbage out' implies, ensuring high-quality data is essential for successful tests; this is particularly pertinent when considering the implications of data quality across various operational models. Therefore, a nuanced understanding of these models, along with a strict adherence to compliance with country requirements for study documents and the critical role of import permits and nationalization of investigational devices, is essential for fostering effective collaboration among network participants and optimizing the overall trial process.
Future Trends and Challenges in Clinical Research Networks
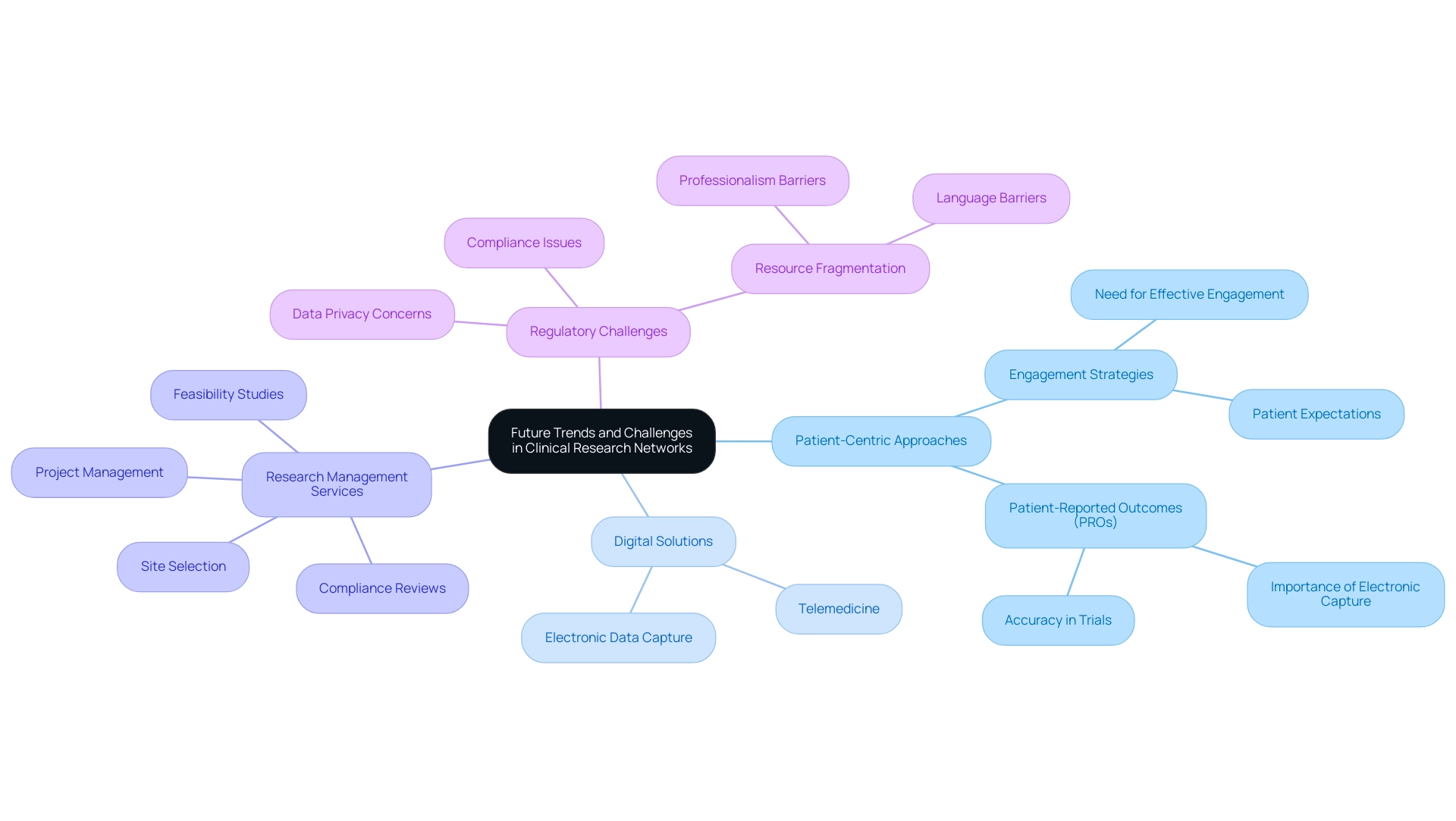
As we look towards the future, the clinical research site network in Latin America is well-equipped to address several significant trends and challenges. A prominent trend is the increasing emphasis on patient-centric approaches, which place patient engagement and experience at the forefront of the inquiry process. This shift necessitates a reevaluation of recruitment and retention strategies to better align with patients' expectations.
For instance, the alarming statistic that about 90% of initial enrollees in Apple's ResearchKit project dropped out within weeks underscores the critical need for effective engagement strategies. Moreover, the research environment is hindered by intricate protocols and restricted research locations, with only 15% of Health Care Organizations (HCOs) performing studies. This saturation pressures locations to meet protocol endpoints within strict timelines, highlighting the necessity for effective enablement strategies.
In tandem with this focus, the adoption of digital solutions such as telemedicine and electronic data capture is transforming the landscape of research studies, allowing for more efficient monitoring and data collection. Comprehensive research management services—ranging from feasibility studies and site selection to compliance reviews, setup, import permits, project management, and reporting—are essential to ensuring successful outcomes. Patient-reported outcomes (Pros) are essential for medical studies and are more precise when recorded electronically, aligning with the emphasis on patient-centered methods.
However, these advancements are not without hurdles; regulatory compliance, data privacy concerns, and the fragmentation of resources, including professionalism and language barriers faced by US Medtech companies in Latin America, continue to pose significant challenges. As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, stated, 'I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.'
Surmounting these challenges will be crucial to guarantee the continuous development and effectiveness of the clinical research site network as we progress into 2024 and beyond, particularly as partnerships like that between Greenlight Guru and bioaccess™ aim to expedite Medtech innovations and health assessments in Latin America.
Leveraging Technology and Collaboration in Clinical Research
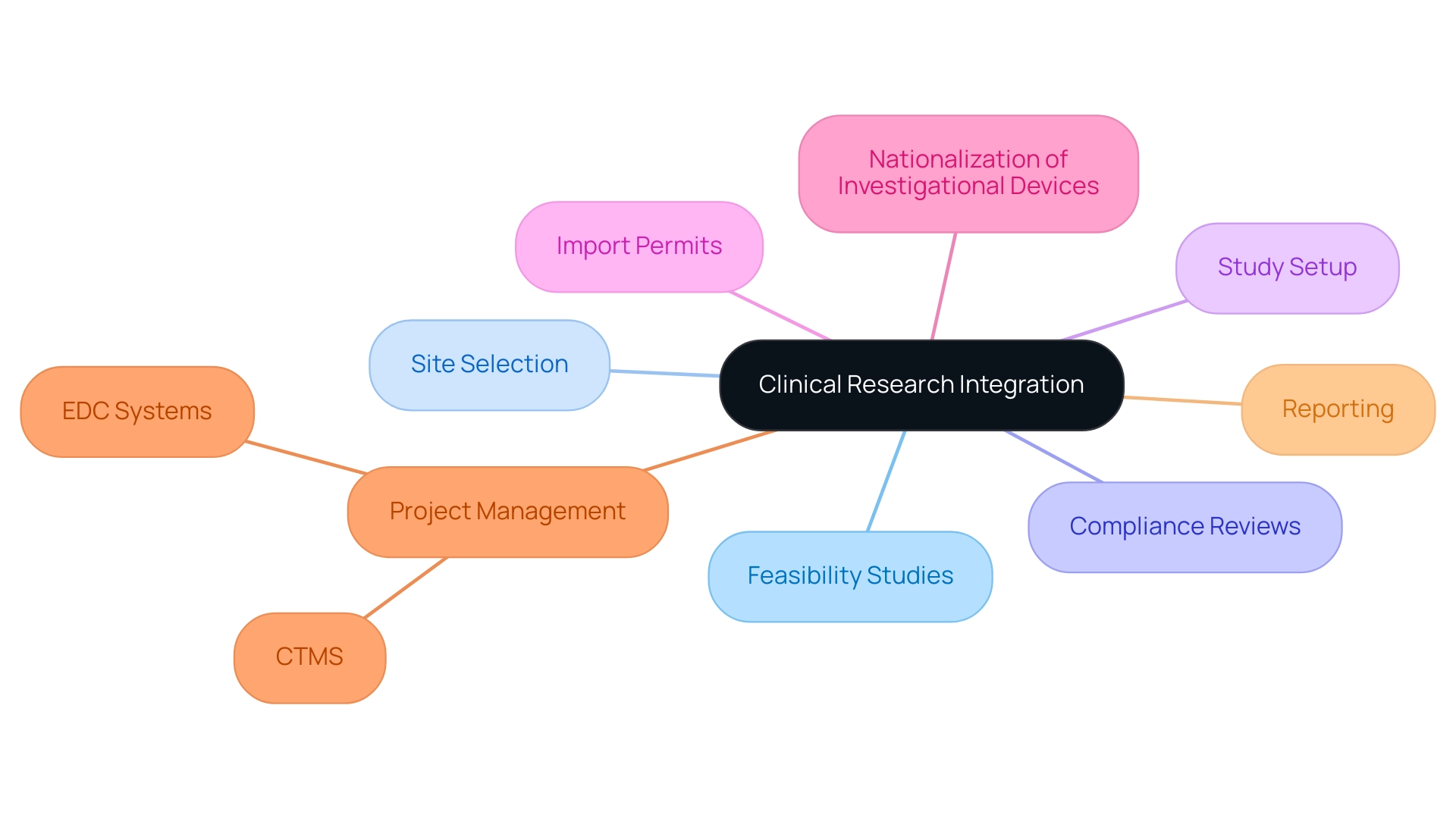
The integration of technology and collaboration is fundamentally transforming the clinical research site network, enhancing efficiency and improving outcomes. Our extensive research study management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting
This ensures that each aspect of the research process is meticulously addressed. This includes navigating the approval processes involving ethics committees and health ministries.
Electronic data capture (EDC) systems and project management systems (CTMS) play crucial roles by facilitating real-time data sharing and optimizing project management. These tools not only facilitate faster participant recruitment but also leverage artificial intelligence (AI) and machine learning to expedite data analysis beyond human capabilities. As noted by Miller, Sovereign, & Krege, effective collaboration involves a combination of assessment, normative feedback, and increasing motivation for modifying use or seeking more intensive treatment services.
Moreover, our collaboration platforms enhance communication among investigators, sponsors, and regulatory entities within the clinical research site network, fostering a unified environment crucial for successful studies. For instance, one team member built three unique studies in just three months, demonstrating the efficiency that these technologies can provide. Additionally, a case study on patient experience in clinical studies illustrates the importance of reducing patient burden through remote data capture technologies, drawing lessons from the hospitality industry to enhance participant satisfaction.
By adopting technology and promoting teamwork, healthcare networks, particularly clinical research site networks, can greatly improve their operational abilities, stimulate local economic growth, and raise the standard of medical studies throughout Latin America.
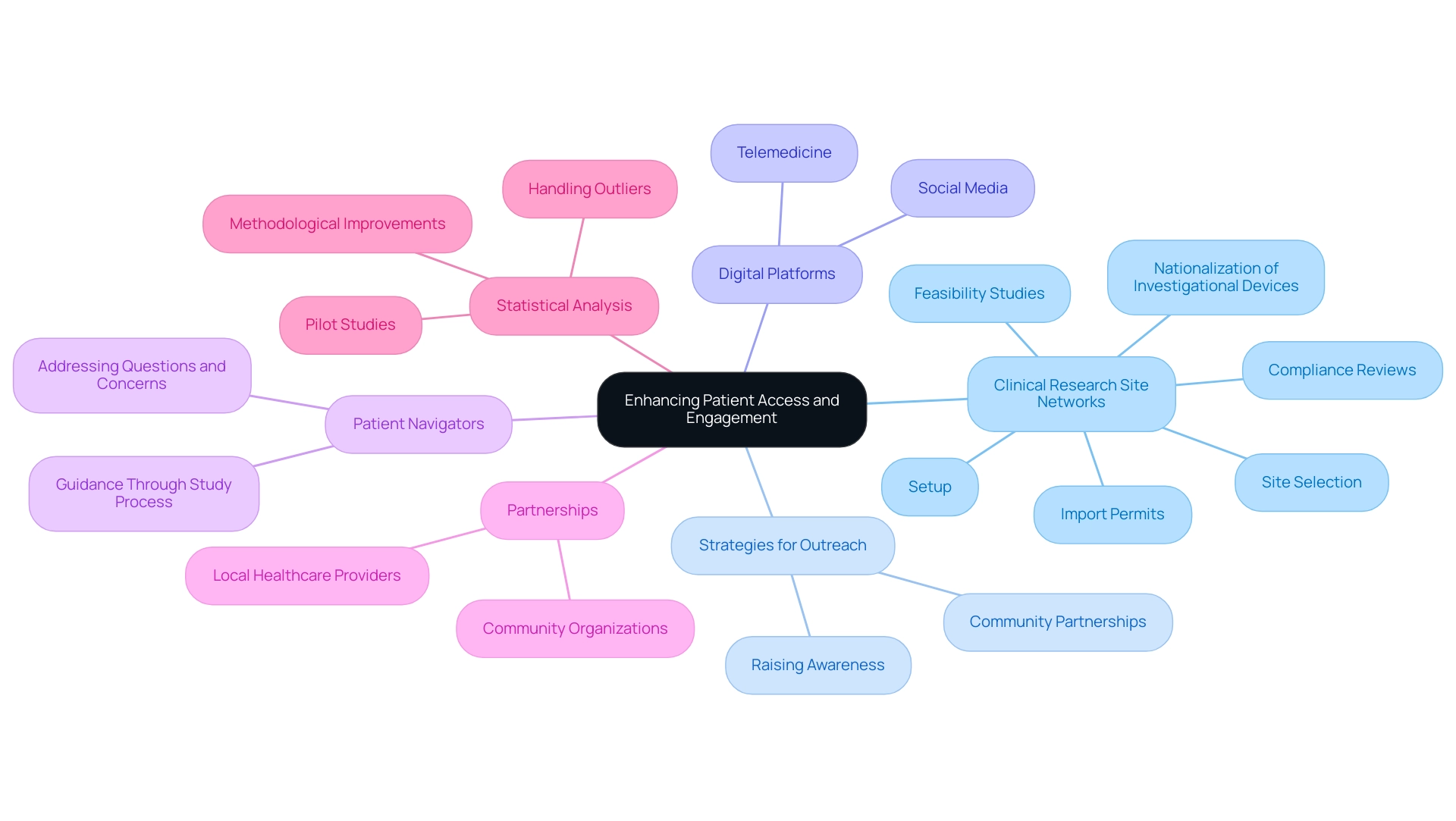
Enhancing Patient Access and Engagement through Site Networks
Clinical research site networks are crucial in improving patient access and involvement, key elements for attaining successful study results. Our comprehensive clinical study management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Nationalization of investigational devices
This ensures a robust foundation for all studies. By forging partnerships with local healthcare providers and community organizations, the clinical research site network can significantly improve outreach and raise awareness of ongoing studies, ultimately contributing to economic growth and healthcare improvement in local economies.
Recent strategies have highlighted the value of utilizing digital platforms, such as social media and telemedicine, to broaden access to diverse populations. Furthermore, the incorporation of patient navigators offers critical support, guiding participants through the study process while addressing their questions and concerns. Regular communication and feedback mechanisms are vital in fostering trust and encouraging participation.
As Georgios D Panos from the Department of Ophthalmology at Nottingham University Hospitals NHS Trust emphasizes,
Collaboration between authors and statisticians is essential for the advancement of medical studies.
Furthermore, regular pilot studies can improve study designs and reduce methodological shortcomings, which is essential in boosting outcome results. It's important to acknowledge that prevention studies are often cumbersome, lengthy, and expensive to conduct, highlighting the need for improved patient access strategies.
The management of outliers in statistical analysis also highlights the importance of careful thought in methodologies, strengthening the theme of improving outcomes through better patient involvement and access. By implementing these strategies alongside our expertise in managing study approvals, import permits, and detailed reporting on study status and adverse events, research can yield more robust and representative outcomes, while also recognizing the importance of patient access statistics in driving engagement. The impact of community partnerships, coupled with innovative approaches to recruitment, cannot be overlooked in the pursuit of enhancing patient access within the clinical research site network for clinical trials.
Conclusion
The advancement of clinical research site networks is transforming the landscape of clinical trials in Latin America. These networks enhance collaboration among sponsors, investigators, and regulatory bodies, leading to improved patient recruitment and retention. The partnership between bioaccess™ and Caribbean Health Group exemplifies how strategic collaborations can create effective infrastructures that streamline processes and enhance outcomes.
Different operational models—centralized, decentralized, and hybrid—each offer specific advantages for optimizing trial efficiency. Focusing on data quality and regulatory compliance is critical, alongside the integration of innovative technologies such as electronic data capture and artificial intelligence, which facilitate real-time data sharing and communication.
Looking ahead, prioritizing patient-centric approaches and digital solutions will be essential for addressing challenges related to complex trial protocols and limited research sites. Effective patient engagement and support strategies will be vital in ensuring successful recruitment and retention.
In conclusion, the future of clinical research in Latin America relies on the continued evolution of site networks. By fostering collaboration, leveraging technology, and enhancing patient access, stakeholders can create a more efficient research environment. This commitment to innovation and partnership will ultimately lead to improved healthcare outcomes and a stronger clinical research ecosystem.
Frequently Asked Questions
What improvements have been made in healthcare study site networks?
The development of healthcare study site networks has seen major improvements aimed at addressing the complexity of medical investigations and regulatory requirements.
What is the partnership between bioaccess™ and Caribbean Health Group?
The partnership aims to establish Barranquilla as a leading location for medical studies in Latin America, supported by Colombia's Minister of Health, and was officially announced on March 29, 2019.
How have medical studies historically been conducted?
Medical studies were often conducted in isolated environments, which led to inefficiencies and disconnected patient recruitment methods.
What role do clinical research site networks play in medical studies?
Clinical research site networks support multi-site studies, enhance patient recruitment, improve data quality, and ensure adherence to regulatory standards.
What services do bioaccess™ and Caribbean Health Group provide?
They offer trial management services including feasibility studies, location selection, compliance reviews, trial setup, import permits, project management, and reporting.
How effective is the partnership with GlobalCare Clinical Trials?
The partnership has achieved over a 50% reduction in recruitment time and a 95% retention rate.
What insights were gained from the 2022 State of Trial Operations survey?
The survey highlighted the significance of clinical research site networks, with data from over 90 locations emphasizing their role in conducting medical studies.
What are the operational models of clinical research site networks?
Clinical research site networks can be centralized, decentralized, or hybrid, each with distinct advantages and challenges.
What are the benefits of centralized networks?
Centralized networks streamline processes, ensure adherence to protocols, and result in reliable data gathering and regulatory compliance.
How do decentralized networks enhance patient recruitment?
Decentralized networks allow individual sites greater autonomy to leverage local expertise and adapt to specific patient populations.
What is the significance of project management in clinical research?
Project management involves monitoring study progress, managing resources, and ensuring timely reporting on study status and adverse events.
What challenges do operational models in clinical research face?
Each model has its own challenges regarding resource management and data integration, necessitating strong leadership and a collaborative culture.
How does medtech research impact local economies?
Medtech research contributes to job creation, economic development, healthcare enhancement, and promotes international cooperation.
Why is data quality important in clinical trials?
High-quality data is essential for successful tests, affecting the integrity of study results across various operational models.

