Overview
Clinical Site Management Organizations (CSOs) are essential for coordinating clinical research studies, providing services like feasibility studies, site selection, and regulatory compliance to enhance research quality and efficiency. The article highlights their role as intermediaries that streamline operations, improve patient recruitment, and adapt to regulatory changes, thereby significantly contributing to the success of clinical trials.
Introduction
In the intricate world of clinical research, Site Management Organizations (SMOs) emerge as essential players, orchestrating the complex dynamics of clinical trials across various landscapes. These organizations are not just facilitators; they are strategic partners that:
- Streamline operations
- Enhance patient recruitment
- Navigate the labyrinth of regulatory compliance
The collaboration between bioaccess™ and Caribbean Health Group exemplifies this role, as they work to establish Barranquilla as a premier clinical trial destination in Latin America. With their comprehensive suite of services, including:
- Feasibility studies
- Project management
SMOs significantly contribute to the integrity and success of clinical trials. As the landscape evolves, particularly with the integration of advanced technologies like AI and telemedicine, the future of clinical research is poised for transformation, making the role of SMOs more pivotal than ever.
Defining Clinical Site Management Organizations (SMOs)
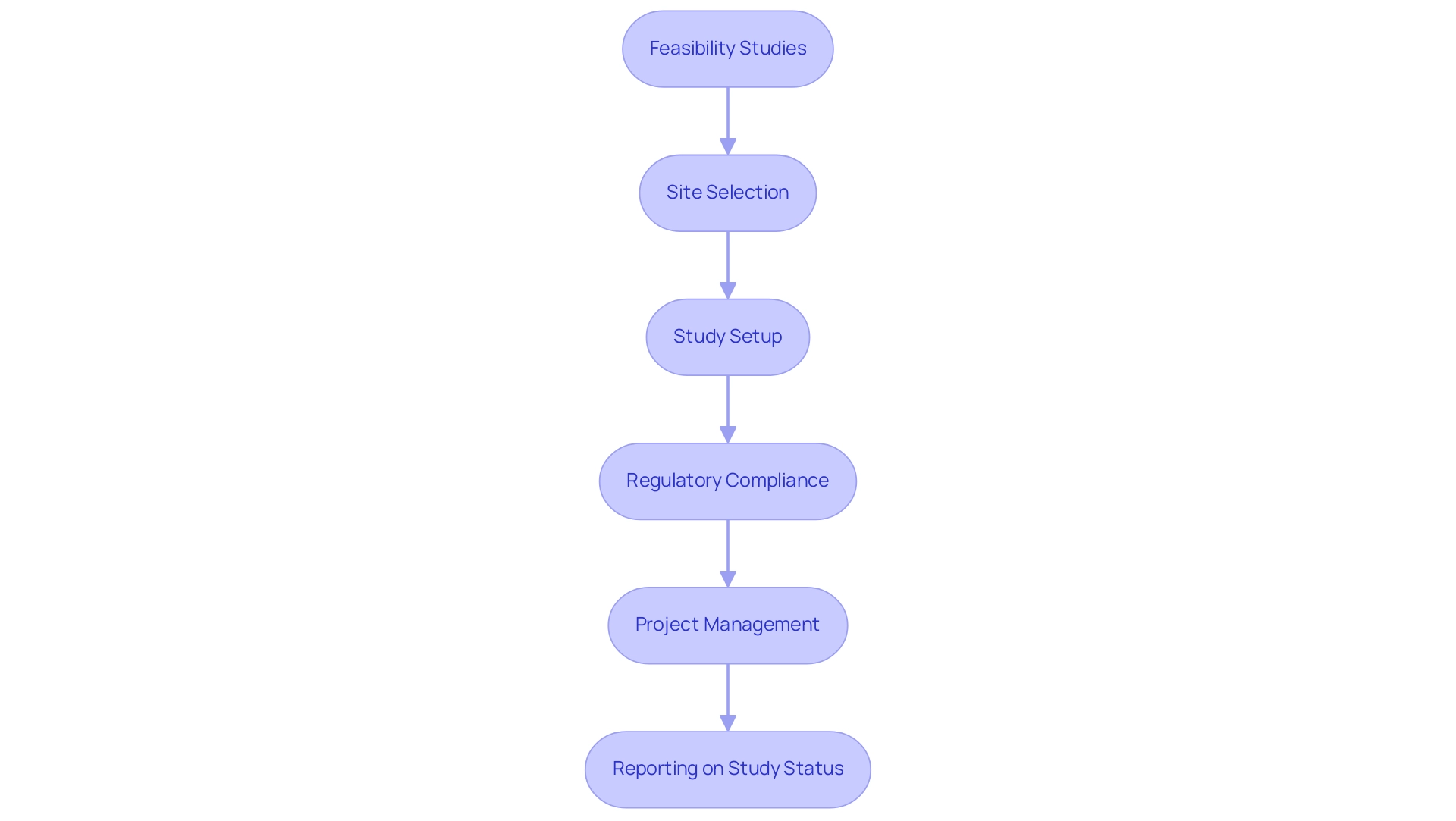
Clinical site management organizations (CSOs) play a crucial role in coordinating research studies across multiple locations, as illustrated by the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a top destination for research in Latin America. This collaboration was announced on March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, where Colombia's Minister of Health openly supported the initiative to improve clinical study projects in the region. Clinical organizations offer a comprehensive range of management services, including:
- Feasibility studies
- Site selection
- Study setup
- Regulatory compliance
- Project management
- Reporting on study status and adverse events
By serving as intermediaries between sponsors and research sites, a clinical site management organization facilitates efficient conduct while ensuring adherence to stringent regulatory standards, ultimately enhancing the integrity and quality of research outcomes. This collaboration has demonstrated promising results, with GlobalCare Clinical Trials partnering with bioaccess™ to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. Recent advancements, such as the launch of myTrialsConnect by Elligo Health Research and Avallano, further illustrate the evolving landscape of research management services.
This AI-driven platform enhances participant enrollment and involvement through automated medical record evaluations and chatbot surveys, highlighting novel methods being embraced by clinical site management organizations to improve study results. As Brian Moore, VP of NICCA USA, Inc., remarked, 'The standard of investigation they have conducted for us has been outstanding,' highlighting the considerable influence that clinical site management organizations have in improving the efficiency and effectiveness of trials.
Exploring Different Models of Site Management Organizations
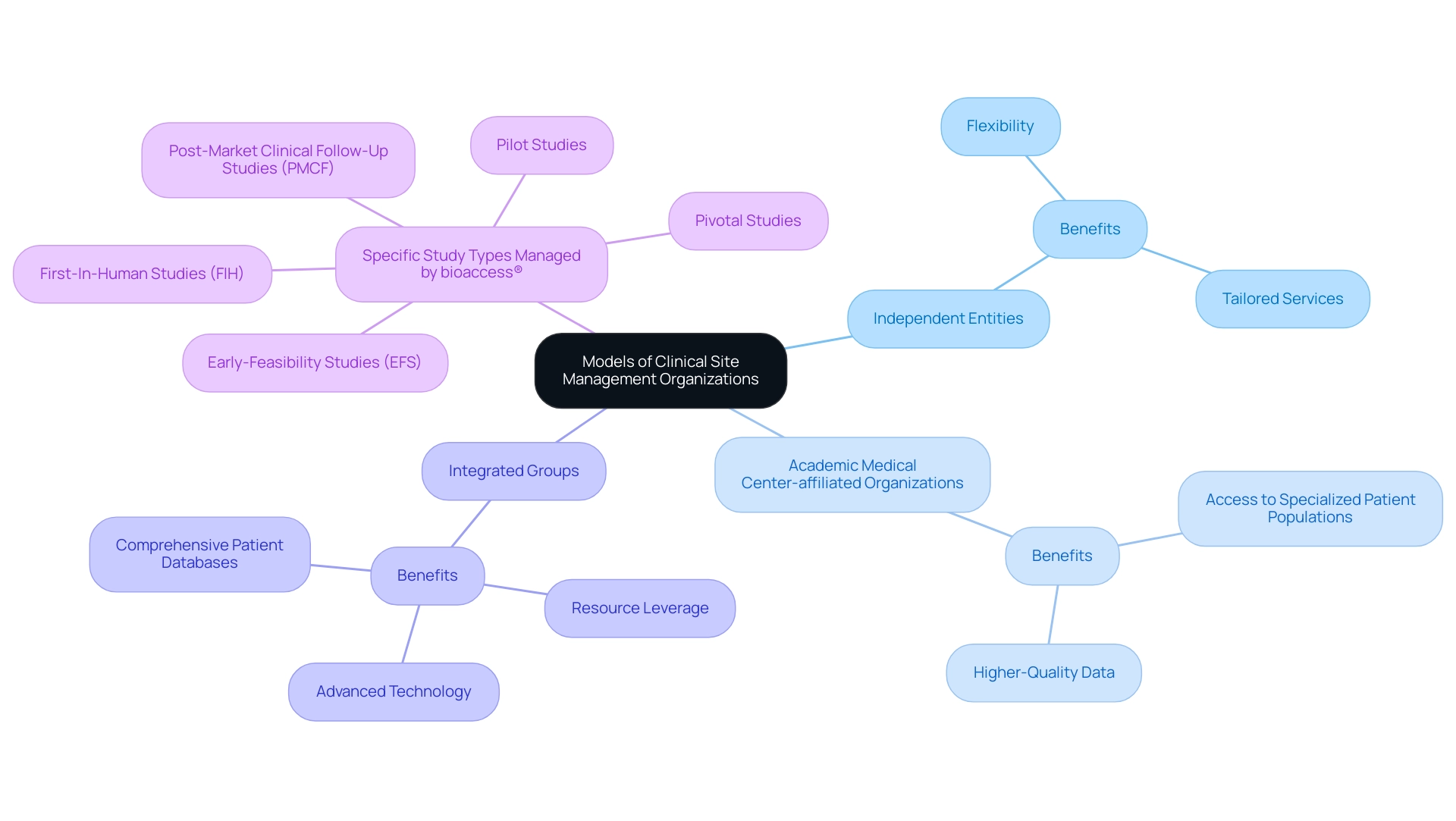
Clinical site management organizations play an essential role in medical studies, functioning under various specific models, including:
- Independent entities
- Academic Medical Center-affiliated organizations
- Integrated groups that are part of larger healthcare systems
Each model offers distinct benefits designed for particular study objectives. Independent organizations are renowned for their flexibility and capacity to tailor services according to the requirements of trials, making them a favored option for many researchers seeking agility in their operations.
In contrast, academic-affiliated organizations provide access to specialized patient populations and can offer higher-quality data, which is vital for the advancement of clinical research, particularly in complex therapeutic areas like advanced non-small cell lung cancer (NSCLC). For example, progression-free survival in patients with advanced NSCLC treated with checkpoint inhibitors has been evaluated using Cox proportional hazards models, underscoring the importance of high-quality data provided by academic-affiliated organizations. Integrated SMOs, which operate within larger healthcare systems, can leverage extensive resources, including advanced technology and comprehensive patient databases, enhancing their operational capabilities and efficiency.
Notably, bioaccess® excels in managing a range of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This ensures compliance with country requirements through feasibility studies, site selection, compliance reviews, and thorough project management. Reporting on study status, inventory, and serious and non-serious adverse events is integral to our service. The effect of these Medtech studies goes beyond investigation, promoting job creation, economic growth, and healthcare enhancement in local economies.
Comprehending these differing models is crucial for researchers intending to align their studies with the most suitable clinical site management organization, thereby ensuring the best possible outcomes for their projects. As George E. P. Box wisely stated, 'All models are wrong, but some are useful,' emphasizing that while each SMO model has its limitations, they can still offer valuable insights and assistance in medical investigation contexts.
The Advantages of Partnering with Site Management Organizations
Working together with Site Management Entities offers researchers a multitude of benefits, such as streamlined operational processes, better patient recruitment, and enhanced regulatory compliance. Organizations adapt to evolving regulatory environments and patient-focused study designs, thereby enhancing their relevance and effectiveness in clinical research. In 2023, North America led the worldwide investigative site network market, possessing a 52% share, highlighting the essential function of clinical site management organizations in this environment.
Their established relationships with healthcare providers facilitate faster recruitment of eligible participants, significantly speeding up study timelines. Furthermore, Sponsor Management Organizations provide crucial services including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup for studies
- Initiation and approval procedures
- Import permits
- Nationalization of investigational devices
- Project management
- Thorough reporting of study status and adverse events
These are essential elements for successful execution. Their deep understanding of regulatory requirements minimizes compliance risks that could impede study progress.
As highlighted by Matthew Wheeler, Managing Director and Partner at L.E.K., it is crucial for sponsors to understand the organizational structure, responsibilities, and central policies/processes of a clinical site management organization for oversight. By utilizing the specialized functions of social media organizations, researchers can focus more on the scientific aspects of their studies, thus improving the chances of successful results.
This collaboration enhances patient enrollment success rates and guarantees studies are carried out efficiently and effectively, paving the way for groundbreaking advancements in research. Just as strategic collaborations in influencer marketing improve visibility and engagement, teaming up with service management organizations can significantly strengthen the effectiveness of research studies and promote economic growth in local communities.
Key Functions and Responsibilities of SMOs in Clinical Trials
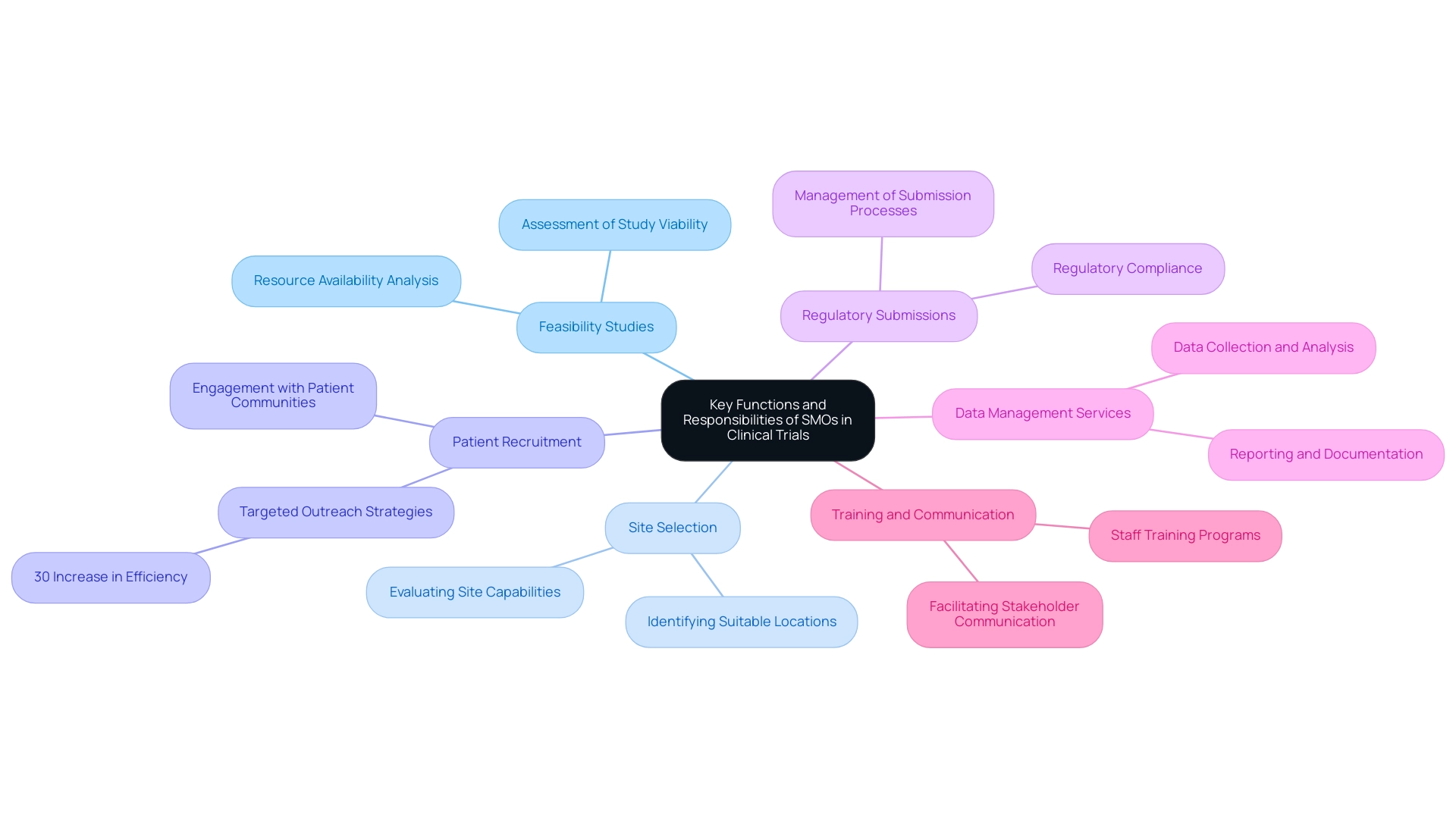
Clinical site management organizations are crucial in the realm of research studies, performing vital tasks that directly affect the success of these investigations. Their responsibilities within a clinical site management organization encompass a broad range of activities, including:
- Feasibility studies
- Site selection
- Patient recruitment
- The meticulous management of regulatory submissions
By ensuring that all regulatory requirements are adhered to, such as those outlined by INVIMA, a Level 4 health authority recognized by PAHO/WHO, clinical site management organizations contribute significantly to the integrity and ethical conduct of research.
As a clinical site management organization, they also oversee study advancement and offer essential data management services, highlighting their extensive research management capabilities. Furthermore, training for site staff and facilitating effective communication among stakeholders—including sponsors, investigators, and ethics committees—are vital aspects of their role within a clinical site management organization. This collaborative approach is essential for the clinical site management organization to maintain integrity and optimize operational efficiency.
For instance, a recent medical trial led by an SMO demonstrated a 30% increase in patient recruitment efficiency through targeted outreach strategies, illustrating the tangible benefits of their expertise. The prospects for specialized clinical site management organizations appear bright, particularly with the growth of technological innovations and patient-focused approaches in medical studies, enhancing their ability to navigate complex regulatory landscapes. Recent reports indicate that specialized medical organizations and clinical site management organizations are increasingly collaborating with regulatory bodies to ensure compliance, proactively addressing regulatory requirements as essential for the success of research endeavors.
Moreover, the function of Contract Research Organizations (CROs) is crucial, as they provide extensive services, including feasibility studies, project management, and reporting, that cover the entire research process and offer strategic advice vital for promoting international cooperation and stimulating economic development in local communities.
Overcoming Challenges in Clinical Research with SMOs
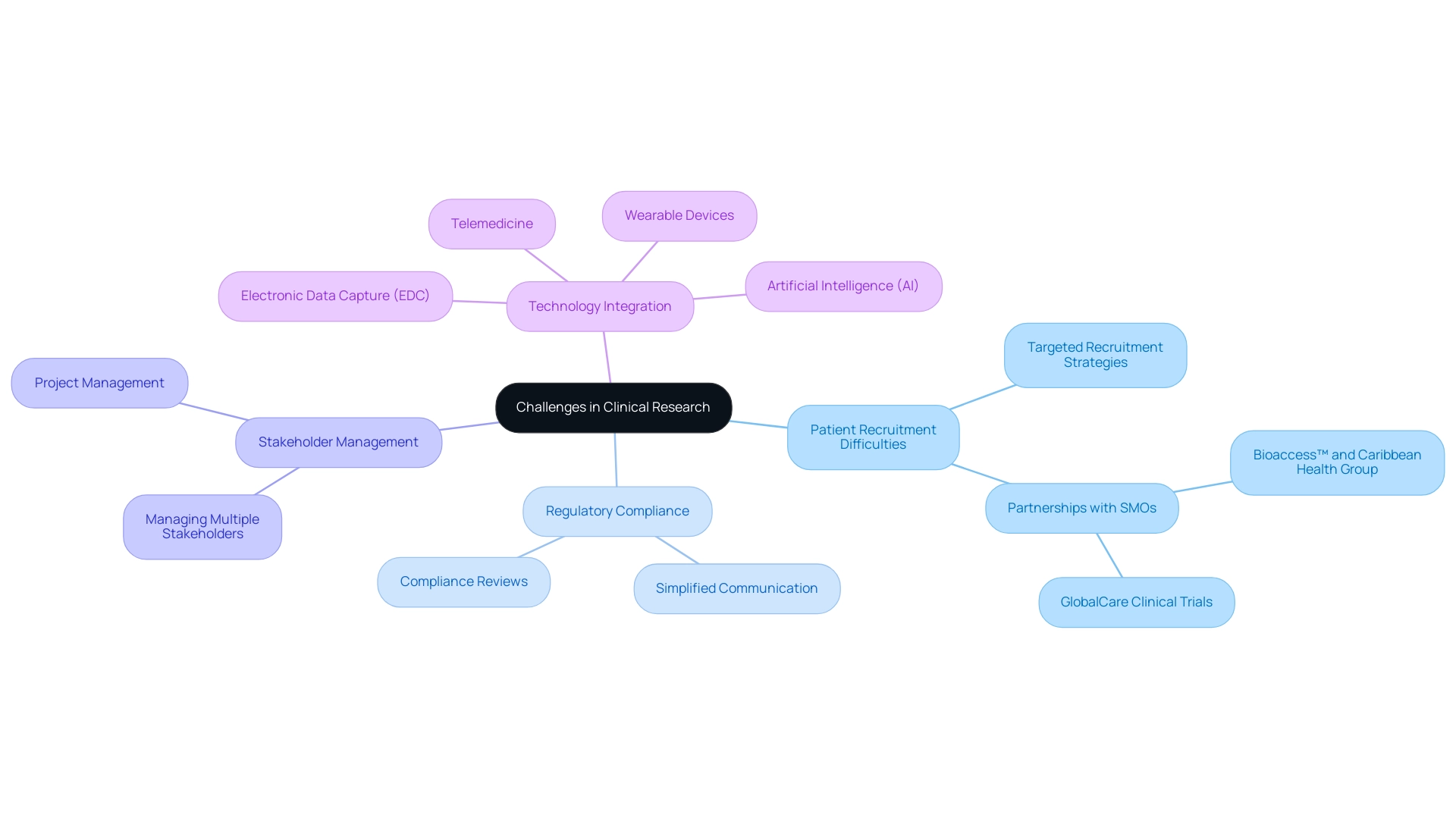
Clinical researchers frequently encounter significant challenges, including:
- Patient recruitment difficulties
- Stringent regulatory compliance
- Complexities of managing multiple stakeholders
In response to these challenges, clinical site management organizations play a pivotal role by employing targeted recruitment strategies that leverage their extensive networks and advanced technologies. A significant instance is the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a leading location for research initiatives in Latin America, backed by Colombia's Minister of Health.
This initiative reflects a commitment to enhancing the research landscape in the region. Additionally, GlobalCare Clinical Trials has partnered with bioaccess™ to expand ambulatory services, achieving over a 50% reduction in recruitment time and maintaining a 95% retention rate. Such outcomes highlight the significance of thorough research management services, which encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events
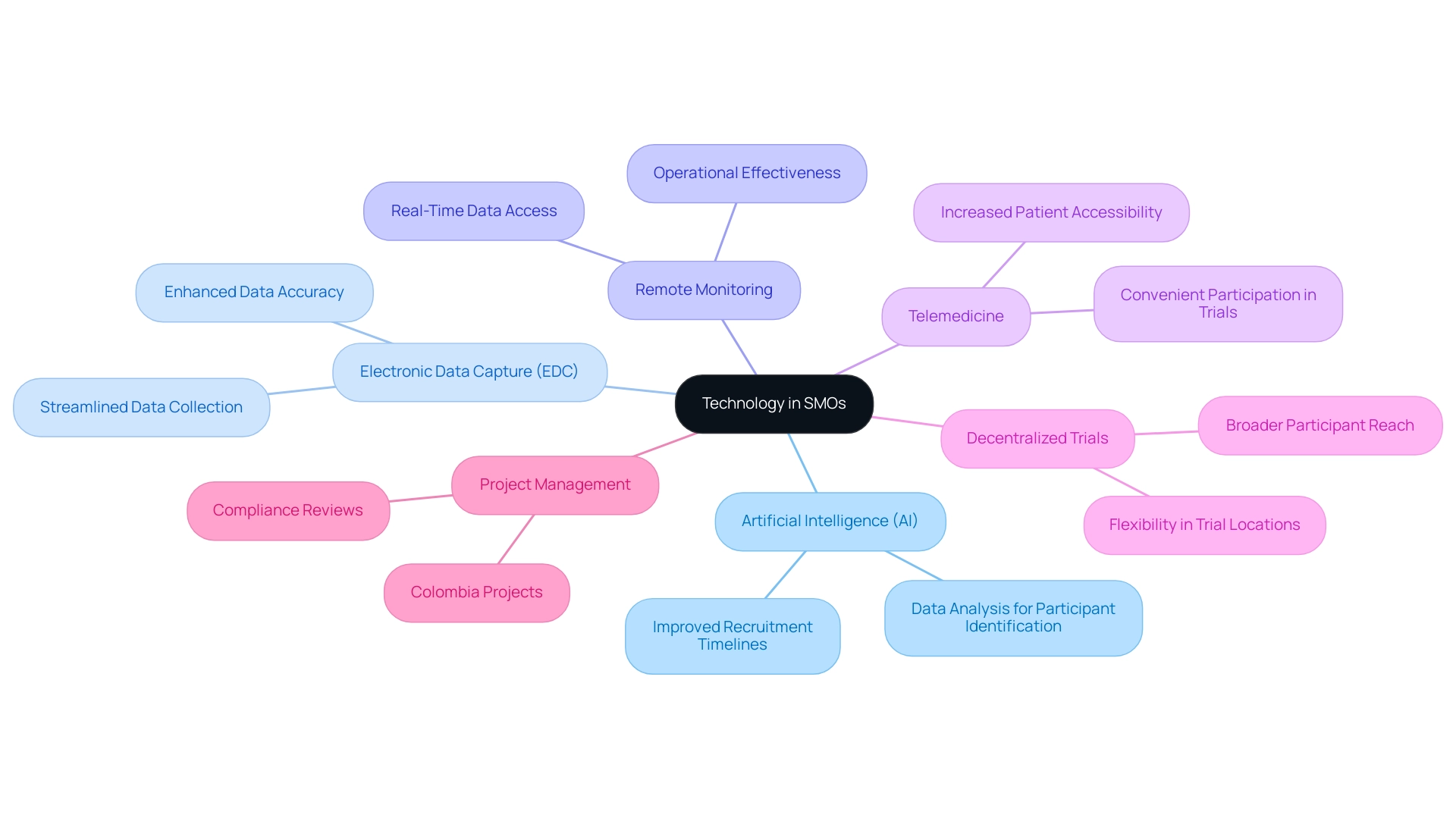
Furthermore, clinical site management organizations excel in navigating regulatory requirements, ensuring that researchers adhere to the necessary guidelines and thereby minimizing the risk of study delays. They simplify communication among various groups involved in research studies, enabling scientists to focus on the scientific aspects of their work. The combination of technologies like electronic data capture (EDC), wearable devices, telemedicine, and artificial intelligence (AI) is essential for contemporary research.
These technologies not only lessen manual efforts and decrease data mistakes but also accelerate timelines, ultimately resulting in more effective studies. As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, articulates, 'I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.'
Her viewpoint highlights the changing environment of medical studies, where clinical site management organizations improve effectiveness and promote a more patient-friendly method to studies, especially through cooperation and technological progress.
Future Trends: The Evolving Role of Technology in SMOs
The incorporation of advanced technology is fundamentally transforming the landscape of site management organizations (SMOs) and the wider field of medical research. Innovations such as electronic data capture (EDC), remote monitoring, and artificial intelligence (AI) significantly enhance the efficiency of clinical studies, particularly in the context of Latin America's unique challenges. For instance, AI's ability to analyze extensive datasets allows for more effective identification of potential participants, optimizing patient recruitment—a crucial factor given the regulatory hurdles and language barriers in the region.
Studies show that AI-driven strategies can improve recruitment timelines by up to 30%, addressing the urgent need for a solution-driven approach in Medtech. Furthermore, specific service capabilities such as initial set-up, project management, and compliance reviews are essential in navigating these challenges effectively. For instance, successful experiments in Colombia have shown the significance of thorough project management and monitoring in ensuring adherence to local regulations.
Furthermore, similar to how self-tinting glass decreases HVAC demands by responding to sunlight, technology in medical studies optimizes resource distribution and operational effectiveness. The rise of telemedicine and decentralized trial models revolutionizes patient accessibility, making participation more convenient and inclusive than ever before. As we approach 2024, it is clear that strategic management organizations will be crucial in steering these technological advancements, ensuring that medical studies not only keep up with innovation but also stay effective and efficient.
As Chris Clausing, a noted authority in construction education, articulates, 'As we move into 2025, these trends will continue to shape the industry, driving innovation and efficiency.' This sentiment resonates in clinical research as well, where the role of SMOs will be critical in adapting to and implementing these transformative changes, ultimately fostering international collaboration and driving economic growth in local communities.
Conclusion
The role of Site Management Organizations (SMOs) in clinical research is increasingly vital as they navigate the complexities of clinical trials, ensuring that they are conducted efficiently and effectively. Through collaborations like that of bioaccess™ and Caribbean Health Group, SMOs are positioning regions such as Barranquilla as prominent clinical trial destinations, exemplifying their commitment to enhancing patient recruitment and regulatory compliance. By providing a comprehensive suite of services, including:
- Feasibility studies
- Project management
- Real-time reporting
SMOs significantly contribute to the integrity and success of clinical trials.
As the landscape of clinical research continues to evolve with advancements in technology, the adaptability of SMOs becomes even more crucial. The integration of artificial intelligence, telemedicine, and electronic data capture not only streamlines operations but also improves patient engagement and recruitment strategies. With innovative approaches, SMOs are not only overcoming traditional challenges but also setting new standards for trial management, ensuring compliance with regulatory requirements while fostering economic growth within local communities.
Ultimately, the future of clinical research rests on the shoulders of SMOs, whose strategic partnerships and technological advancements will drive the industry forward. By embracing these changes, SMOs can enhance the effectiveness of clinical trials and contribute to groundbreaking medical advancements, solidifying their role as indispensable allies in the pursuit of better healthcare outcomes.
Frequently Asked Questions
What is the role of Clinical Site Management Organizations (CSOs)?
CSOs coordinate research studies across multiple locations, facilitating efficient conduct while ensuring adherence to regulatory standards, which enhances the integrity and quality of research outcomes.
What services do CSOs provide?
CSOs offer a comprehensive range of management services, including feasibility studies, site selection, study setup, regulatory compliance, project management, and reporting on study status and adverse events.
What was the partnership between bioaccess™ and Caribbean Health Group about?
The partnership aims to establish Barranquilla as a top destination for research in Latin America, supported by Colombia's Minister of Health to improve clinical study projects in the region.
What advancements have been made in clinical site management services?
Recent advancements include the launch of myTrialsConnect by Elligo Health Research and Avallano, an AI-driven platform that enhances participant enrollment through automated medical record evaluations and chatbot surveys.
What are the different models of CSOs?
CSOs function under various models, including independent entities, academic medical center-affiliated organizations, and integrated groups within larger healthcare systems, each offering distinct benefits for specific study objectives.
How do independent CSOs differ from academic-affiliated organizations?
Independent CSOs are known for their flexibility and ability to tailor services, while academic-affiliated organizations provide access to specialized patient populations and higher-quality data crucial for advancing clinical research.
What types of studies does bioaccess® manage?
Bioaccess® manages various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Why is understanding the different CSO models important for researchers?
Comprehending these models helps researchers align their studies with the most suitable CSO, ensuring the best possible outcomes for their projects.
What impact do Medtech studies have beyond investigation?
Medtech studies promote job creation, economic growth, and healthcare enhancement in local economies.




