Overview
The article focuses on understanding the various factors influencing clinical trial costs in Latin America, highlighting the region's growing significance in global research due to its diverse patient populations and regulatory environments. It details how elements such as regulatory requirements, site selection, operational expenses, and technological advancements collectively shape the financial landscape of clinical trials, ultimately underscoring the importance of strategic planning and local engagement to optimize costs and enhance research outcomes.
Introduction
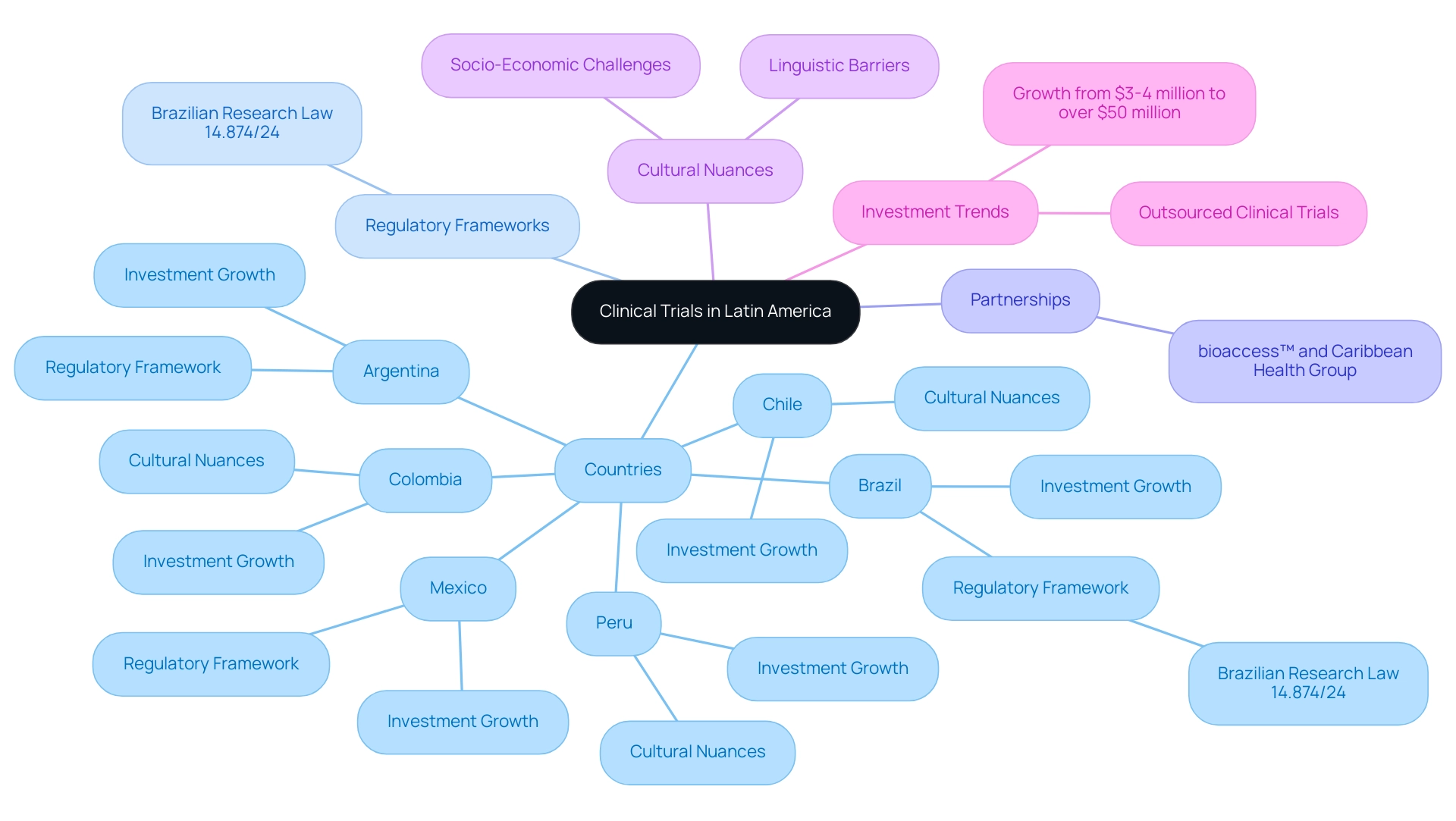
Latin America is emerging as a crucial hub for clinical trials, characterized by its diverse patient populations and cost-effective research environments. Countries like Brazil, Mexico, and Argentina are leading the charge with strong regulatory frameworks and skilled investigators, making the region increasingly attractive to global sponsors. Recent initiatives, such as Brazil's Clinical Research Law 14.874/24, aim to streamline the trial process, while collaborations like that of bioaccess™ and the Caribbean Health Group are positioning cities like Barranquilla as prime locations for clinical research.
However, the success of these trials hinges on a nuanced understanding of local cultures, healthcare systems, and the unique challenges of patient recruitment. As investments in clinical trials soar, with dramatic increases in funding and interest, the landscape is ripe for exploration—offering both opportunities and obstacles that must be navigated with care.
The Landscape of Clinical Trials in Latin America
The region has firmly established itself as a crucial participant in the worldwide research field, primarily because of its diverse patient groups and the clinical trial costs in Latin America. Countries such as Brazil, Mexico, and Argentina stand out for their robust regulatory frameworks and the presence of highly qualified investigators, which significantly influence clinical trial costs in Latin America. Significantly, Brazil is enhancing its research environment with the enforcement of the Brazilian Research Law 14.874/24, which seeks to simplify assessments and reduce regulatory timelines.
Furthermore, the region showcases an appealing combination of quality and affordability, which has resulted in heightened interest from global sponsors in clinical trial costs in Latin America. A recent partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as the premier location for medical studies in Latin America, supported by Colombia's Minister of Health, Juan Pablo Uribe, who publicly backed the initiative during a meeting on March 29, 2019, at PROCOLOMBIA's office in Miami, FL. This initiative is set to attract more clinical research projects to the region, which could help reduce clinical trial costs in Latin America and enhance its reputation further.
Successful testing execution necessitates a deep understanding of local contexts, including cultural nuances and the intricacies of healthcare systems. This awareness is essential for navigating linguistic barriers, such as language differences, cultural challenges related to patient involvement, and socio-economic obstacles that impact patient access to studies, particularly in countries like Peru, Colombia, and Chile. These nations are also witnessing substantial growth in research investments, which have surged dramatically from $3-4 million to over $50 million annually over the last decade, and this trend emphasizes the importance of clinical trial costs in Latin America, contrasting with the decrease of 4% and 12% in registrations in upper middle income and high income countries, respectively, between 2020 and 2023.
As emphasized by Mariana Bei, Sr. Director of Clinical Operations and Brazil GMBA at Parexel, 'Having a local presence and leadership that is based in the LATAM region strengthens the relationships that Parexel builds with local talent, regulatory authorities based in the area, local associations and the global network of sites overall.' This comprehensive method boosts the likelihood of favorable results in research studies across the southern continent, illustrated by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, who shared positive experiences with bioaccess® during its initial human study in Colombia, highlighting a notable rise in recruitment efficiency and participant retention.
Key Factors Influencing Clinical Trial Costs
Numerous essential elements influence study expenses in Latin America, each playing a role in the overall financial scenery:
-
Regulatory Requirements: Compliance with local regulations can significantly increase expenses, especially when studies are subjected to extensive ethical reviews. The recent approval of Law 14.874/24 in Brazil is set to positively influence these expenses by simplifying the evaluation process for studies, minimizing bureaucratic challenges, and improving predictability in study timelines. This not only leads to cost efficiencies but also ensures adherence to the regulations set forth by entities like INVIMA, the Colombia National Food and Drug Surveillance Institute, which functions as a Level 4 health authority by PAHO/WHO.
-
Site Selection: The choice of research locations plays a pivotal role in cost management. Selecting sites with established infrastructure can minimize expenses, while those needing extensive setup and training can escalate costs considerably. bioaccess® offers expertise in feasibility studies and site selection, ensuring optimal choices that align with project needs.
-
Operational Expenses: Variability in operational costs is considerable across different regions and study complexities. Elements like personnel, equipment requirements, and material procurement can vary significantly, affecting the overall budget for research studies. Effective project management services provided by bioaccess® can help mitigate these expenses, ensuring that all operational aspects are efficiently managed from start to finish.
-
Recruitment Strategies: Effective participant recruitment strategies are essential for controlling costs. Successful recruitment can mitigate expenses, whereas ineffective strategies can extend timelines and lead to increased spending. The strong patient-physician relationships and urban concentrations in Latin America contribute to lower dropout rates, enhancing the feasibility of recruitment efforts. For instance, the high incidence of gallbladder cancer in Peru underscores the importance of targeted recruitment in regions with pressing health needs.
-
Study Project Management and Reporting: Comprehensive project management is essential for supervising all stages of a research study. bioaccess® ensures that all aspects, from feasibility to reporting, are meticulously handled, providing detailed reports on study status, inventory, and adverse events. This systematic approach not only enhances compliance but also supports timely decision-making.
-
Import Permits and Nationalization: Navigating the regulatory landscape for import permits and nationalization of investigational devices is essential for successful execution of the study. bioaccess® aids clients in comprehending and acquiring the required permits, making certain that all investigational products adhere to local regulations.
Understanding the clinical trial costs in Latin America, estimated at approximately $1.5 million per project in 2024, is crucial for strategic planning. This comprehensive perspective on the elements affecting research costs in the region uncovers a complex interaction of regulatory frameworks, site logistics, operational requirements, and recruitment efficiencies, ultimately emphasizing the influence of Medtech studies on local economies, including job creation, economic growth, and healthcare enhancement.
Navigating Recruitment Challenges in Clinical Trials
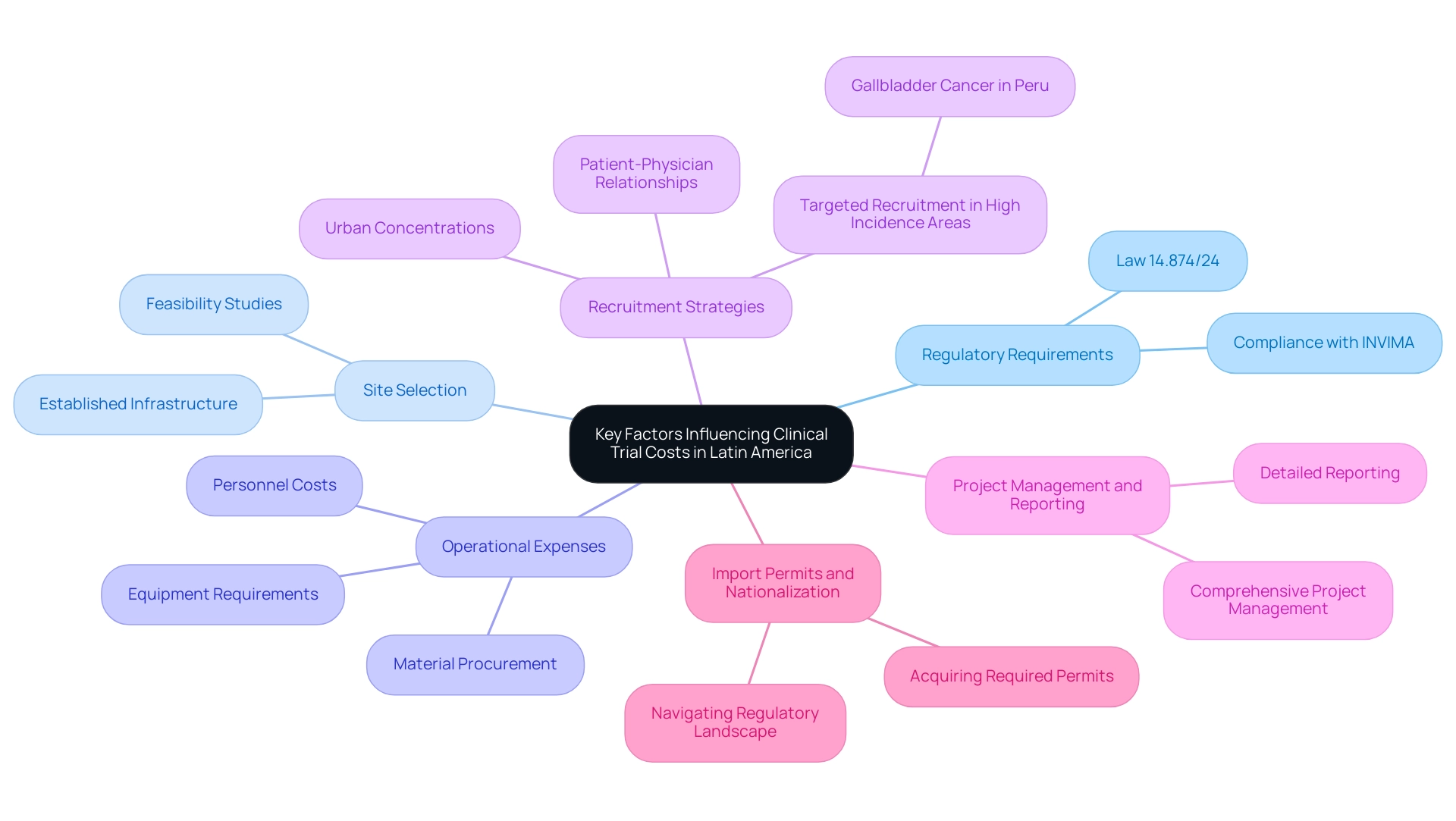
Recruitment difficulties in Latin America stem from various interconnected elements that must be effectively tackled to improve involvement in research studies. Cultural obstacles frequently appear in misconceptions regarding research protocols or a widespread distrust of medical studies, greatly obstructing recruitment efforts. Notably, recent studies indicate that among 86 investigations, a concerning 21% did not report sample sizes by racial and ethnic groups, while 64% failed to analyze data based on these demographics, complicating effective outreach.
Furthermore, health literacy plays an essential role; a lack of awareness about the advantages and procedures of medical studies can restrict participation rates among potential candidates.
Geographical disparities pose considerable barriers, with many patients encountering difficulties in accessing healthcare services. This issue is compounded by limited resources, as highlighted by recent reports indicating that a lack of government research grants poses a barrier to investigator-initiated research in the region, directly impacting recruitment efforts.
To navigate these complexities, researchers should prioritize engaging with local communities to build trust and establish rapport. Partnerships such as the one between bioaccess™ and the Caribbean Health Group are crucial in establishing Barranquilla as a prominent location for research studies, backed by Colombia's Minister of Health.
Such partnerships can enhance outreach efforts, ensuring that information reaches potential participants effectively. Furthermore, using culturally appropriate materials for participant education can clarify the trial process and promote informed decision-making. Insights from GlobalCare Clinical Trials highlight that the region enjoys excellent patient retention, with strong bonds between physicians and patients resulting in high rates of compliance and study retention.
Utilizing these relationships is crucial in overcoming recruitment obstacles and promoting successful research initiatives. Moreover, the case study entitled 'The Opportunities For Conducting Vaccine Clinical Research In South America' highlights how clinical trial costs in Latin America can be significantly reduced, offering considerable prospects for recruitment when effectively tackling these obstacles. By concentrating on community involvement, addressing health comprehension, and employing strategic alliances, the recruitment environment for research studies in Latin America can be significantly enhanced.
Leveraging Technology to Reduce Trial Expenses
Technological advancements are essential in reducing research expenses, with several strategies arising as especially effective:
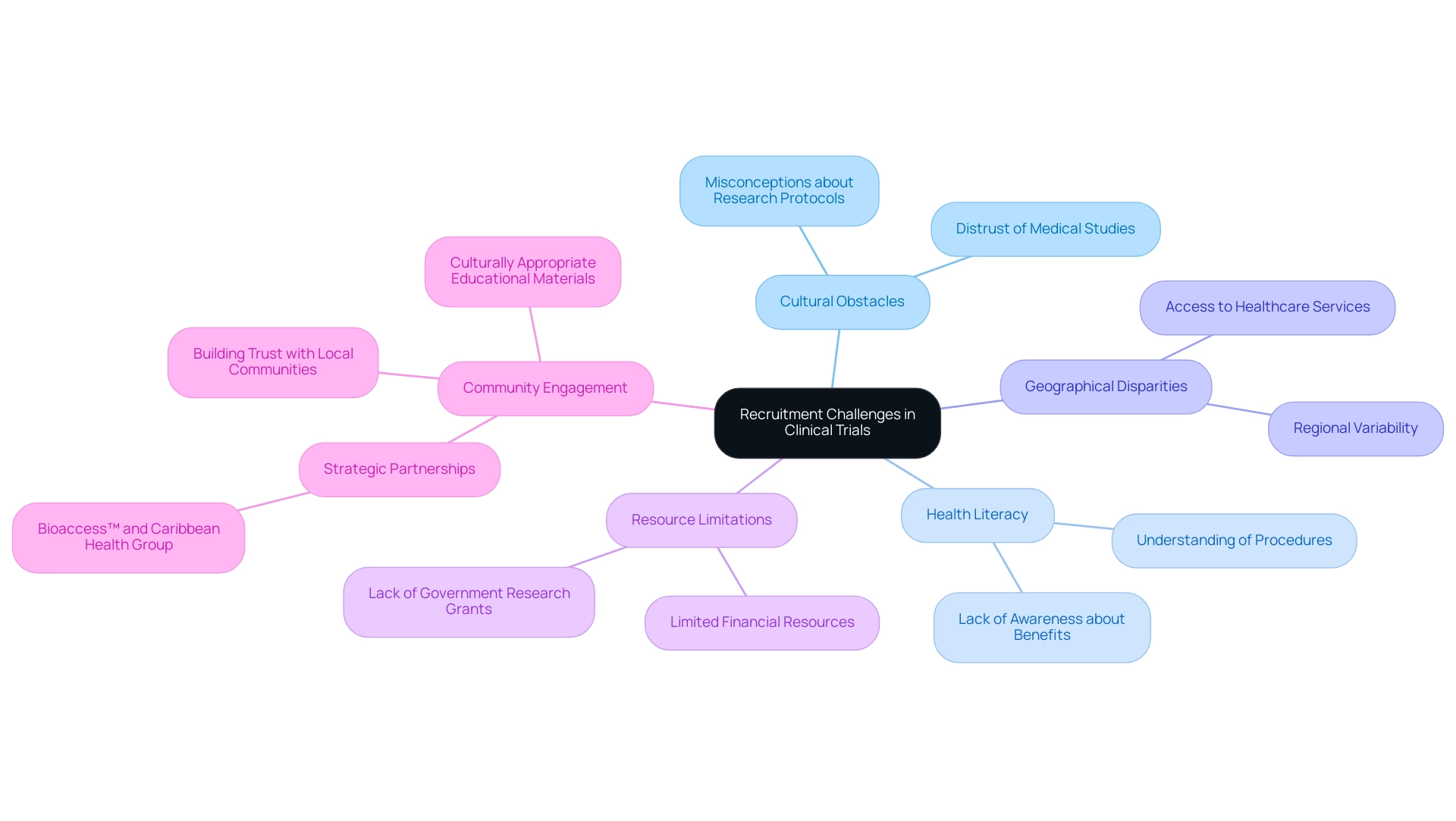
- Electronic Data Capture (EDC): The adoption of EDC systems, which currently stands at 27.5% in randomized studies registered in India, significantly reduces the reliance on paper-based processes. This transition not only minimizes data entry errors but also enhances data management efficiency. By leveraging EDC, researchers can streamline their workflows and improve the accuracy of their data collection. Significantly, the EDC system presently hosts 64 projects and includes 102 users, with 43 active projects, 19 in production status, and 24 in development status, illustrating its expanding presence in research.
- Telemedicine: The use of telemedicine facilitates remote consultations, effectively lowering travel costs for participants while improving accessibility for individuals in rural areas. This approach broadens the participant pool, often making clinical trial costs in Latin America more inclusive and cost-effective.
- Patient Recruitment Platforms: Digital platforms for patient recruitment expedite the process of finding and engaging suitable participants. By utilizing technology, researchers can reach a wider audience and enhance the efficiency of their recruitment efforts.
- Project Management Software: Tools that support collaboration and monitoring are essential for streamlining project timelines. These technologies ensure that resources are allocated effectively, thereby reducing unnecessary expenditures on clinical trial costs in Latin America.
Alongside these technological innovations, our extensive study management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup involving ethics committee and health ministry approvals
- Import permits
- Thorough project management and reporting on study status and adverse occurrences
A notable example of EDC's impact is illustrated by the integration capabilities of REDCap, which offers an API and Data Transfer Service (DTS) for seamless data management. This functionality enables researchers to consolidate and manage data from various external sources, ultimately enhancing the robustness of their research projects.
The integration of external data sources has been shown to enhance the overall quality and reliability of results. Furthermore, the role of the 'honest broker' in collecting and providing Protected Health Information (PHI) data to research investigators is critical, as it ensures data privacy and ethical considerations are upheld during the data collection process. As the field continues to develop, adopting these technological solutions alongside our extensive services will be crucial for optimizing clinical trial costs in Latin America and enhancing overall research effectiveness.
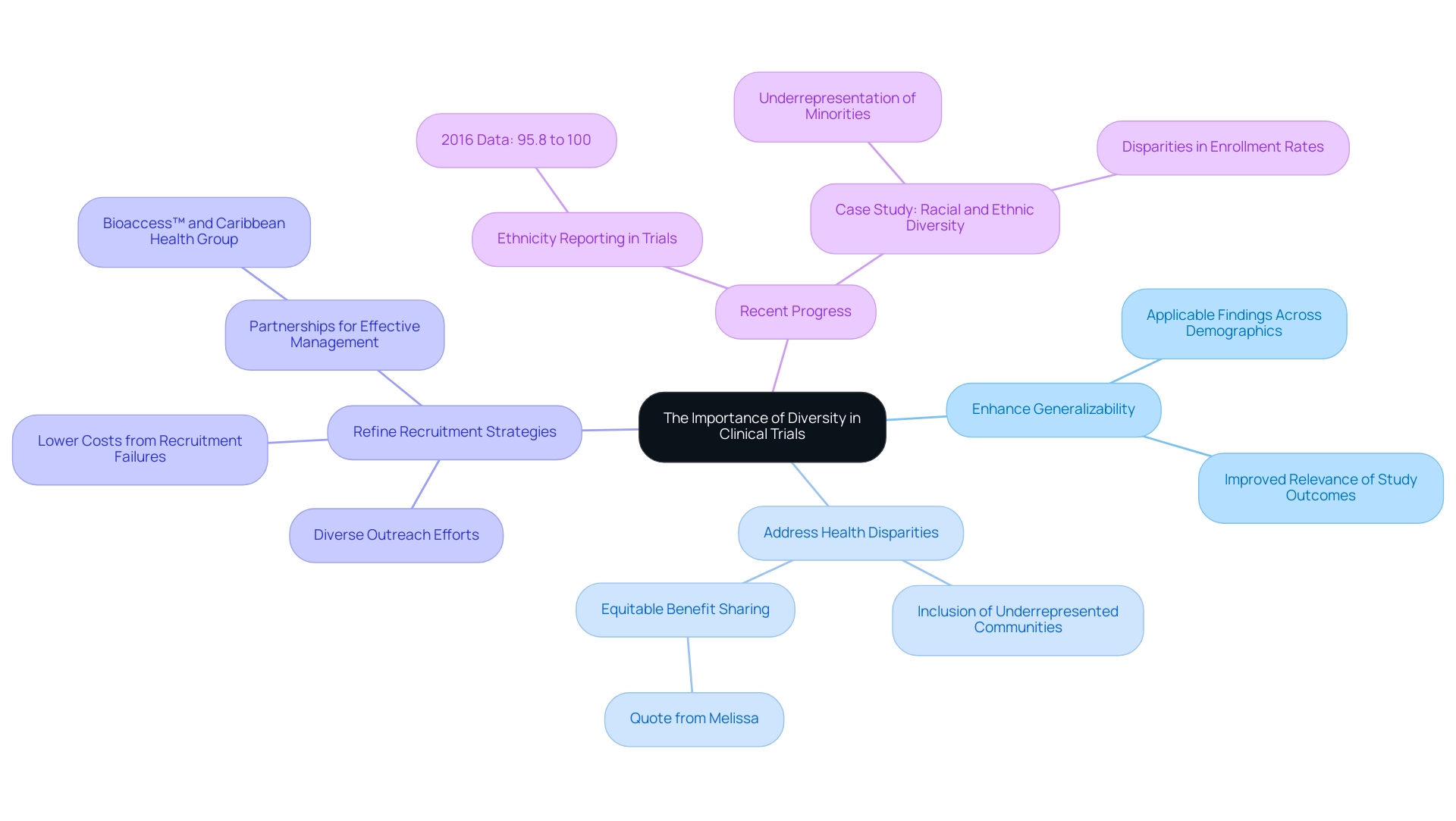
The Importance of Diversity in Clinical Trials
Ensuring variety in participant demographics is crucial for generating research findings that represent the wider population, a requirement that is especially relevant in the region, where ethnic and cultural diversity is significant. The demographic distribution of study participants often does not reflect the population affected by the conditions being examined, underscoring the critical need for inclusive research practices. By actively pursuing a diverse participant base, researchers can achieve several important objectives:
-
Enhance Generalizability: Diverse participants allow for findings that are more applicable across different demographic groups, thereby improving the relevance of study outcomes.
-
Address Health Disparities: By including underrepresented communities, researchers can help identify and address significant health disparities.
This is a sentiment echoed by Melissa, who states,
As we strive for equitable benefit sharing with the communities we serve, we can actively address the disparities in access to healthcare.
-
Refine Recruitment Strategies: Implementing diverse outreach efforts not only attracts a wider array of participants but also helps lower costs associated with recruitment failures that often arise from narrow targeting.
Recent partnerships, like the one between bioaccess™ and Caribbean Health Group, promote the initiative to make Barranquilla a top location for medical studies, which will ultimately impact clinical trial costs in Latin America. This partnership has successfully led to a significant reduction in recruitment times and enhanced retention rates, demonstrating a model for effective clinical research management services that address clinical trial costs in Latin America, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. Additionally, the support from Colombia's Minister of Health underscores the importance of these initiatives in fostering an environment conducive to diverse and inclusive research.
Despite historical underrepresentation, recent data indicates that from 2016 onwards, 95.8% to 100% of participants in Phase 3 trials had their ethnicity reported, showcasing progress in acknowledging the importance of diversity. However, ongoing initiatives are essential to ensure that recruitment practices adapt to represent the diverse demographic fabric of the region. A case study titled 'Racial and Ethnic Diversity in Clinical Trials' highlights that racial and ethnic minority groups are often underrepresented, with significant disparities in enrollment rates compared to white participants.
As the industry progresses, innovative strategies are essential to engage diverse populations effectively, ensuring that research benefits all segments of society.
Understanding the Regulatory Landscape for Clinical Trials
Navigating the regulatory environment for research studies in Latin America presents distinct challenges, particularly concerning the clinical trial costs in Latin America, as guidelines vary significantly from nation to nation. Grasping this intricate network of regulations is crucial for successful market entry and can generate various opportunities for organizations like bioaccess®, a prominent Contract Research Organization (CRO) that focuses on supporting medical device research studies in the region. Key considerations include:
-
Ethics Committees: Obtaining consent from local ethics boards is a vital step in the research process. However, delays can occur if these processes are not managed efficiently. The average time for ethics committee approval in Latin America can vary widely, often impacting clinical trial costs in Latin America, taking several weeks to months. Therefore, understanding local timelines and expectations is vital for researchers aiming to navigate this landscape effectively.
-
Research Study Registries: Numerous nations mandate the registration of research studies, which can increase administrative expenses. Effectively navigating these requirements is crucial for maintaining compliance and ensuring successful study initiation, particularly in the dynamic Medtech environment.
-
Reporting Obligations: Researchers must stay informed about ongoing reporting requirements to avoid potential fines and ensure adherence to regulations. The recent endorsement of Law 14.874/24 in Brazil, which seeks to simplify the evaluation process for research studies, illustrates the significance of staying updated with changing regulations. This law is anticipated to greatly improve the effectiveness of medical studies, positioning Brazil as a more appealing location for medtech research and benefiting the pharmaceutical sector by lessening bureaucratic hurdles.
-
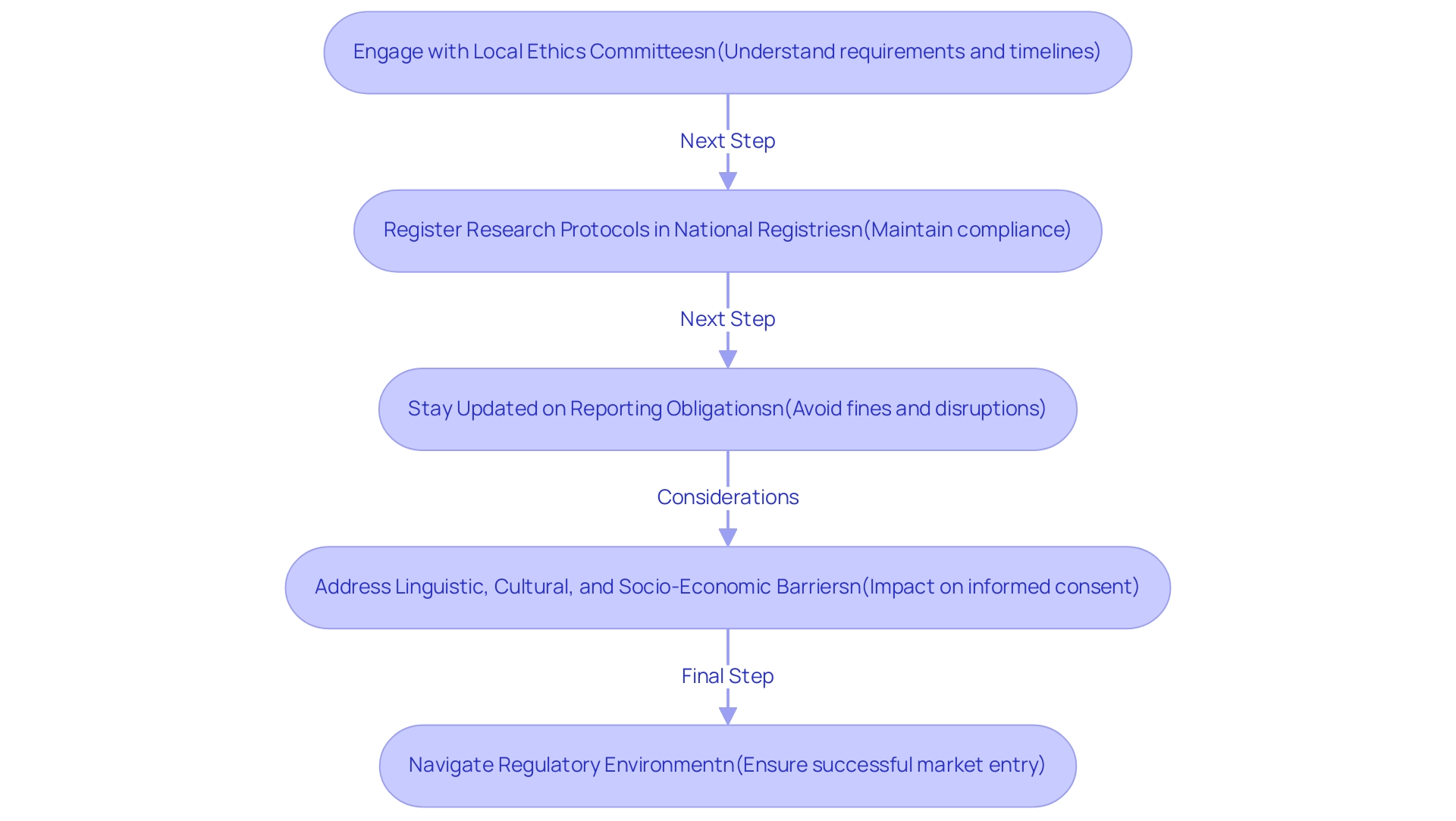
Practical Steps for Success: To effectively navigate these challenges, bioaccess® recommends the following:
- Engage early with local ethics committees to understand their specific requirements and timelines.
- Ensure that all research protocols are registered in the appropriate national registries to maintain compliance.
- Stay informed about changes in local regulations and reporting obligations to avoid any disruptions during the process.
Moreover, as outsourced medical studies grow in Peru, Colombia, and Chile, sponsors encounter obstacles related to clinical trial costs in Latin America, including linguistic, cultural, and socio-economic barriers impacting informed consent. Addressing these obstacles is vital for conducting successful medical studies in these countries.
As Julio G. Martinez-Clark, CEO of bioaccess®, noted, "Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy." This emphasizes the increasing acknowledgment of the significance of regulatory adherence and the strategic benefits it provides in the competitive environment of medical research in the southern region, reinforcing bioaccess®'s dedication to being your reliable ally in expediting medical device evaluations throughout the area. If you have any questions or issues regarding the research process, feel free to contact our Grievance Officer at info@bioaccessla.com.
Future Trends in Clinical Trials and Their Cost Implications
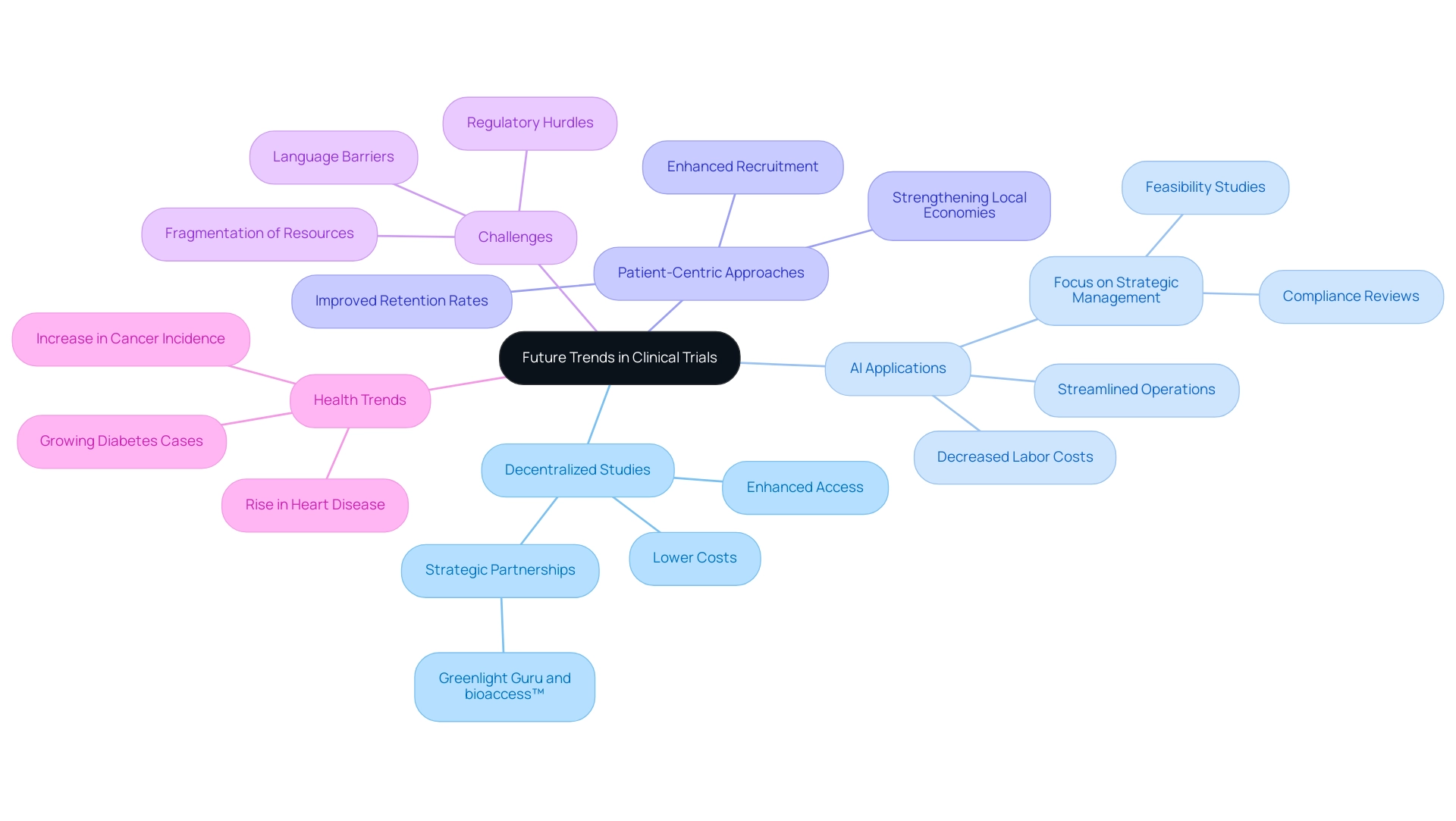
The future of clinical trials in Latin America is poised for significant transformation, driven by several key trends:
- Increased Use of Decentralized Studies: Embracing decentralized study models promises to substantially lower site costs while enhancing participant access. This shift not only reduces expenses but also aligns with patient needs, making participation in clinical trials more feasible for a broader demographic, particularly considering the clinical trial costs in Latin America. Moreover, strategic partnerships, such as the collaboration between Greenlight Guru and bioaccess™, are essential for accelerating Medtech innovations and ensuring seamless execution of these studies.
- Artificial Intelligence (AI) Applications: The integration of AI in data management and analytics holds the potential to streamline operational processes, thereby decreasing labor costs. By automating routine tasks, AI can free up resources, allowing clinical teams to focus on strategic aspects of management, including feasibility studies and compliance reviews, which are critical for understanding clinical trial costs in Latin America and navigating the regulatory landscape.
- Patient-Centric Approaches: A greater emphasis on patient-centric methodologies can significantly enhance recruitment and retention rates. By prioritizing the needs and experiences of participants, studies can become more efficient, ultimately leading to faster and more reliable outcomes. This patient-first focus not only enhances study success but also helps strengthen local economies through job creation and healthcare advancements.
However, Medtech firms in the region encounter significant challenges, including regulatory hurdles, language barriers, and fragmentation of resources, all of which affect clinical trial costs in Latin America. Tackling these socio-economic obstacles is vital for boosting patient safety and enhancing the overall standard of research studies and healthcare provision in the area, especially considering the clinical trial costs in Latin America, as mentioned by Gotuzzo in 'Research in South Region: Constraints and Opportunities.' Furthermore, with the Asia Pacific market projected to reach USD 12,687.3 million by 2030, Latin America must leverage these trends to remain competitive.
The area has also observed a significant rise in the occurrence of cancer, heart disease, and diabetes because of lifestyle modifications, highlighting the necessity for research approaches that tackle these particular health issues.
As these trends unfold, it is crucial for researchers to recalibrate their strategies, leveraging innovations and comprehensive clinical trial management services to optimize clinical trial execution and outcomes, especially considering the impact of clinical trial costs in Latin America while bridging the gap between innovation and execution.
Conclusion
Latin America is rapidly establishing itself as a key player in clinical trials, thanks to its diverse patient populations and robust regulatory frameworks. Countries like Brazil, Mexico, and Argentina are at the forefront, with Brazil's Clinical Research Law 14.874/24 enhancing efficiency and attracting global sponsors.
Successful trial execution relies on overcoming recruitment challenges and understanding local cultures. By engaging communities and utilizing technology such as electronic data capture and telemedicine, researchers can improve participation rates and reduce costs.
Diversity in clinical trials is essential for generating relevant findings. Implementing inclusive recruitment strategies allows researchers to address health disparities effectively and improve the applicability of results.
As trends like decentralized trials and artificial intelligence emerge, Latin American organizations must adapt to remain competitive. These innovations promise to streamline processes and enhance healthcare outcomes.
In conclusion, with strategic planning and a focus on diversity, Latin America is well-positioned to lead in clinical research, paving the way for significant advancements in healthcare on a global scale.
Frequently Asked Questions
Why is Latin America considered a crucial participant in the global research field?
Latin America is important in global research due to its diverse patient groups and lower clinical trial costs, with countries like Brazil, Mexico, and Argentina having strong regulatory frameworks and qualified investigators.
What recent changes are being made to improve the research environment in Brazil?
Brazil is enhancing its research environment through the enforcement of the Brazilian Research Law 14.874/24, which aims to simplify assessments and reduce regulatory timelines.
How does the combination of quality and affordability in Latin America affect clinical trials?
The appealing combination of quality and affordability has led to increased interest from global sponsors in conducting clinical trials in Latin America, thereby influencing clinical trial costs positively.
What initiative is being taken to establish Barranquilla as a key location for medical studies in Latin America?
A partnership between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, aims to make Barranquilla the premier location for medical studies in Latin America, attracting more clinical research projects.
What factors are essential for successful clinical trial execution in Latin America?
Successful execution requires a deep understanding of local contexts, including cultural nuances, healthcare systems, and navigating linguistic barriers, particularly in countries like Peru, Colombia, and Chile.
How have research investments changed in Latin America over the last decade?
Research investments in Latin America have dramatically increased from $3-4 million to over $50 million annually, contrasting with a decrease in clinical trial registrations in higher-income countries.
What role does local presence play in the success of clinical trials in Latin America?
Having a local presence and leadership strengthens relationships with local talent, regulatory authorities, and associations, which enhances the likelihood of favorable research outcomes.
What are the key elements influencing clinical trial costs in Latin America?
Key elements include regulatory requirements, site selection, operational expenses, recruitment strategies, project management, and navigating import permits and nationalization of investigational devices.
How does the Brazilian Research Law 14.874/24 impact study expenses?
The law simplifies the evaluation process for studies, reducing bureaucratic challenges and improving predictability in study timelines, which can lead to cost efficiencies.
What is the estimated cost of clinical trials in Latin America for 2024?
Clinical trial costs in Latin America are estimated to be approximately $1.5 million per project in 2024.

