Introduction
Clinical trials play a crucial role in advancing medical treatments and improving patient outcomes. However, there are various aspects that must be carefully considered and addressed to ensure the success and integrity of these trials.
This article will delve into four key challenges faced by clinical trial companies: study design, recruitment and enrollment, data collection and analysis, and regulatory compliance and ethical considerations. By understanding and navigating these challenges effectively, researchers and organizations can optimize the process and pave the way for impactful medical advancements.
The Importance of Study Design
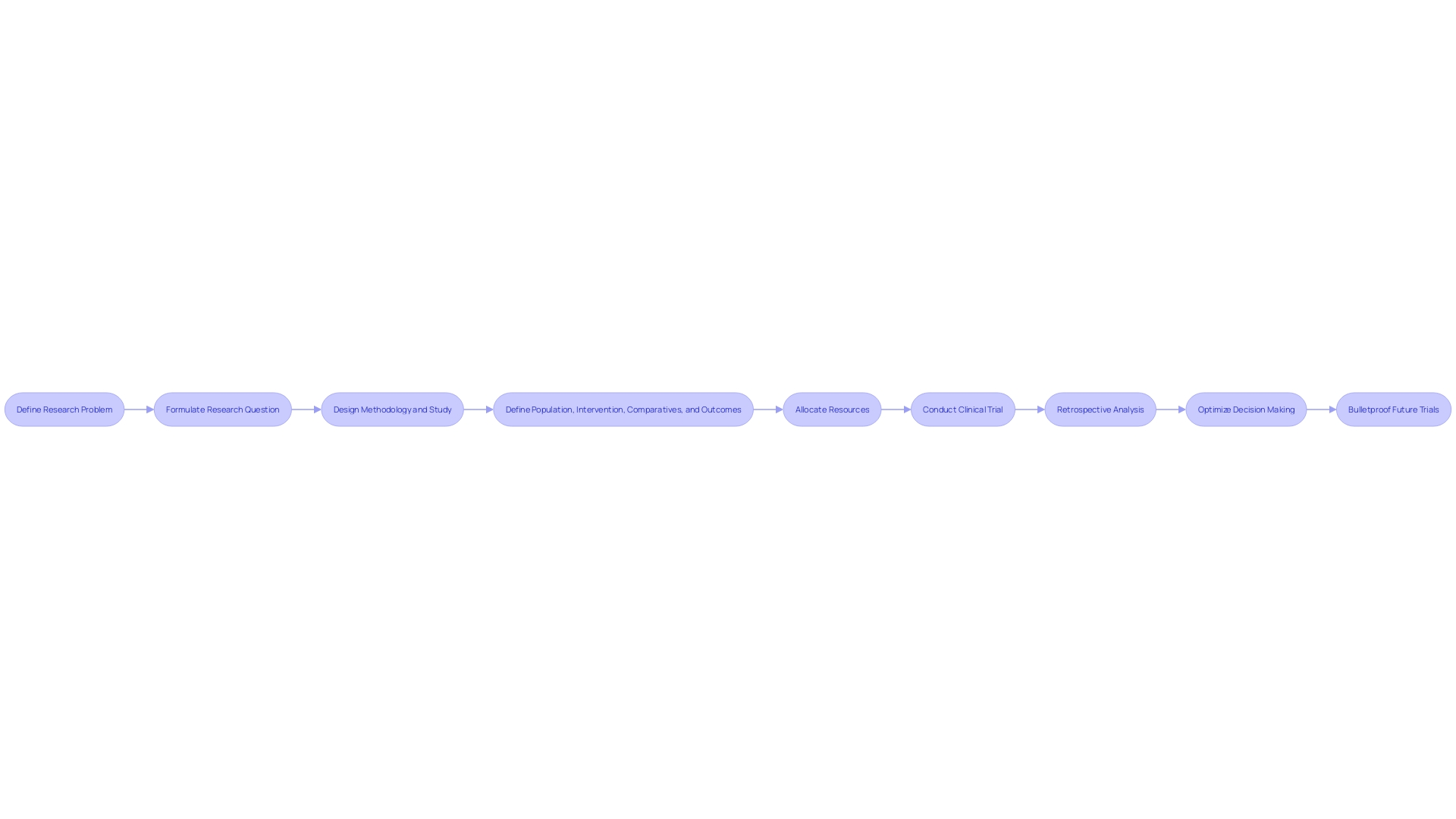
Expert problem solvers in the realm of clinical trial management emphasize the pivotal role of first delineating the research problem rather than jumping straight to the methods or design. The essence of this approach is aptly captured in the formulation of the research question, which is instrumental in shaping the entire study. A research question of excellent caliber not only informs the methodology but also guides the strategic design of the study.
To construct such a research question, a dual focus is paramount: it must be both pointedly focused and unequivocally answerable. This necessitates a sharp definition of the population, the intervention or exposure, the comparative measures, and the desired outcomes. In observational studies, an 'exposure' refers to a variable experienced by a subset of the population, such as smoking, whose correlation with lung cancer outcomes is of interest.
The research question is the compass by which the clinical trial's cost and design are ultimately directed; it ensures that resources are intelligently and efficiently allocated, forestalling unnecessary expenditure arising from a trial that is overextended in duration or scope. An insight from the industry highlights the importance of this rigorous approach: an adviser from Tree hill, reflecting on two decades of consulting experience, noted that retrospective analysis of Phase I, II, or III trials revealed that a significant majority of decisions could be optimized if more energy were devoted to 'bulletproofing' them at earlier stages. This analogy likens the clinical trial process to a chain, with the implication that strengthening each link, particularly through meticulous research questioning, is an essential strategy for operational and financial efficiency in clinical trials.
Recruitment and Enrollment Challenges
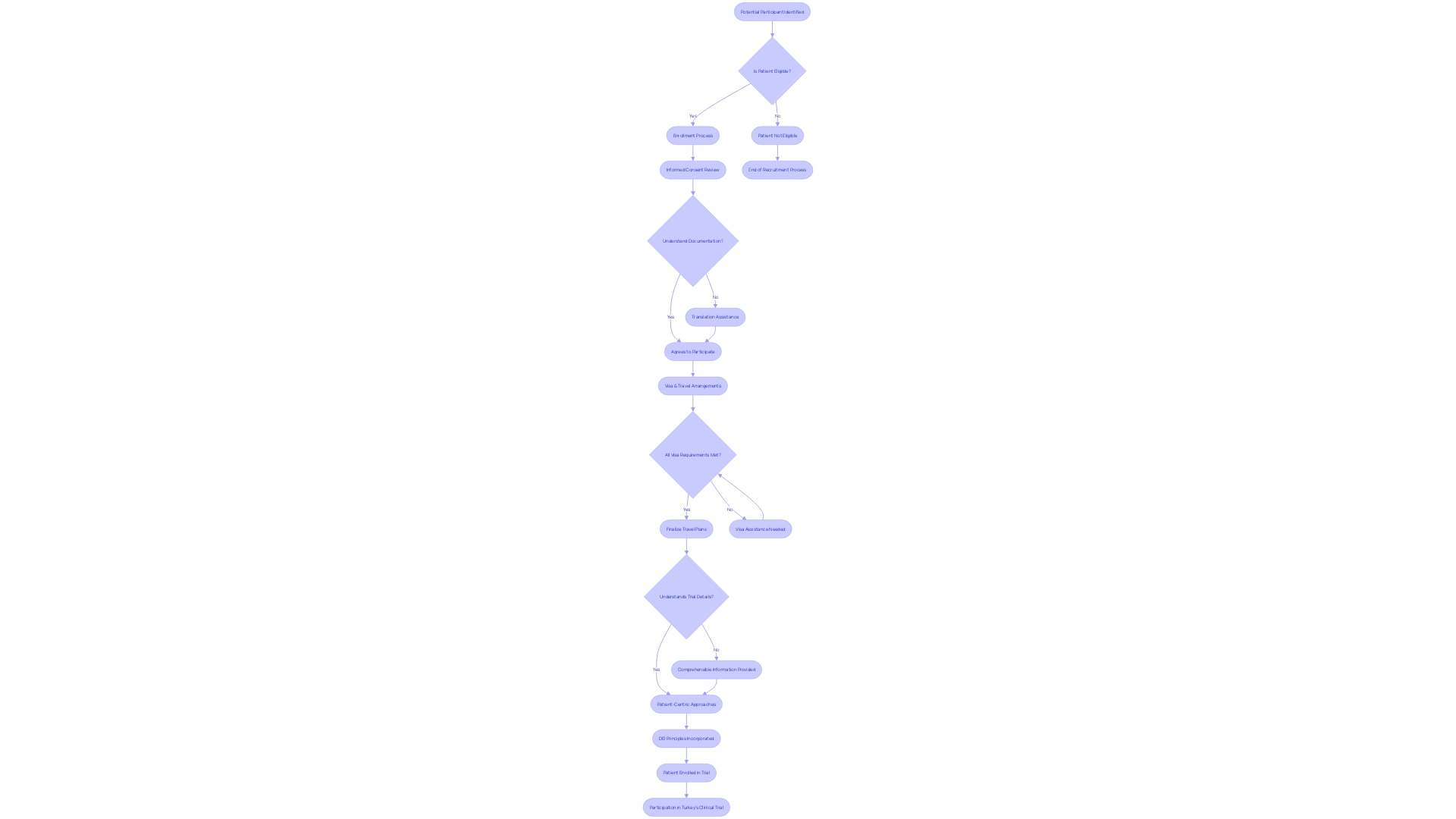
The logistics of patient recruitment and enrollment in clinical trials cannot be underestimated. Consider the poignant scenario of a patient residing in rural Pennsylvania, who suffers from an ultra-rare disease without any FDA-approved treatments available.
When presented with the chance to participate in a clinical trial based in Turkey, not only is the beacon of hope ignited, but also a cascade of logistical and communication challenges. Navigating visa processes, understanding foreign-language documentation, and coordinating international travel arrangements are just a few of the substantial hurdles they face.
Patient centricity is increasingly recognized as fundamental for the success of clinical trials, emphasized by insights from Daniel J Herron, Vice President at RWS. Herron illuminates the core of patient-centric approaches: actively involving patients in the trial's planning and ensuring they receive comprehensible information.
The significance of incorporating diverse perspectives through a commitment to diversity, equity, and inclusion (DEI) is imperative. Crucially, it acknowledges that diseases manifest and affect individuals differently due to varying lived experiences, conditions, and demographic factors such as race, ethnicity, age, sex, and sexual orientation. Clinical trial companies must, therefore, strategically prioritize patient-centricity. By embedding DEI principles and addressing the multifaceted challenges of patient recruitment, they not only streamline the patient experience but also work towards eliminating enrollment-related delays that inflate trial costs.
Data Collection and Analysis
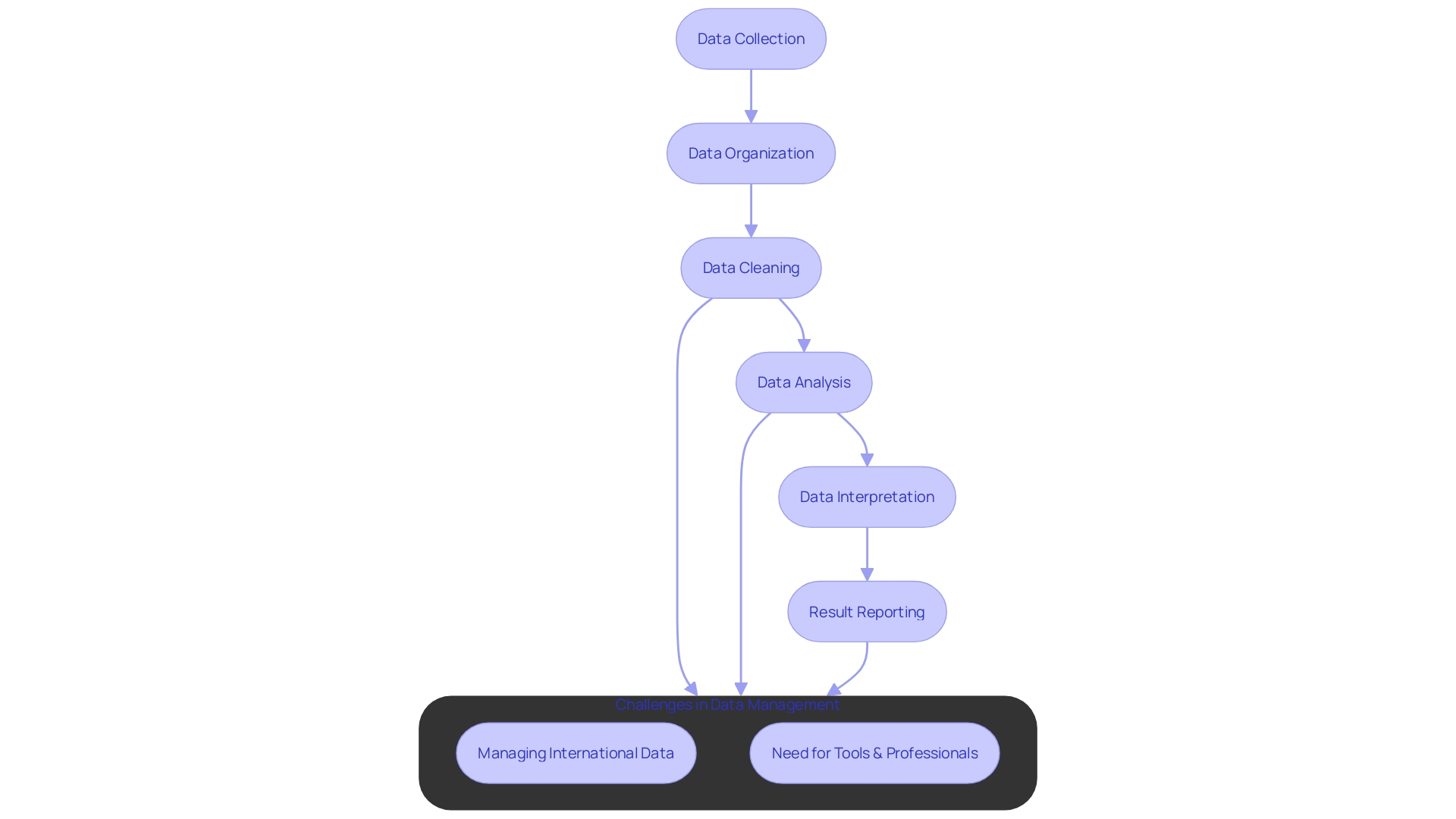
At the heart of every clinical trial is data, serving not just as a beacon of insight but as the foundation upon which the validity and efficacy of the trial rests. Implementing the best practices in data analysis upholds reproducibility, fortifies data integrity, and guarantees precise interpretation.
A systematic framework is paramount in curbing errors and ensuring data of the highest order. This framework entails meticulous structuring of data using matrices, where rows encapsulate singular observations—often a patient—and columns denote participant attributes.
Such an organization simplifies the intricate journey from data collection to analysis and result reporting. With a keen focus on maintaining the data's essence, clinical trial companies are tasked with adopting methodologies that serve as a bulwark against common pitfalls.
They need to deploy sophisticated tools and employ adept professionals to expeditiously manage data. Case in point, a patient from rural Pennsylvania with an ultra-rare disease may partake in a trial abroad, carrying hopes of a cure. Here, the complexity balloons not just in the medical arena but in the logistical domain too. Ensuring the seamless integration of data from international participants encapsulates the gravity of the challenge at hand. Efficient and accurate data management is not a luxury but a necessity, paving the way for trials that shape the future of medicine.
Regulatory Compliance and Ethical Considerations
Navigating the intricate landscape of regulatory compliance is a formidable challenge for clinical trial companies. These entities are critical in advancing medical treatments, but must meticulously adhere to strict regulations mandated by agencies like the U.S. Food and Drug Administration. This oversight ensures the safety, efficacy, and security of medical interventions.
For example, CMIC Group, Japan's pioneering Contract Research Organization, extends a comprehensive suite of services to cover the entire pharmaceutical value-chain, demonstrating the significant investment and oversight necessary to meet regulatory criteria. Furthermore, ethical considerations are paramount; compensating clinical trial participants justly acknowledges their integral role and personal costs incurred. This evolving ethical standpoint reflects a growing recognition of fair treatment as essential to the progress of clinical research.
It's a delicate balance to maintain participant safety while also protecting their rights, without which research progress and public health advancements could be compromised. Recent discussions highlight the duality of 'safe and effective'—terms that may serve as benchmarks for compliance but are multifaceted and context-dependent. As the regulatory environment becomes increasingly sophisticated, clinical trial companies must be adept, aligning operations with both substantial financial and ethical commitments to navigate the complexities of modern drug development and to achieve their ultimate objectives.
Conclusion
In conclusion, clinical trial companies face four key challenges: study design, recruitment and enrollment, data collection and analysis, and regulatory compliance and ethical considerations. These challenges require strategic navigation to optimize the process and pave the way for impactful medical advancements. Study design is crucial in shaping a study, with a well-defined research question guiding resource allocation and preventing unnecessary expenditure.
By strengthening trials at earlier stages, clinical trial companies can enhance decision-making processes and improve operational efficiency. Recruitment and enrollment pose logistical and communication hurdles, particularly for patients in different locations or with language barriers. Patient-centric approaches and a commitment to diversity, equity, and inclusion are essential.
Involving patients in the planning process and addressing enrollment delays streamline the patient experience and reduce trial costs. Data collection and analysis form the foundation of clinical trials, ensuring data integrity and precise interpretation. Implementing systematic frameworks, accurate data structuring, and sophisticated tools are key.
Seamless integration of data from international participants is crucial for shaping the future of medicine. Regulatory compliance and ethical considerations are paramount, ensuring the safety, efficacy, and security of medical interventions. Fair compensation for participants acknowledges their role and personal costs.
Balancing participant safety with protecting their rights is vital for achieving research objectives. By prioritizing study design, patient-centric approaches, data integrity, and regulatory compliance, clinical trial companies can optimize the process and contribute to impactful medical advancements. Navigating these challenges effectively improves patient outcomes and paves the way for the progress of clinical research.




