Overview
The article focuses on the significance of clinical trial diversity in Latin America, emphasizing its essential role in enhancing the validity and applicability of research findings across different demographic groups. It supports this by illustrating how diverse participant inclusion can lead to improved health outcomes and more effective therapies, while also addressing the challenges and strategies for recruiting a varied participant pool in the region.
Introduction
In the realm of clinical research, the significance of diversity cannot be overstated. As medical advancements progress, the need for inclusive clinical trials becomes increasingly critical, particularly in heterogeneous regions like Latin America. This article delves into the multifaceted importance of diversity in clinical trials, exploring how varied demographic representation not only enriches the validity of research outcomes but also enhances the relevance of findings across diverse patient populations.
With Latin America emerging as a prime location for clinical trials due to its vibrant and diverse patient base, the challenges and opportunities within this landscape are ripe for exploration. From navigating regulatory frameworks to implementing effective recruitment strategies, understanding the dynamics of this region is essential for fostering innovation and improving health outcomes for all.
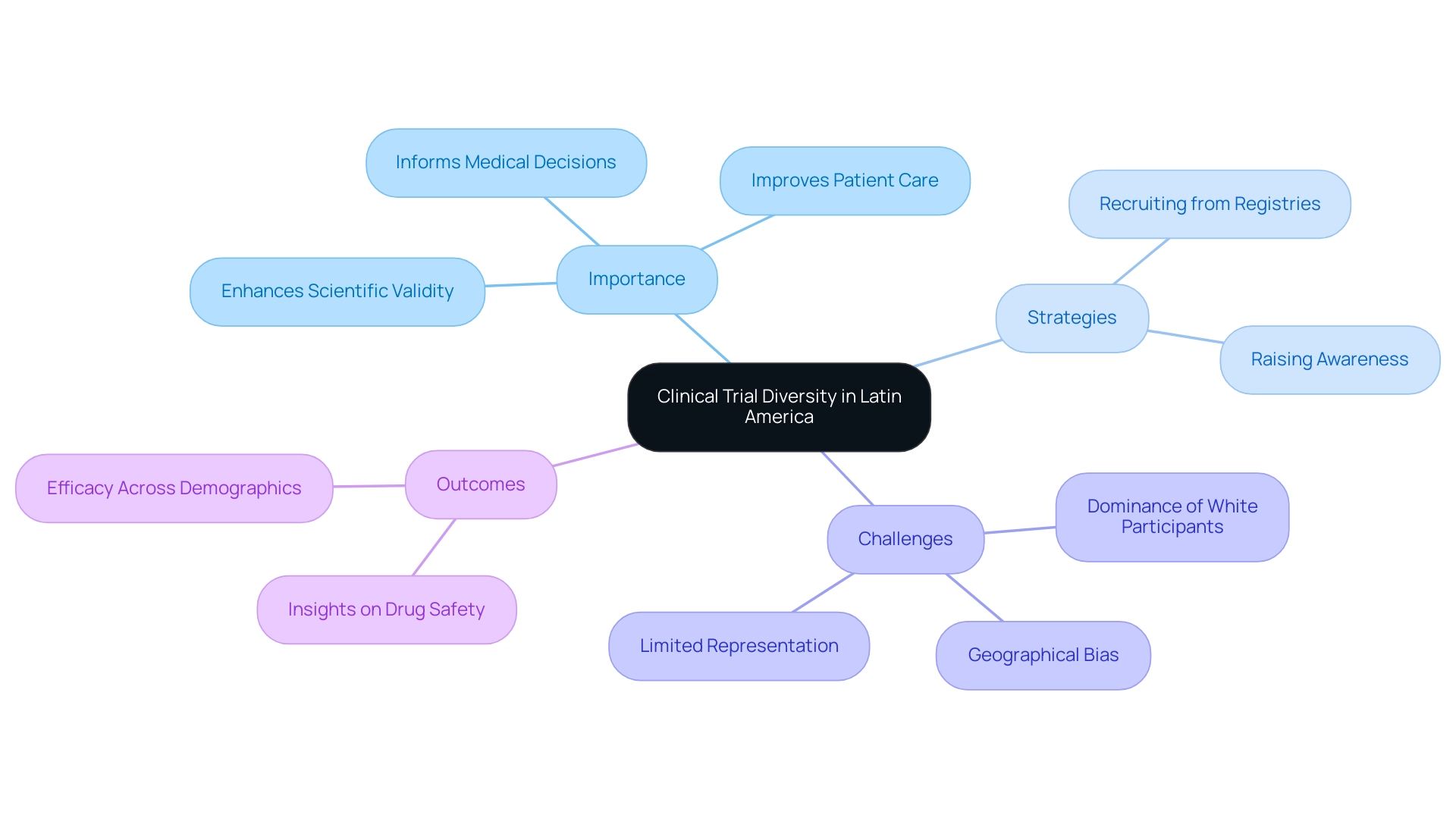
1. The Importance of Diversity in Clinical Trials
Diversity in clinical studies encompasses the inclusion of participants from a wide range of demographic backgrounds, including various races, ethnicities, genders, and age groups. In Latin America, known for its diverse groups, ensuring clinical trial diversity in Latin America is not just beneficial but essential. The inclusion of diverse demographics enhances the external validity of study results, enabling findings to be relevant across different patient populations.
Significantly, varied research studies can uncover differences in drug effectiveness and safety that might remain overlooked in more uniform participant groups. For example, the case study on enrollment disparities in NCI research from 2005 to 2020 highlighted significant underrepresentation of older adults, women, and Hispanic participants, which emphasizes the critical need for more inclusive research practices. As Rachael Fones, the director of government and public affairs at IQVIA, articulates,
'If we learn that one genetic marker impacts one race differently or more prevalently, that not only helps you develop your drug better, but it helps advance the science, advance the practice of medicine, and advance new therapies.'
Alarmingly, just over half of research studies have been found to lack the necessary information regarding the intended target group, reflecting the issues highlighted in the case study. This lack of representation can hinder the understanding of how treatments perform across different demographics, ultimately impacting health outcomes for all. Additionally, specific cancer medications are recognized to focus on genes that are more common in groups of Asian ancestry, further highlighting the significance of diversity in research studies.
By prioritizing diverse participant inclusion, clinical trial diversity in Latin America can lead to better reflection of real-world populations, resulting in improved health outcomes and more effective therapies. In this context, partnerships such as that of bioaccess™ and Caribbean Health Group are crucial in establishing Barranquilla as a prominent location for research in Latin America, strengthened by the backing of Colombia's Minister of Health. Such partnerships not only enhance ambulatory services for studies but also aim for significant advancements in recruitment and retention rates, making the region more appealing for diverse research opportunities.
Additionally, the media coverage from Clinical Leader highlights the increasing attention on research studies in Latin America and Colombia, underscoring the essential role of clinical trial diversity in Latin America in achieving significant health results and ensuring that the conclusions of research studies are relevant to all segments of society.
2. Why Latin America is an Attractive Destination for Clinical Trials
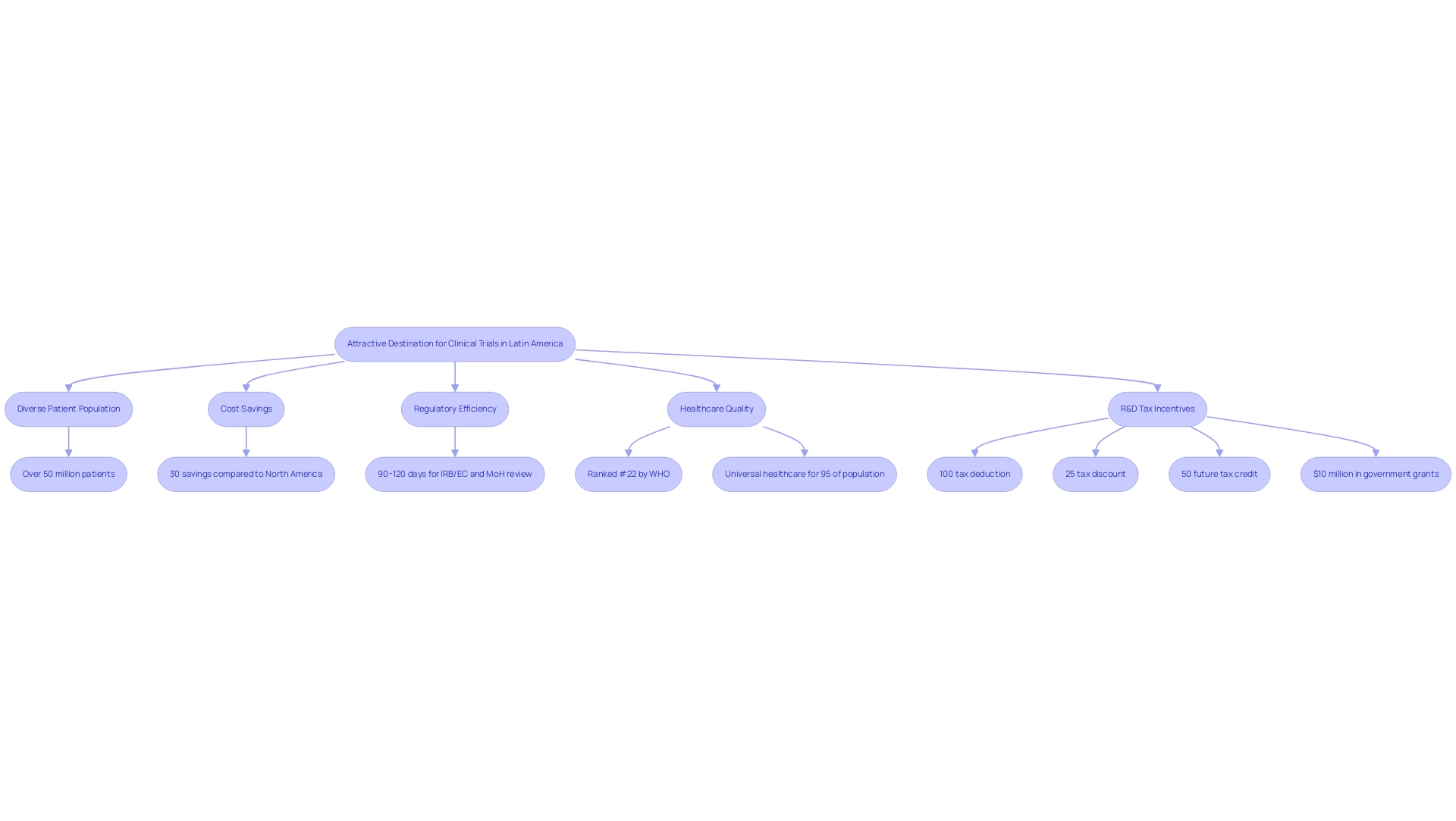
Clinical trial diversity in Latin America is exemplified by the compelling environment for clinical studies in the region, particularly in Colombia, which showcases significant competitive advantages for first-in-human studies. With a large and diverse patient population of over 50 million, Colombia offers enhanced generalizability of research findings. Significantly, the nation reports savings of approximately 30 percent compared to expenses in North America and Western Europe, making it an economically appealing choice.
The total IRB/EC and MoH (INVIMA) review process is completed within a swift 90-120 days, reflecting Colombia's commitment to regulatory efficiency. The process for obtaining trial approval involves:
- Initial IRB/EC approval
- INVIMA review, which ensures compliance with international standards
Additionally, the World Health Organization ranks Colombia's healthcare system as #22 globally, and the nation boasts some of the best hospitals in Latin America, which contribute to clinical trial diversity in Latin America. These factors, combined with universal healthcare coverage for about 95 percent of the population, facilitate patient recruitment and ensure access to a broad demographic.
Furthermore, Colombia provides substantial R&D tax incentives, including:
- A 100% tax deduction
- A 25% tax discount
- A 50% future tax credit
- Around $10 million in free government grants
These incentives create a supportive environment for research investments. As highlighted by Mariana Bei, Senior Director of Clinical Operations and Brazil GMBA at Parexel, local presence and leadership foster strong relationships with regulatory authorities and local talent. It is essential to address language and cultural barriers to ensure compliance with ethical guidelines, which can be managed through careful translation of regulatory materials and ongoing training for local staff.
bioaccess® is your reliable CRO for expediting medical device research in Latin America, guaranteeing a dedication to information security and client assistance, which is essential considering the inherent risks linked to data transmission in medical research.
3. Challenges in Conducting Clinical Trials in Latin America
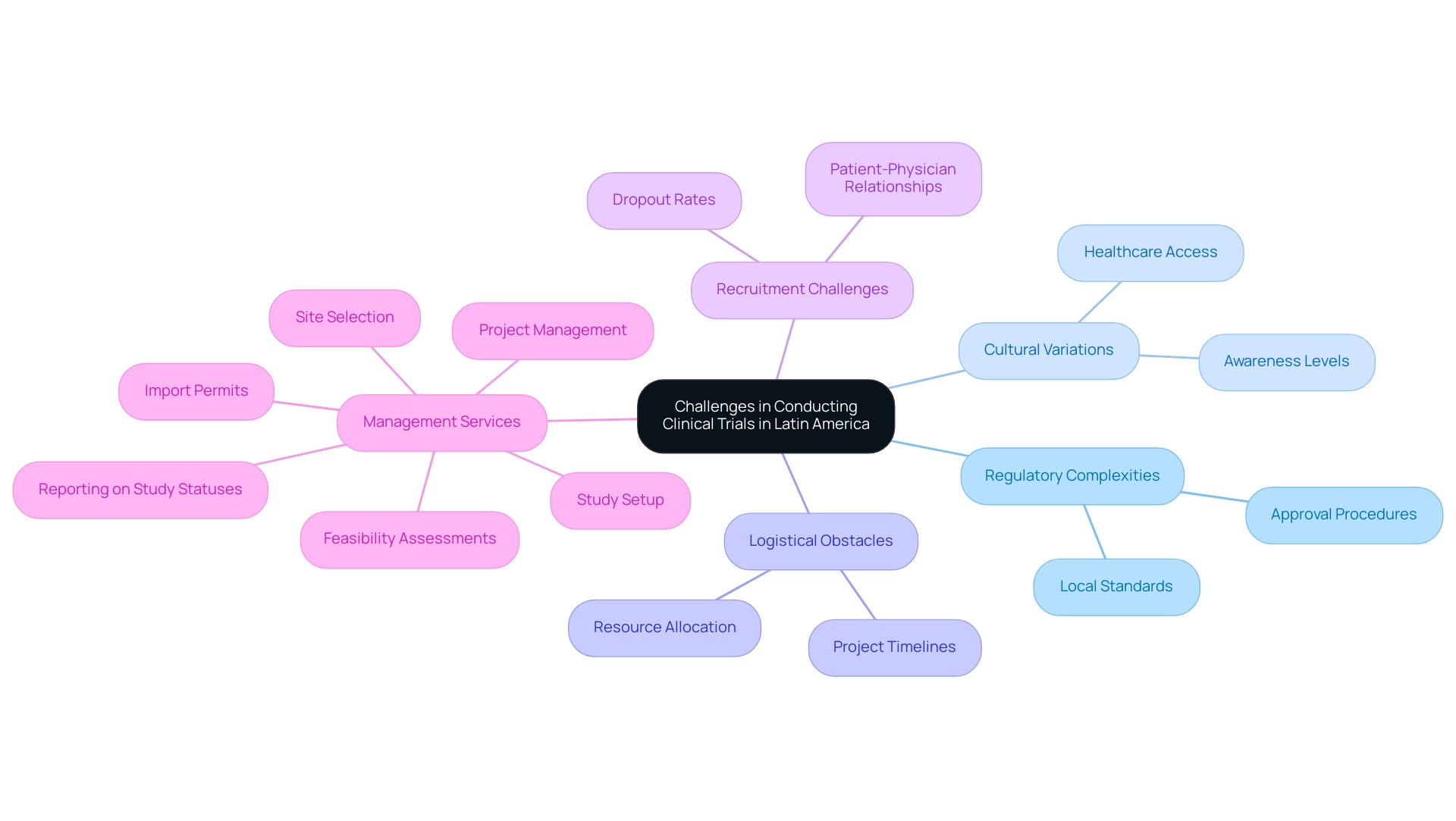
Carrying out research studies in Latin America involves navigating distinct challenges that impact clinical trial diversity in Latin America, including regulatory intricacies, cultural variations, and logistical obstacles. Most Latin American nations have established regulatory frameworks for clinical studies; however, the lengthy and inconsistent approval procedures can impact clinical trial diversity in Latin America, often necessitating thorough compliance reviews to meet local standards. For instance, Colombia’s INVIMA, classified as a Level 4 health authority by PAHO/WHO, plays a critical role in overseeing medical device regulations, ensuring that all trial setups comply with established guidelines.
The time required for approvals can vary significantly between countries, complicating project timelines and resource allocation. Moreover, recruitment efforts for clinical trial diversity in Latin America often face obstacles, as varying levels of healthcare access and awareness can impede participant engagement. The recent observation that dropout rates in Latin America are one-third of those in the U.S. and EU highlights the potential benefits of strong patient-physician relationships and concentrated urban populations.
Significantly, there is a gradual rise in the number of Latin Americans engaging in research studies, indicating the potential for future expansion in clinical trial diversity in Latin America despite the obstacles. This trend is further supported by the economic impact of Medtech research studies, contributing to job creation and healthcare improvements within local economies. Nonetheless, researchers must understand specific laws and guidelines within each country, as these differences necessitate careful navigation and local partnerships to support clinical trial diversity in Latin America.
Moreover, financial support for research studies in low- and middle-income nations remains scarce, further complicating the situation. As Julio G. Martinez-Clark, CEO of bioaccess, notes, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.' This forward-thinking approach emphasizes the significance of thorough research study management services, including:
- Feasibility assessments
- Site selection
- Study setup
- Import permits
- Project management
- Reporting on study statuses and adverse events
These services are essential for overcoming the inherent challenges of conducting research in the region.
4. Navigating the Regulatory Framework for Clinical Trials in Latin America
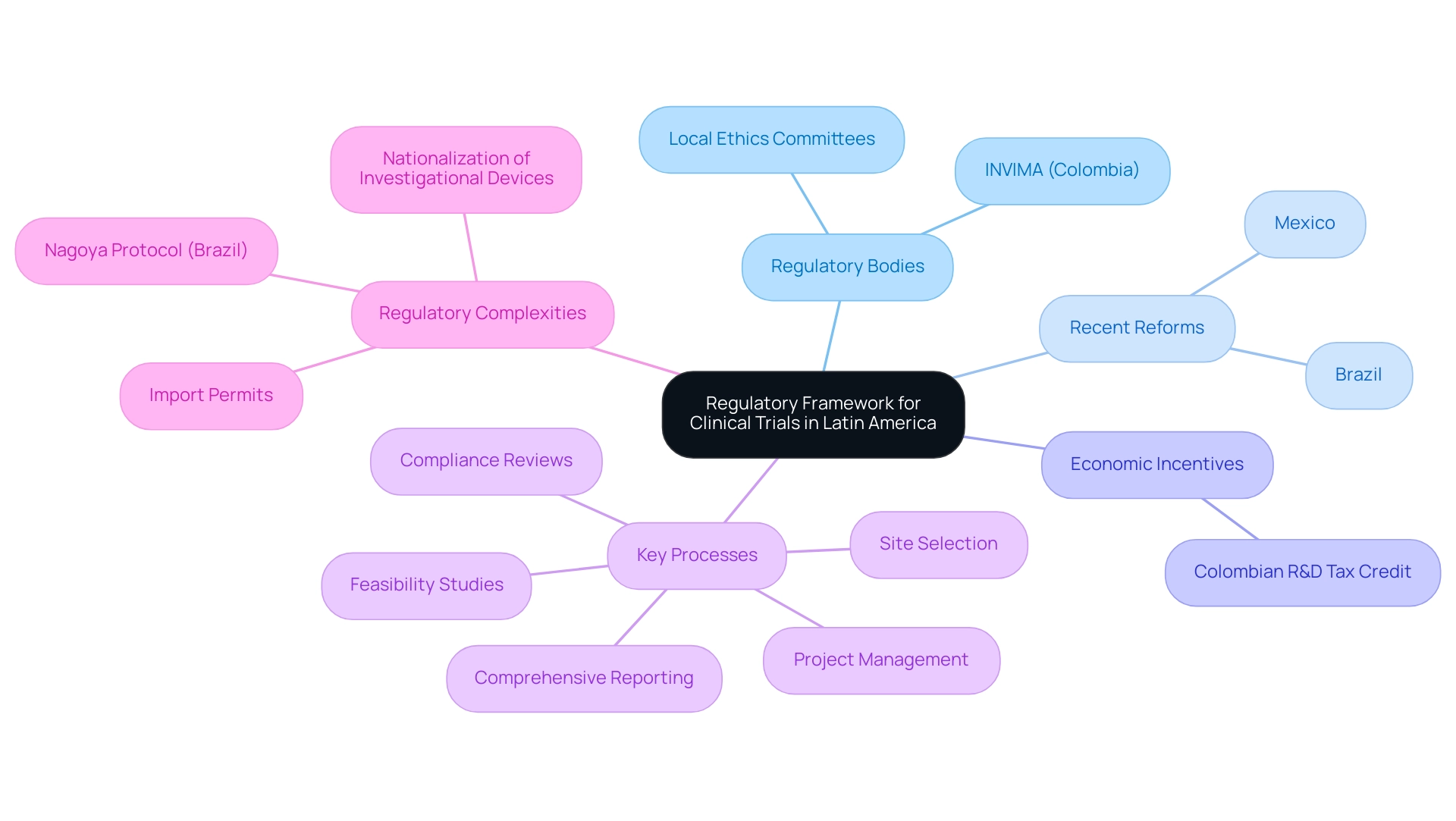
The regulatory framework governing clinical trial diversity in Latin America is characterized by significant variation across countries, typically involving oversight from national health authorities such as INVIMA in Colombia, which plays a crucial role in ensuring compliance and medical device supervision as a Level 4 health authority recognized by PAHO/WHO. Recent reforms in Brazil and Mexico have notably streamlined the approval processes, which is essential for enhancing clinical trial diversity in Latin America and facilitating a more efficient pathway for conducting experiments. Engaging with local regulatory bodies during the initial planning stages is crucial for researchers, as it helps ensure compliance with evolving regulations, particularly concerning clinical trial diversity in Latin America.
Furthermore, fostering relationships with local ethics committees can greatly enhance the approval process, which is crucial for achieving clinical trial diversity in Latin America and establishing trust within the communities participating in the trials. For instance, Colombia's proactive approach to medical research, which emphasizes clinical trial diversity in Latin America, has led to remarkable outcomes such as job creation and enhanced access to innovative treatments, thereby contributing to economic growth. The country’s ambitious science, technology, and innovation plan for 2022–2031 reflects its commitment to becoming a knowledge economy, as emphasized by Julio G. Martinez-Clark, CEO of bioaccess, who stated, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.'
Furthermore, the new Colombian R&D tax credit enables small and midsize firms to claim a 50% tax credit on their R&D and innovation initiatives, offering tangible financial incentives for research. It is also important to note that Brazil's participation in the Nagoya Protocol may affect studies involving certain non-human genetic resources, highlighting the need for researchers to navigate these regulatory complexities. Effective regulatory navigation not only advances medical research but also fosters broader economic development by enhancing healthcare improvement and encouraging international collaboration, particularly in promoting clinical trial diversity in Latin America.
To completely support research management, services must encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Project management
- Comprehensive reporting of study status and adverse events
Furthermore, acquiring import permits and nationalization of investigational devices are essential steps in the setup process that must be addressed.
5. Enhancing Clinical Trial Outcomes Through Diversity
Incorporating clinical trial diversity in Latin America into clinical studies transcends ethical considerations; it fundamentally enhances the scientific validity of research outcomes. Clinical trial diversity in Latin America is crucial for uncovering variations in drug metabolism, efficacy, and side effects across different demographic groups. Strategies for improving clinical trial diversity in Latin America include:
- Recruiting from independent disease registries and community organizations
- Increasing awareness among researchers about the importance of diverse enrollment
For example, recent evaluations of FDA-approved studies from 2014 to 2021 have revealed a significant trend towards gender equality, with women making up an average of 51% of those involved. However, challenges persist in achieving clinical trial diversity in Latin America, as white participants continue to dominate many studies. Significantly, 57% of research findings still come from study locations in the US, which underscores the necessity for clinical trial diversity in Latin America to enhance geographical representation.
By reflecting the true demographics of the populations that will ultimately utilize the drug or therapy, researchers can gain crucial insights into safety and efficacy across various groups, emphasizing the need for clinical trial diversity in Latin America. This approach not only leads to more informed medical decisions but also significantly improves patient care and outcomes. As Bibbins-Domingo highlights, increased clarity in reporting enrollment and results can improve the informational worth of studies, aiding both researchers and patients equally.
Ultimately, actively seeking varied contributor groups in research is essential for advancing the scientific integrity and relevance of findings, which underscores the importance of clinical trial diversity in Latin America.
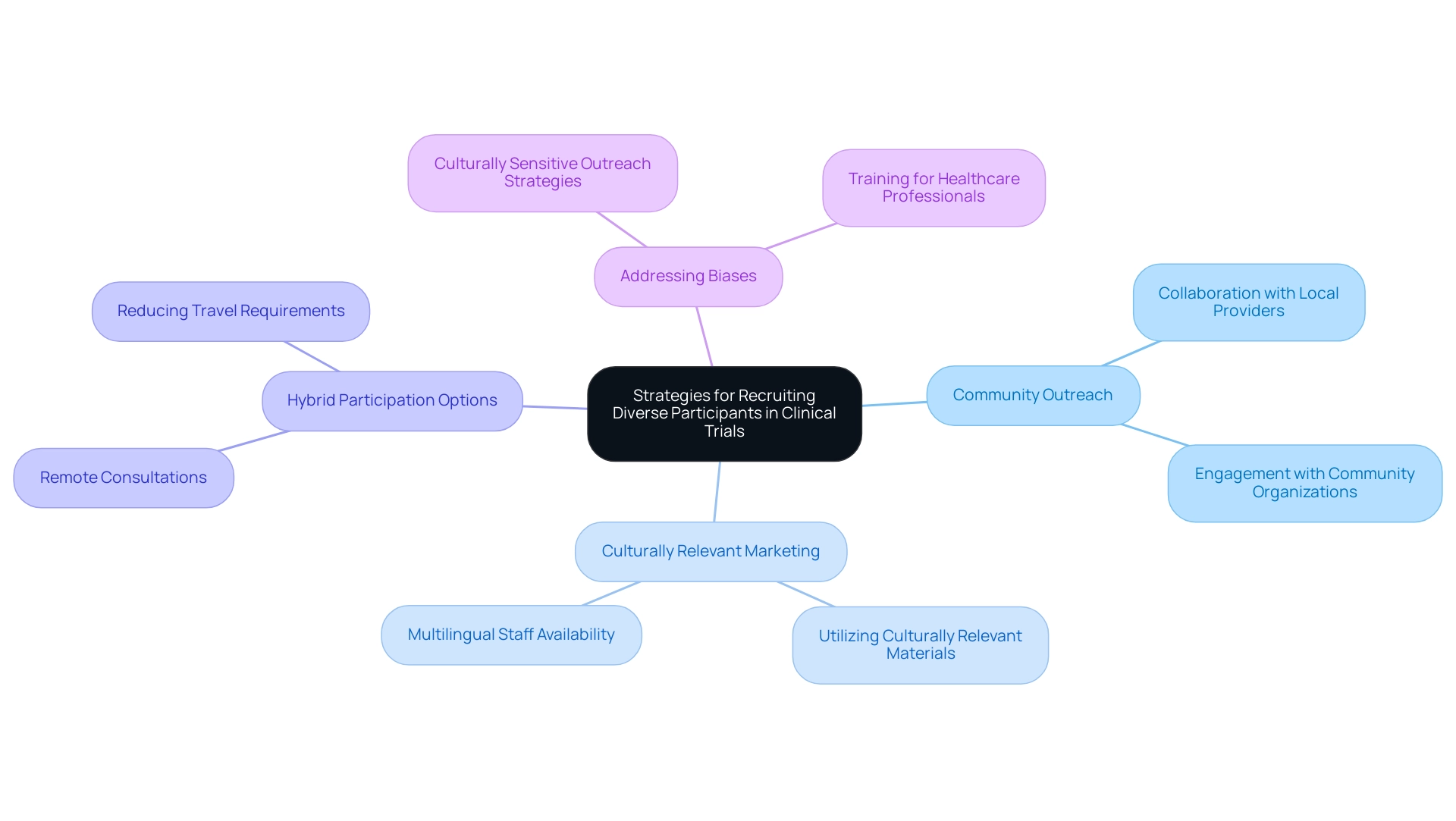
6. Strategies for Recruiting Diverse Participants in Clinical Trials
To successfully recruit a diverse array of individuals for clinical trials, it is crucial for researchers to implement outreach strategies that are thoughtfully tailored to resonate with various communities, thereby promoting clinical trial diversity in Latin America. Forming collaborations with local healthcare providers and community organizations can significantly enhance trust and encourage involvement. Furthermore, utilizing culturally relevant marketing materials and ensuring the availability of multilingual staff can greatly enhance accessibility for potential attendees.
Recent statistics reveal that hybrid participation options are particularly important for non-white individuals, highlighting the necessity for flexibility in study designs. Accommodating participants' needs through options such as remote consultations and reducing travel requirements can effectively address barriers to participation. Additionally, the case study titled 'Provider Perceptions and Language Differences' illustrates how unconscious biases among healthcare professionals can lead to differential treatment and lower quality of care for minority patients, underscoring the importance of culturally sensitive outreach strategies.
As Allison Kalloo, founding partner and communications lead of Clinical Ambassador and iParticipate, notes,
The underrepresentation that we encounter today is much more about current affairs and implicit bias, access to healthcare, practice of medicine, and less about historical issues.
This understanding strengthens the necessity for creative approaches that not only expand involvement but also enhance clinical trial diversity in Latin America within research groups. Moreover, utilizing targeted digital marketing strategies can help reach specific patient populations effectively during online outreach for clinical trials.
Conclusion
The exploration of diversity in clinical trials reveals its critical role in enhancing the validity and applicability of research outcomes. By ensuring that clinical trials include participants from varied demographic backgrounds, particularly in heterogeneous regions like Latin America, researchers can uncover vital differences in drug efficacy and safety. This inclusivity not only enriches the scientific integrity of studies but also ensures that health solutions are relevant and effective for diverse patient populations.
Latin America, with its rich tapestry of cultures and a large patient base, stands out as a promising location for clinical trials. The economic advantages, efficient regulatory processes, and a commitment to improving health outcomes make this region an attractive destination for researchers. However, navigating the unique challenges, such as regulatory complexities and cultural differences, remains essential for successful trial execution.
Implementing effective recruitment strategies that resonate with local communities is paramount. By fostering partnerships with healthcare providers and utilizing culturally sensitive outreach, researchers can enhance participant engagement and overcome barriers to diversity. Ultimately, prioritizing diversity in clinical trials is not just a matter of ethical responsibility; it is essential for advancing medical science and improving health outcomes for all populations. The future of clinical research in Latin America is bright, driven by a commitment to inclusivity and innovation that promises to transform healthcare for diverse communities.
Frequently Asked Questions
What is meant by diversity in clinical studies?
Diversity in clinical studies refers to the inclusion of participants from a wide range of demographic backgrounds, including various races, ethnicities, genders, and age groups.
Why is clinical trial diversity particularly important in Latin America?
Clinical trial diversity in Latin America is essential because it enhances the external validity of study results, making findings relevant across different patient populations and uncovering differences in drug effectiveness and safety that might be overlooked in more uniform groups.
What are some demographics that have been historically underrepresented in clinical studies?
Historically, older adults, women, and Hispanic participants have been significantly underrepresented in clinical studies, as highlighted by a case study on enrollment disparities in NCI research from 2005 to 2020.
How can diverse participant inclusion improve health outcomes?
By prioritizing diverse participant inclusion, clinical trials can better reflect real-world populations, resulting in improved health outcomes and more effective therapies.
What are some competitive advantages of conducting clinical trials in Colombia?
Colombia offers a large and diverse patient population, cost savings of approximately 30% compared to North America and Western Europe, a swift IRB/EC and MoH review process, and a highly ranked healthcare system.
What is the typical timeline for obtaining trial approval in Colombia?
The total IRB/EC and MoH (INVIMA) review process in Colombia is completed within 90-120 days.
What R&D tax incentives are available in Colombia for clinical trials?
Colombia provides several R&D tax incentives, including a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and around $10 million in free government grants.
How does language and culture affect clinical trials in Latin America?
Addressing language and cultural barriers is essential for compliance with ethical guidelines, which can be managed through careful translation of regulatory materials and ongoing training for local staff.
What role do partnerships play in enhancing clinical trial diversity in Latin America?
Partnerships, such as that of bioaccess™ and Caribbean Health Group, are crucial in establishing prominent research locations in Latin America, improving recruitment and retention rates, and enhancing the overall research environment.

