Introduction
Understanding the intricacies of first-in-human (FIH) and early-feasibility studies (EFS) is pivotal in the realm of drug development. These initial phases are the bedrock upon which new treatments are meticulously sculpted, setting the groundwork for potential breakthroughs in medicine. FIH studies signify the first time a new therapy is tested in humans, marking a critical transition from laboratory research to clinical application.
EFS, on the other hand, are exploratory studies, often conducted with a small number of patients to assess the feasibility, safety, and initial efficacy of a treatment.
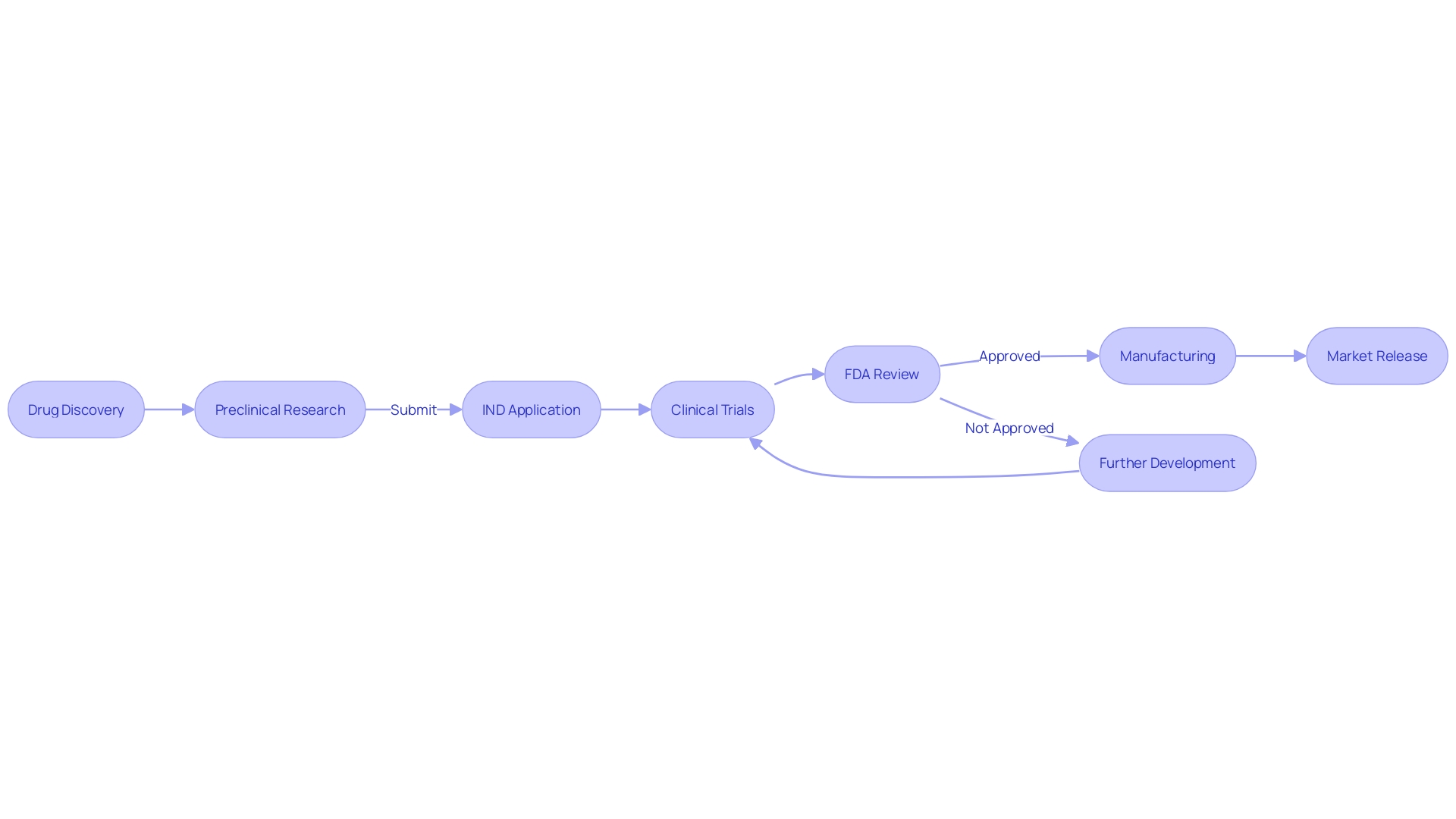
The journey from a promising molecule to a marketed medication is fraught with complexity and requires a substantial investment of time and resources. Typically, the drug development pipeline can be segmented into three major phases: discovery and optimization, preclinical assessment, and clinical testing. The latter is a monumental undertaking, often spanning decades and costing upwards of $2.6 billion.
However, the pace of clinical development is critical. Delays can have profound implications, from diminished financial returns to, more importantly, delayed patient access to potentially life-saving treatments. Recent analyses have shown that the average length of clinical trials has increased, with up to 80 percent of trials not completing on schedule.
This pressure is compounded in the face of urgent health crises, where every month counts in the race to deliver new treatments. Innovations in artificial intelligence and integrated team approaches are among the strategies being leveraged to expedite this process, demonstrating the industry's commitment to evolving and improving the path from discovery to delivery.
What are First-in-Human and Early-Feasibility Studies?
Understanding the intricacies of first-in-human (FIH) and early-feasibility studies (EFS) is pivotal in the realm of drug development. These initial phases are the bedrock upon which new treatments are meticulously sculpted, setting the groundwork for potential breakthroughs in medicine. FIH studies signify the first time a new therapy is tested in humans, marking a critical transition from laboratory research to clinical application.
EFS, on the other hand, are exploratory studies, often conducted with a small number of patients to assess the feasibility, safety, and initial efficacy of a treatment.
The journey from a promising molecule to a marketed medication is fraught with complexity and requires a substantial investment of time and resources. Typically, the drug development pipeline can be segmented into three major phases: discovery and optimization, preclinical assessment, and clinical testing. The latter is a monumental undertaking, often spanning decades and costing upwards of $2.6 billion.
It begins with the identification of a biological target and proceeds through rigorous screenings and optimizations. Once a lead molecule emerges, it undergoes preclinical testing to ascertain its pharmacological properties, safety, and dosage parameters.
However, the pace of clinical development is critical. Delays can have profound implications, from diminished financial returns to, more importantly, delayed patient access to potentially life-saving treatments. Recent analyses have shown that the average length of clinical trials has increased, with up to 80 percent of trials not completing on schedule.
This pressure is compounded in the face of urgent health crises, where every month counts in the race to deliver new treatments. Innovations in artificial intelligence and integrated team approaches are among the strategies being leveraged to expedite this process, demonstrating the industry's commitment to evolving and improving the path from discovery to delivery.
Regulatory Framework in Latin America
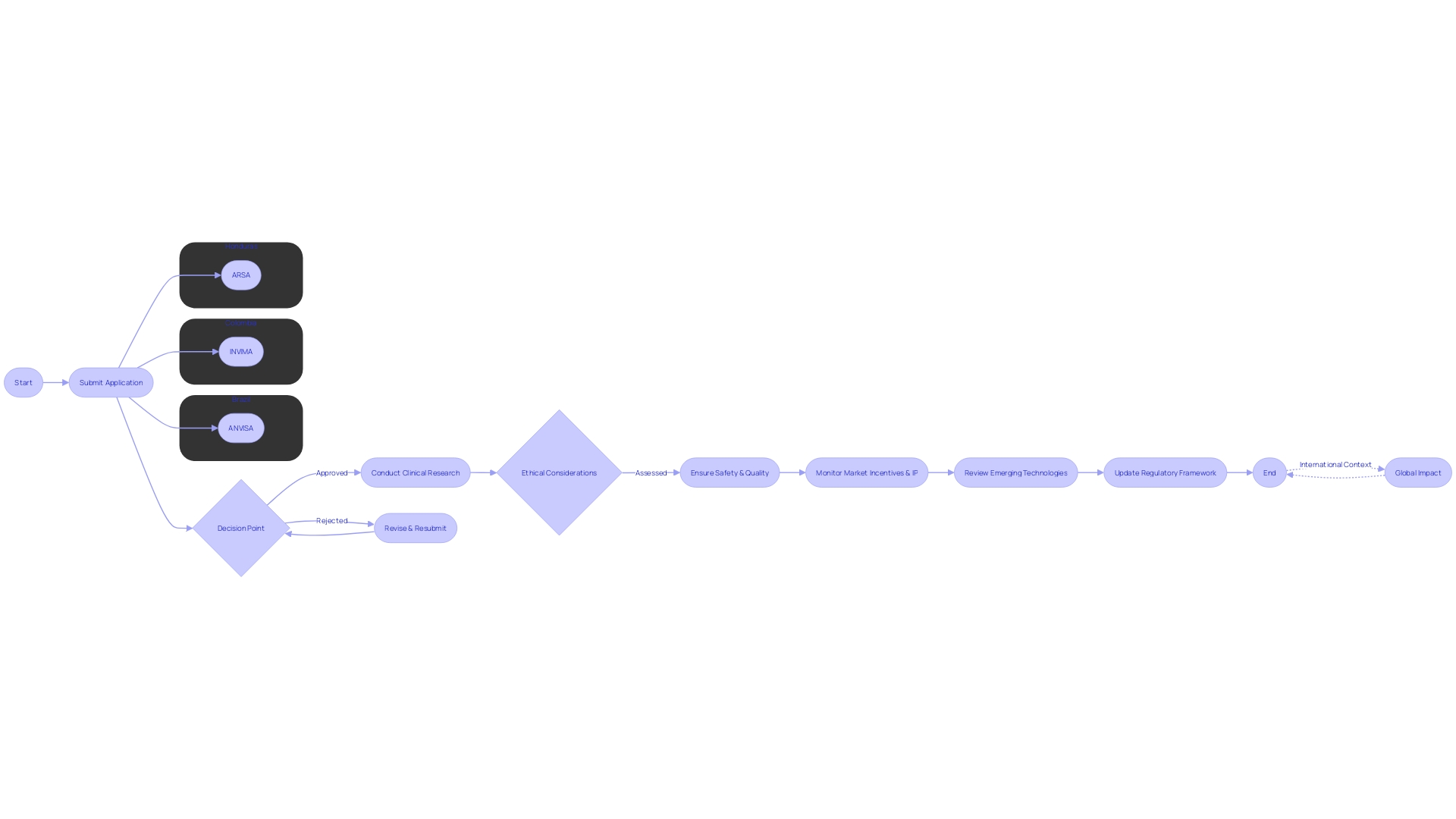
Latin America presents a dynamic regulatory landscape for clinical research, particularly concerning First-in-Human (FIH) and Early Feasibility Studies (EFS). Key regulatory bodies such as ANVISA in Brazil, INVIMA in Colombia, and ARSA in Honduras play pivotal roles in ensuring the safety, quality, and effectiveness of medical products and services. With responsibilities that include the establishment of sanitary standards, evaluation of food additives, and control over pharmaceuticals, these agencies are central to navigating the regulatory environment.
In Brazil, for instance, the Law # 6360 is foundational to understanding the regulation of products with potential health risks. It mandates registration with health authorities before marketing products like medicines and cosmetics, ensuring transparency in labeling and the communication of dosage, side effects, and precautions. This is evidenced by ANVISA's efforts to improve medication label accessibility through digital means, reflecting a commitment to promoting responsible usage.
Furthermore, recent developments in the region underscore the complexity and fluidity of regulatory governance. The uncertainty surrounding the appointment of a new head of INVIMA in Colombia, and ANVISA's struggle with maintaining its controlled substances database, are indicative of the challenges faced within these systems. Honduras is responding to such challenges by increasing inspection actions and enhancing pharmacovigilance training, demonstrating a proactive approach to regulatory compliance.
Case studies, such as those developed to understand governance ecosystems, emphasize the interplay of ethical, legal, and social considerations in health and medicine technologies. They reveal the importance of a cross-sectoral governance framework that can adapt to evolving technologies and market incentives.
As Latin American countries continue to focus on improving their regulatory frameworks, understanding these systems' historical contexts and current statuses is crucial for stakeholders, from healthcare providers to policymakers. The governance of emerging technologies, therefore, requires a nuanced appreciation of the ethical issues and market dynamics that shape their development and implementation.
Key Considerations for Conducting FIH and EFS in Latin America
Embarking on First-in-Human (FIH) and early-feasibility studies (EFS) in Latin America involves navigating a complex regulatory landscape. Ethical considerations are paramount, as they must align with the four bioethical principles of respect for autonomy, nonmaleficence, beneficence, and justice. These foundational principles, elaborated by Beauchamp and Childress, now extend to emerging technologies, demanding a thorough ethical, legal, and social framework.
Informed consent in this region must be transparent and culturally sensitive, ensuring that participants fully understand the implications of their involvement. Safety monitoring is crucial, with stringent oversight to protect participant welfare, while robust data management practices must comply with local and international standards to guarantee data integrity and confidentiality.
Latin America faces unique challenges, such as the need for improved biometric data protection highlighted in comparative studies with the European Union. The absence of specific laws in the region could jeopardize privacy and security, suggesting an urgent requirement for enhanced regulatory measures, including encryption and multi-factor authentication, to align with advanced EU practices.
Moreover, the global increase in decentralized clinical trials (DCTs) indicates a 30.1% compound annual growth rate from 2021 to 2026, but it brings challenges like data security and varying technological literacy. Researchers must also consider the implications of pharmacovigilance systems, which play an essential role in policy making and regulation, ensuring drug safety through evidence-based actions.
Local stakeholder engagement, involving communities, researchers, and institutions, is essential for upholding ethical standards and for the successful adoption of open science practices. This collective effort can foster an environment where scientific discovery is accelerated through the rapid sharing of research inputs and outputs, as per the mission of organizations like CZ Science.
The recent nomination of a health policy expert as the head of INVIMA, despite initial delays, demonstrates a progressive stride towards strengthening the region's regulatory framework. This move signals a commitment to advance the oversight and quality of clinical research in Colombia, setting a precedent for other Latin American countries.
Navigating the Clinical Trial Approval Process
The pathway to obtaining clinical trial approval in Latin America is a multifaceted process, requiring adherence to specific submission requirements and a clear understanding of the local regulatory timelines and authorities. For First-in-Human (FIH) and Early-Feasibility Studies (EFS), it's essential to recognize that the landscape of clinical research, particularly regarding mental health, is both vast and intricate. Latin America, bearing 80% of the mental health disease burden in low- and middle-income countries, presents unique challenges and opportunities for clinical trials.
Depression alone, with its extensive implications on socio-economic factors like productivity and absenteeism, is a significant contributor to the overall disease burden. The implications of these disorders extend into daily life, affecting not only individual health but also economic stability and professional participation. In this context, the approval process for clinical trials must be navigated with a comprehensive strategy that takes into account both the scientific and societal factors at play.
The FDA's commitment to efficient, well-designed clinical research that advances the development of safe, effective medical products is one we share in Latin America. Harmonizing our regulations with international standards, such as the U.S. HHS Common Rule, helps facilitate this goal while ensuring the protection of human subjects. The process of obtaining approval is not only about meeting the regulatory requirements but also about contributing to the broader public health mission.
Recent updates in the regulatory environment, such as the European Medicines Agency's ongoing review of certain medications for potential psychological side effects, underscore the importance of robust and transparent clinical research. In Latin America, as we strive to improve mental health outcomes, it is paramount that clinical trials are conducted with the utmost rigor to generate reliable data that informs regulatory decisions.
As we journey through the approval process for clinical trials in this region, it's worth noting the words of Adolfo Garcia, PhD, a prominent figure in neurodegeneration research in Latin America. His work underscores the potential for clinical research to transform patient care through early disease detection. The collaboration between disciplines—neurodegeneration, language, and computer science—exemplifies the innovative approaches necessary to navigate the complex regulatory environments we face.
In conclusion, the clinical trial approval process in Latin America is not just a procedural hurdle; it is an opportunity to improve mental health care, address societal challenges, and advance scientific knowledge. By understanding the nuances of the local regulatory landscape and aligning our efforts with international standards, we can successfully navigate First-in-Human and Early-Feasibility studies, ultimately contributing to the betterment of public health in the region.
Ensuring Compliance with Good Clinical Practice (GCP) Guidelines
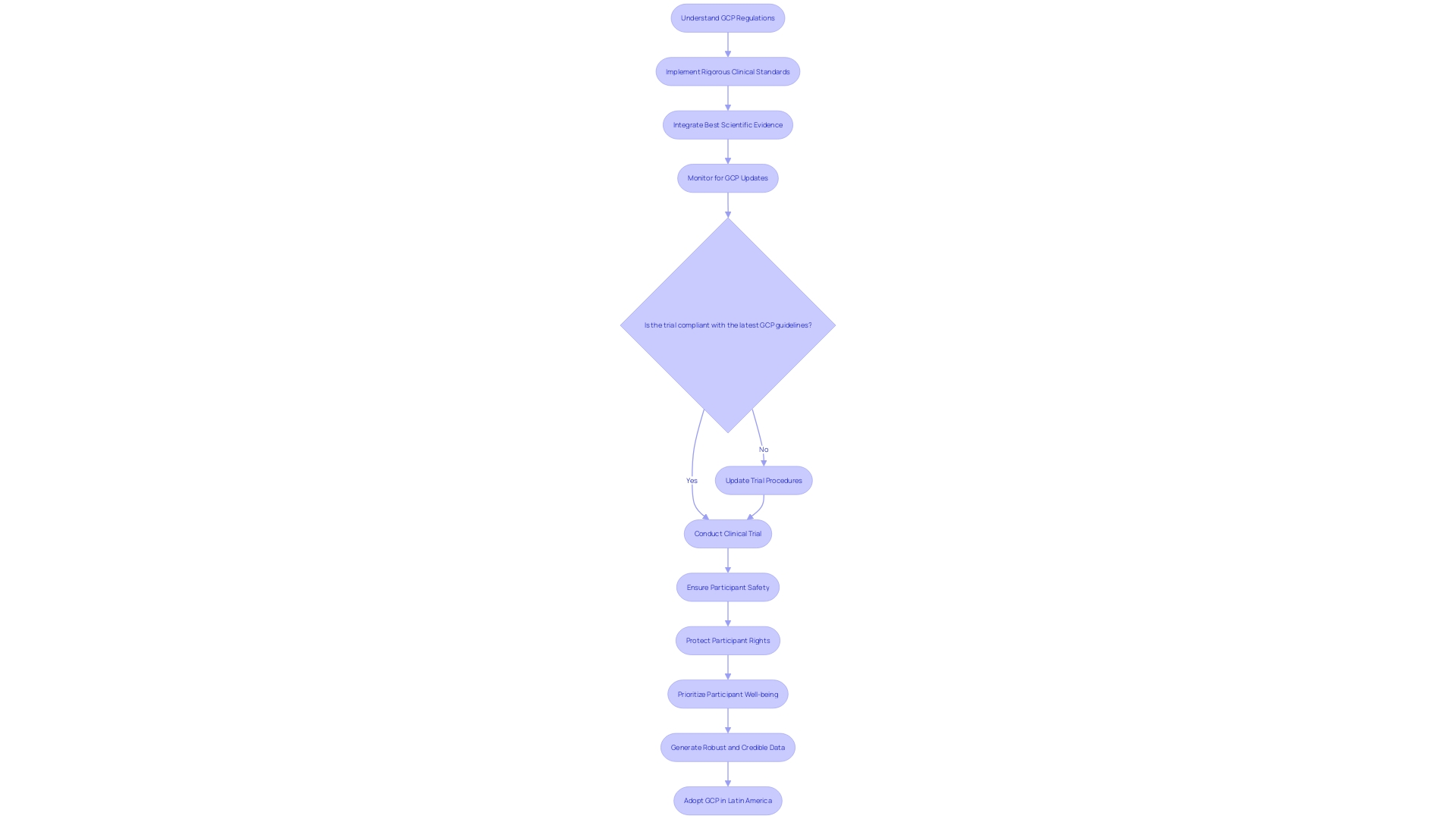
Good Clinical Practice (GCP) is a cornerstone of clinical research, providing a framework for high-quality trials that prioritize participant safety, rights, and well-being. GCP ensures the integrity and credibility of data which is especially critical in First-in-Human (FIH) and Early-Feasibility Studies (EFS) in Latin America. GxP regulations and guidelines, of which GCP is a part, are essential for maintaining product and process quality across various industries, including life sciences.
Ensuring compliance with GCP during FIH and EFS in Latin America requires an understanding of these regulations and a commitment to rigorous clinical standards.
A multi-disciplinary approach, as evidenced by the implementation science study in a university hospital, emphasizes the importance of collective construction of protocols and their evaluation. This approach aligns with GCP guidelines by integrating best scientific evidence into the workflow, thus enhancing the quality and safety of neonatal care. Similarly, the Golden Hour strategy in neonatology demonstrates the benefits of standardizing care based on evidence, which can be applied to clinical trial protocols to improve outcomes and maintain consistency.
Recent updates in the European GCP guidance, with a focus on data governance, underline the evolving nature of clinical trial regulation, aiming to protect participant rights and the reliability of results. These updates are expected to influence global GCP standards and practices, including those in Latin America.
As Brazil steps into the G20 presidency, it highlights Latin America's growing role in global health leadership. The region's unique challenges and advancements in medical and scientific expertise present an opportunity for clinical research to adopt open science practices, as encouraged by stakeholders seeking to increase the adoption of these practices in Latin America.
Incorporating GCP in Latin America not only fosters ethical research but also contributes to the region's capacity to address unmet medical needs. By embracing GCP and the latest guidelines, clinical research in Latin America can ensure the safety of participants and the generation of robust, credible data.
Addressing Ethical Considerations and Informed Consent
Ethical considerations in clinical research are the cornerstone of participant protection and the integrity of the study. For First-in-Human (FIH) and Early-Feasibility Studies (EFS) in Latin America, these considerations take on unique dimensions due to the region's diverse cultural, economic, and political landscapes. Ethics committees play a crucial role in safeguarding the rights and well-being of study participants by rigorously reviewing the study protocols.
They ensure that the research adheres to the four fundamental bioethical principles: respect for autonomy, nonmaleficence, beneficence, and justice.
The informed consent process is another pillar of ethical research. It involves not just the provision of information, but also ensuring that participants fully understand the implications of their involvement. This is where the collective construction of understanding, considering the best available scientific evidence, becomes pivotal.
Implementation science, like the multi-professional care protocol studies carried out in university hospitals, emphasizes the translation and application of scientific evidence into the work process, thereby promoting ethical awareness among professionals.
Approaches to ensure participant understanding and autonomy are therefore deeply embedded in the fabric of clinical research. Quality improvement interventions driven by implementation research, as seen in the Golden Hour strategy in neonatology, highlight the systematic use of effective interventions and standardization of clinical practices. These interventions contribute not just to improved patient outcomes but also to ethical research conduct by reinforcing integrated work through effective communication and multidisciplinary teamwork.
As Latin America continues to evolve as a hub of medical and scientific expertise, the ethical landscape is also transforming. The region's leadership in health, especially during the G20 presidency year, underscores the importance of robust ethical standards that resonate with global practices. This evolution is evident in the growing emphasis on protecting biometric data in the wake of Artificial Intelligence advancements, underscoring the urgency for enhanced privacy and security measures.
In summary, ethical considerations in FIH and EFS in Latin America encompass a multi-faceted approach involving ethics committees, informed consent, and participant autonomy within an evolving regulatory and scientific environment that is increasingly cognizant of the global standards of ethical research.
Data Management and Reporting Requirements
In the dynamic realm of clinical trials, the management of data is not merely a supporting function—it is a cornerstone of the entire process. The orchestration of First-in-Human (FIH) and Early-Feasibility Studies (EFS) in Latin America demands a robust data infrastructure, capable of handling the intricacies of data collection, ensuring impeccable data quality, and fulfilling rigorous reporting commitments to regulatory authorities.
The advent of Electronic Health Records (EHR) has revolutionized data acquisition, providing a treasure trove of information that can be harnessed for research. Yet, this wealth of data comes with its own challenges. As revealed by Raman et al.
in their Trials article, EHR-sourced trials necessitate a framework that can effectively operationalize study goals within the existing trial sites and their infrastructure. A demonstration project highlighted in the same study underscores the successful integration of EHR data into a multi-center pharmaceutical industry outcomes trial, emphasizing the critical role of a central coordinating center in navigating the technical, governance, and operational aspects of EHR utilization.
Moreover, the burgeoning use of connected devices, wearables, and decentralized trial solutions has exponentially increased the volume and variety of data sources. This digital evolution demands an intentional approach to data management, one that is strategic and well-defined before the onset of protocol design. As the data landscape broadens, it is pivotal to establish a data strategy that charts out the optimal collection methods from both traditional and digital sources, ensuring that data insights are leveraged to inform decisions while upholding patient safety and data quality.
Recent developments in the Latin American regulatory environment further emphasize the importance of maintaining data integrity and compliance. The delay in confirmation of a new head of INVIMA, as reported by El Tiempo, and the instability of Brazil's controlled substance database, as noted by ANVISA, serve as cautionary tales underscoring the need for stringent data management practices.
In light of these evolving conditions, data protection authorities across Latin America have been proactive in issuing guidelines, as outlined in the Issue Brief titled 'Regulatory Strategies and Priorities of Data Protection Authorities in Latin America: 2024 and Beyond.' These frameworks are instrumental in guiding organizations through the complexities of personal data processing in an increasingly digital world.
In conclusion, the landscape for FIH and EFS in Latin America is one that requires a multi-faceted approach to data management, with a keen eye on the evolving regulatory requirements and the innovative use of technology to enhance data quality and integrity. As we navigate this landscape, we must be mindful of the data's origin, its journey, and the processes that ensure its recency and reliability. With these considerations at the forefront, we can forge ahead in the pursuit of scientific discovery and patient-centric drug development, informed by data that is as robust as it is revelatory.
Post-Authorization and Paediatric Study Requirements
Following authorization, the importance of post-authorization and pediatric studies emerges as a key facet in ensuring the ongoing safety and efficacy of drugs and medical devices in Latin America. These studies are fundamental in post-marketing surveillance, where they serve to monitor outcomes and detect any long-term adverse effects that might not have been apparent during the initial clinical trials. In pediatric populations, the challenges are amplified due to the ethical, legal, and economic implications of conducting clinical trials on children.
Pediatric studies often rely on observational data and expert consensus, given the ethical concerns and the complexity of interpreting symptoms in children, who are reliant on caregivers to manage their medication.
Amidst the global health landscape, Latin America has faced significant public health challenges, such as the decline in vaccination coverage rates exacerbated by the COVID-19 pandemic. The region has shown resilience and innovation, with countries developing local capacities and health cooperation strategies to address such challenges. As Brazil takes on the G20 presidency in 2024, there is an unmatched opportunity to highlight the region's public health issues and showcase its growing medical and scientific expertise.
The experiences and solutions emerging from Latin America's vaccination policy and public health strategies, including efforts to increase vaccination coverage and prevent outbreaks, could provide valuable insights for the global community.
The need for extensive post-authorization studies, which includes pediatric research, is underscored by the complexities of health issues in Latin America. These studies are not just a regulatory requirement but also an ethical imperative to ensure the well-being of populations, especially the most vulnerable. By comprehensively evaluating the safety and efficacy of medical interventions post-authorization, Latin America continues to contribute to the global effort of enhancing health outcomes and advancing medical knowledge.
Common Questions and Answers
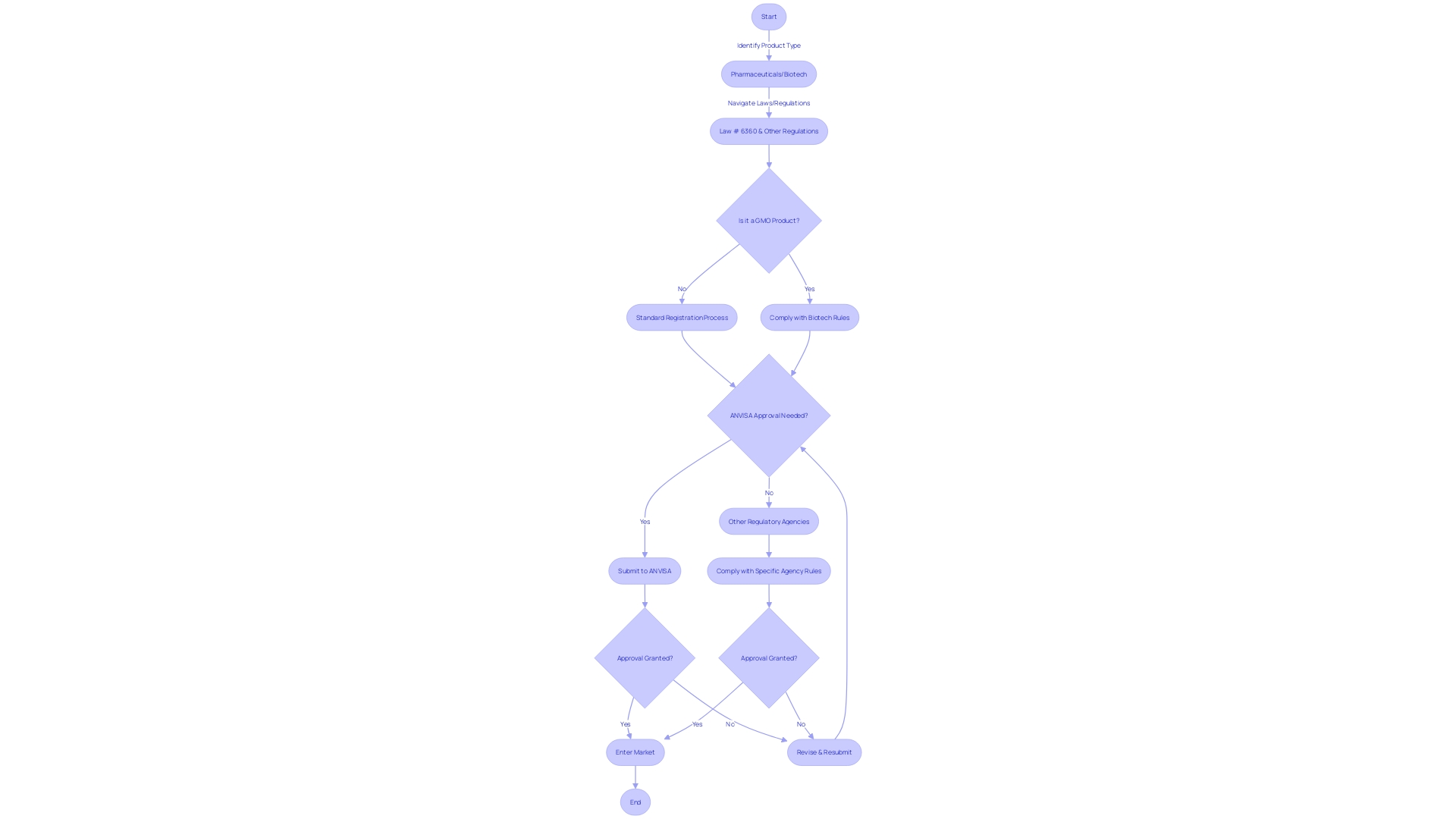
Navigating the intricate regulatory environments across Latin America for first-in-human (FIH) and early-feasibility studies (EFS) requires an understanding of the diverse regulatory frameworks that exist. Each country may present a unique set of challenges, influenced by factors such as market incentives, intellectual property laws, and the specific requirements of emerging technologies. A key agency in this respect is ANVISA in Brazil, which oversees a broad spectrum of health-related regulations, including the safety and efficacy of food and pharmaceuticals, as per Law # 6360.
It's essential to be conversant with various regulatory milestones and understand the significance of having products registered by health authorities before entering the market.
The regulatory scenario is further complicated by products that involve biotechnology, such as genetically modified organisms (GMOs), or those that arise from genetic resources or associated traditional knowledge. In such cases, the regulatory landscape becomes more complex, demanding a thorough navigation of the rules to ensure quality, safety, and efficacy.
Furthermore, swift and successful regulatory interactions can benefit from adopting strategies akin to those used in rapid drug development during emergencies, as noted by the executives at Alvea, where the goal was to accelerate the process from design to human trials. This was achieved by employing small, integrated teams that worked in parallel to expedite the application process, reducing it from the typical four months to a mere 48 hours.
In light of the evolving technologies in health and medicine, case studies from the United States, with relevant international context, reveal the importance of addressing ethical, legal, and social issues that arise in governance across various sectors. These studies emphasize the dynamic nature of governance and the challenges it faces in keeping pace with technological advancements.
Finally, real-time regulatory updates are crucial for staying informed about the latest developments in drug submissions, approvals, and other decisions that can impact clinical research projects. This awareness is vital for clinical research directors who manage multiple projects with the objective of improving patient outcomes through medical advancements.
Conclusion
Understanding the intricacies of first-in-human (FIH) and early-feasibility studies (EFS) is pivotal in drug development. These initial phases set the groundwork for potential breakthroughs in medicine. Delays in clinical development can hinder patient access to life-saving treatments.
Innovations like artificial intelligence and integrated team approaches expedite the process.
Latin America's regulatory landscape, led by agencies like ANVISA in Brazil, ensures the safety and effectiveness of medical products. Stakeholders must navigate this complex environment.
Embarking on FIH and EFS in Latin America requires adhering to ethical principles. Transparent informed consent, safety monitoring, and robust data management practices are essential. Challenges include biometric data protection and decentralized clinical trials.
Local stakeholder engagement fosters ethical standards and open science practices.
The clinical trial approval process in Latin America is an opportunity to improve mental health care and advance scientific knowledge. Harmonizing regulations with international standards ensures participant protection. Collaboration between disciplines and innovative approaches transform patient care.
Good Clinical Practice (GCP) prioritizes participant safety. Compliance with GCP requires understanding regulations and rigorous standards. Recent updates in European GCP guidance emphasize data governance.
Ethical considerations in Latin American clinical research are influenced by diverse landscapes. Ethics committees safeguard participant rights. Informed consent and quality improvement interventions driven by implementation research improve patient outcomes.
Strengthening privacy and security measures is a priority.
Data management is vital in clinical trials. Electronic Health Records (EHR) and connected devices increase data volume. Recent developments in the Latin American regulatory environment emphasize data integrity and compliance.
Post-authorization and paediatric studies ensure ongoing safety and efficacy. Latin America's experiences provide valuable insights for global health. Comprehensively evaluating medical interventions post-authorization enhances health outcomes.
Navigating the regulatory environments for FIH and EFS in Latin America requires understanding diverse frameworks and staying informed about updates. Evaluating the safety and efficacy of medical interventions contributes to advancing medical knowledge.




