Introduction
Latin America has emerged as a prominent hub for clinical trials, drawing attention from researchers and pharmaceutical companies alike. This region offers a unique combination of cost-effectiveness, diverse patient populations, and regulatory advantages that make it an attractive destination for conducting high-quality clinical research. As the demand for innovative treatments grows, understanding the intricacies of navigating the clinical trials process in Latin America becomes essential.
From engaging with local experts to comprehending the financial landscape and identifying funding opportunities, stakeholders must be well-informed to capitalize on the benefits this region has to offer. The following exploration delves into the factors that contribute to Latin America's appeal for clinical trials while addressing the challenges that still need to be overcome.
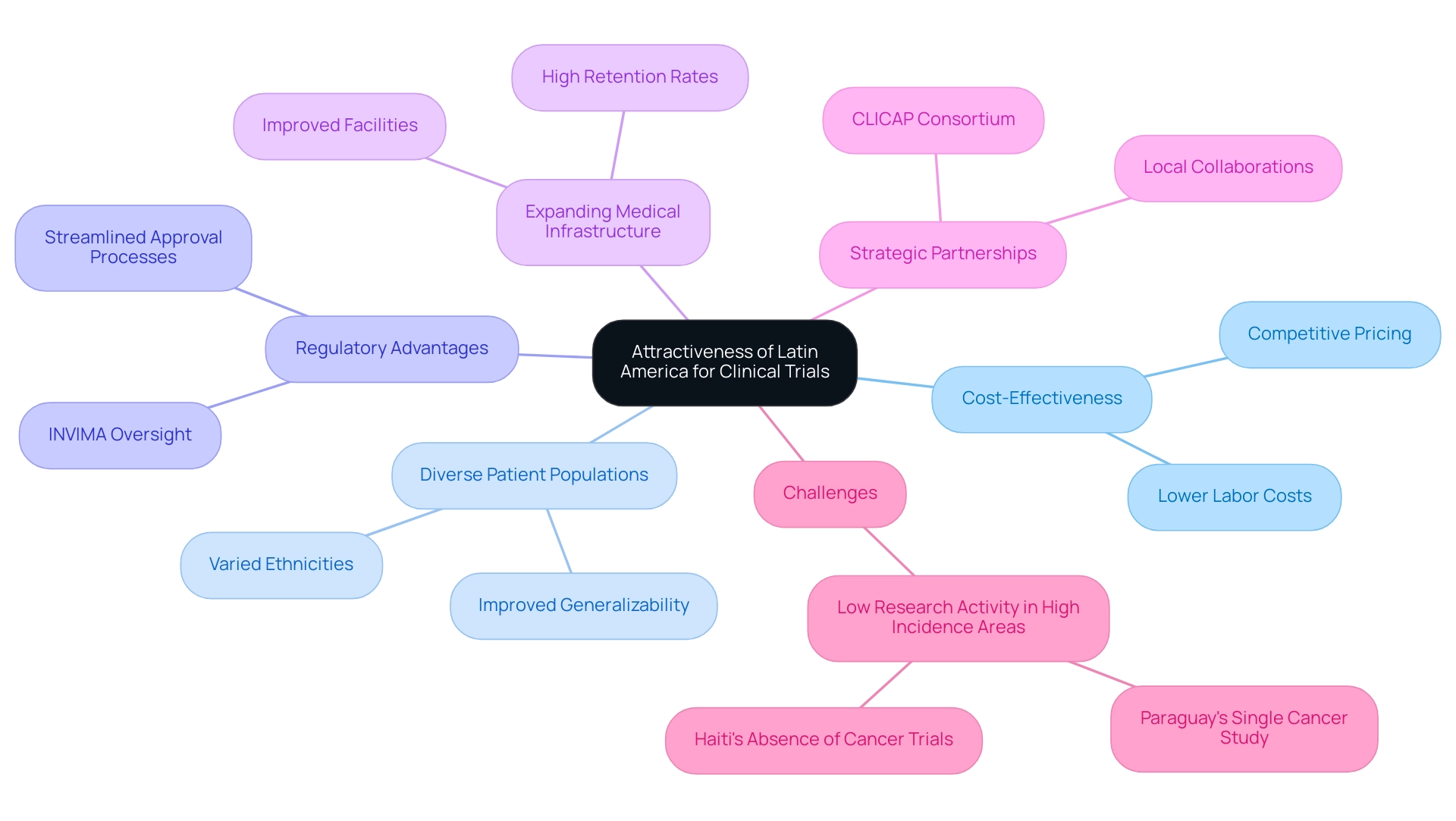
Why Latin America is an Attractive Destination for Clinical Trials
Latin America has increasingly become a prime location for conducting clinical studies, driven by several compelling factors:
-
Cost-Effectiveness: Clinical studies in Latin America can be conducted at a fraction of the cost compared to North America and Europe. Lower labor costs and competitive pricing for services contribute significantly to operational savings.
-
Diverse Patient Populations: The region's rich diversity in ethnicities and genetic backgrounds is invaluable for studies that require a varied participant pool. This diversity improves the generalizability and applicability of research findings.
-
Regulatory Advantages: Streamlined regulatory processes in numerous Latin American nations enable quicker approval times for research studies. For example, Colombia's Minister of Health backs efforts such as the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for research studies. Additionally, the regulatory framework provided by INVIMA, the National Food and Drug Surveillance Institute, ensures that health products meet rigorous safety and efficacy standards. INVIMA oversees the marketing and manufacturing of health products, identifies violations of health standards, and provides medical approval for imports and exports. It is classified as a Level 4 health authority by PAHO/WHO, indicating its competence in health regulation.
-
Expanding Medical Infrastructure: Swift progress in medical facilities and investigative capabilities throughout Latin America has made it possible to carry out high-quality trials. The region’s improving healthcare facilities and research institutions enhance the overall quality and dependability of studies. 'GlobalCare Clinical Trials' collaboration with bioaccess™ illustrates this expansion, showing a decrease in research participant recruitment time by more than 50% and an impressive retention rate exceeding 95%. Bioaccess™ provides extensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting, which improve the efficiency of clinical trials.
-
Strategic Partnerships: Collaborating with local researchers and institutions can lead to successful partnerships, ensuring adherence to local regulations and enhancing study execution. The Latin American Consortium for the Investigation of Lung Cancer (CLICAP), established in 2011, illustrates such collaborative efforts aimed at enhancing lung cancer studies in the region.
Despite the potential, challenges remain. For example, nations such as Uruguay, Cuba, and Paraguay report high cancer occurrence and death rates yet perform relatively few research studies. Paraguay, for instance, has just one cancer study, highlighting the necessity for enhanced investigative efforts. Likewise, Haiti's absence of cancer research studies, as shown by its lack of investigation activity in this area, implies significant obstacles that require attention.
Overall, the combination of cost-effectiveness, varied patient demographics, regulatory benefits, expanding medical infrastructure, and strategic alliances makes Latin America an increasingly appealing location for research studies, providing both economic and scientific advantages. The impact of medical studies extends to local economies, creating jobs, promoting economic growth, improving healthcare, and enhancing international collaboration.
Navigating the Clinical Trials Process in Latin America
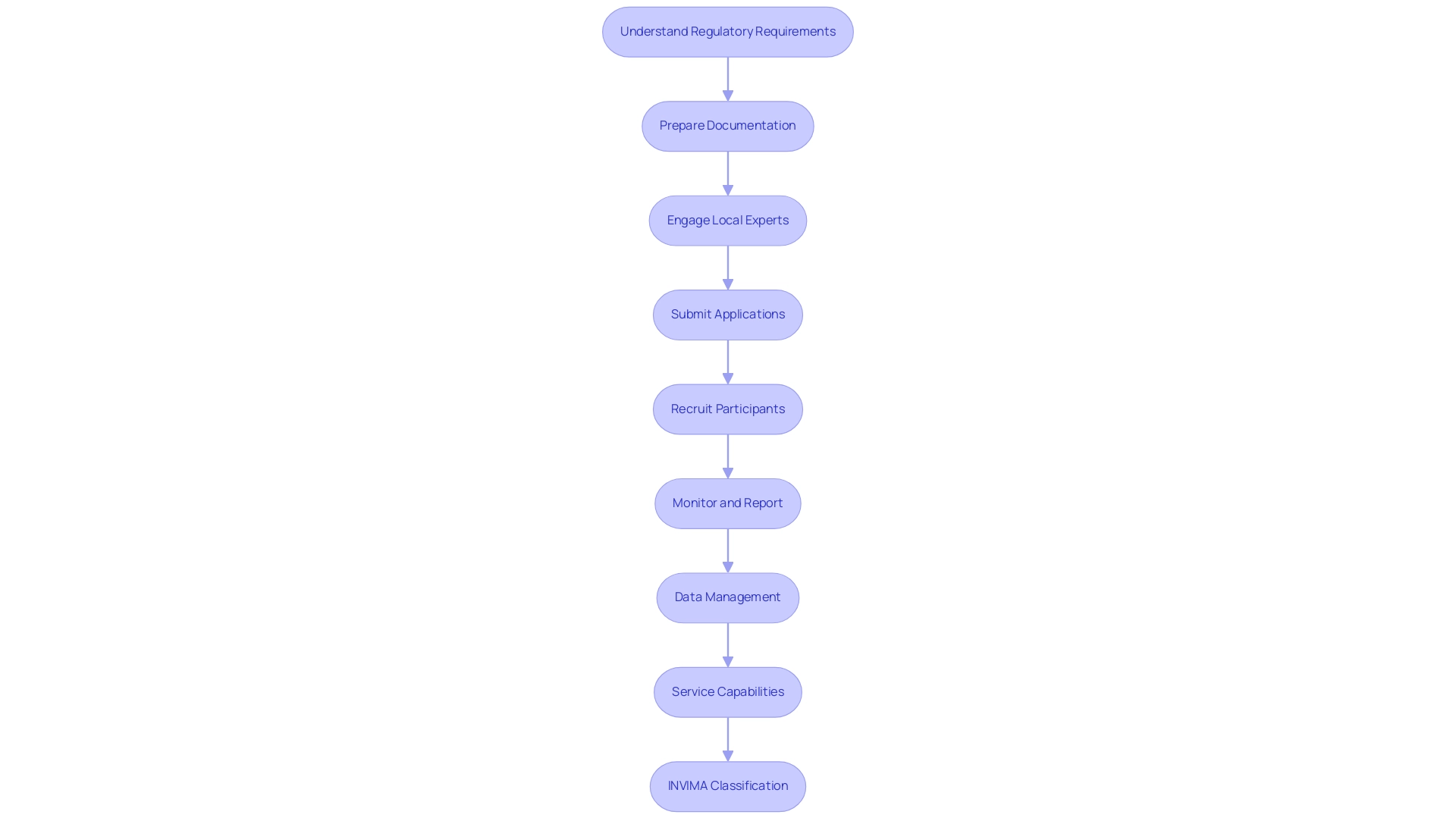
Navigating the clinical research process in Latin America involves several key steps:
- Understand Regulatory Requirements: Each country has its own regulatory body and requirements. Familiarize yourself with the specific regulations of the country where you plan to conduct the trial, such as those enforced by INVIMA, the Colombia National Food and Drug Surveillance Institute, which ensures compliance with health standards and oversees medical device regulations.
- Prepare Documentation: Develop comprehensive research protocols, informed consent documents, and investigator brochures that comply with local regulations, ensuring that all documents are reviewed and tailored to meet INVIMA's requirements.
- Engage Local Experts: Collaborate with local clinical research organizations (CROs) or consultants, such as Katherine Ruiz, a Regulatory Affairs expert with extensive knowledge of obtaining market clearance for medical devices and clinical trials in Colombia, to facilitate smoother processes.
- Submit Applications: File applications with INVIMA and obtain ethics committee approval before enrolling participants, ensuring that all submissions are thorough and meet the regulatory criteria set forth by the health ministry.
- Recruit Participants: Utilize local networks and databases to identify potential participants, ensuring that recruitment strategies are culturally sensitive and ethical.
- Monitor and Report: Establish robust monitoring systems to ensure compliance with the protocol and regular reporting to regulatory authorities, including tracking of serious and non-serious adverse events as part of the project management services.
- Data Management: Implement efficient data collection and management processes to handle the data generated during the study, ensuring it meets international standards and is prepared for submission to INVIMA.
- Service Capabilities: Our service capabilities encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and comprehensive reporting on study status, inventory, and adverse events.
- INVIMA Classification: Understanding INVIMA's classification as a Level 4 health authority by PAHO/WHO is crucial, as it signifies the agency's competence and efficiency in regulating health products, ensuring the safety, efficacy, and quality of medicines in Colombia.
Understanding Cost Factors in Clinical Trials
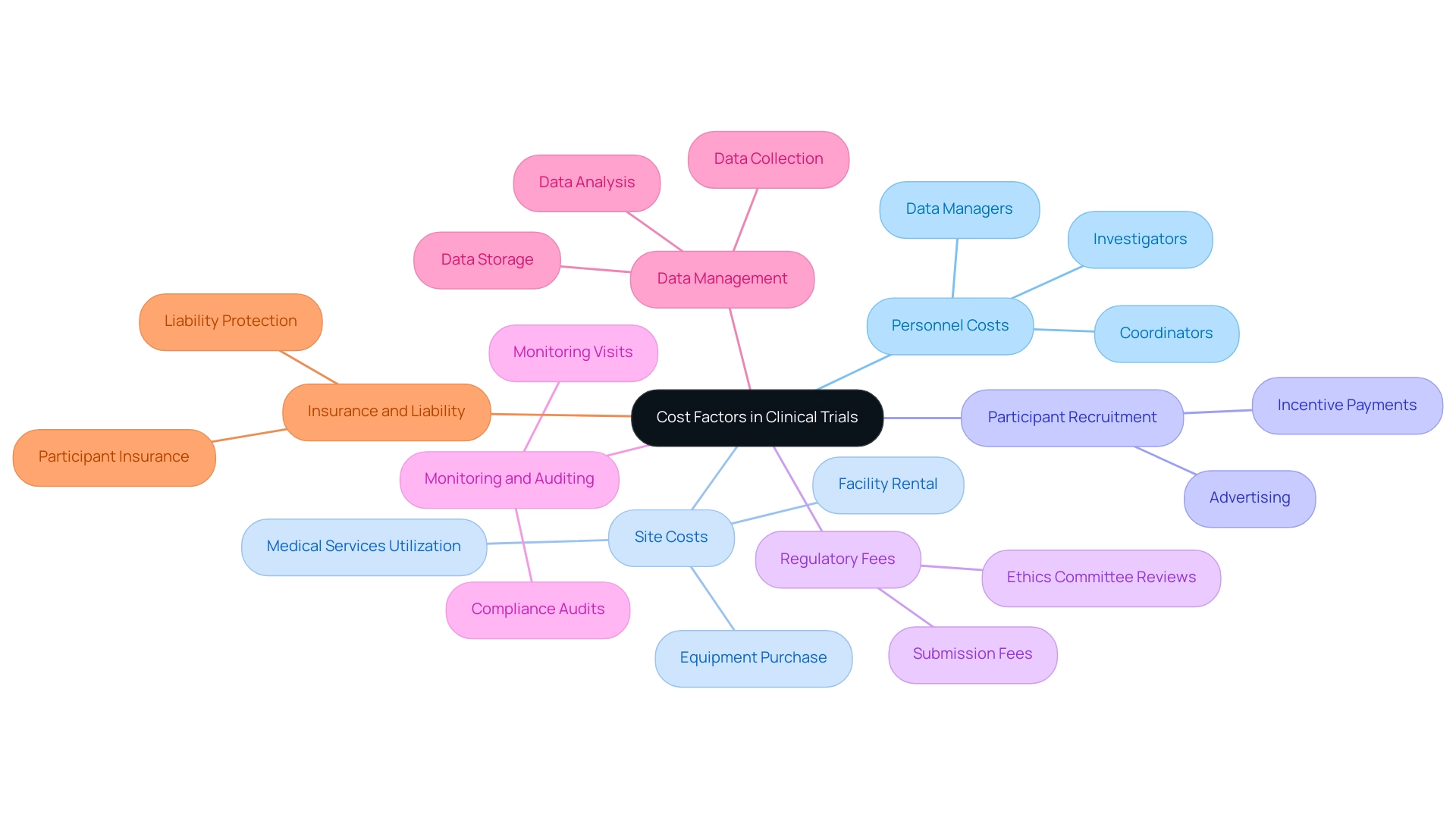
Multiple expense elements can influence medical studies in Latin America:
- Personnel Costs: Salaries for clinical study staff, including investigators, coordinators, and data managers, can vary significantly by country.
- Site Costs: The cost of renting facilities, purchasing equipment, and utilizing medical services can influence overall study expenses.
- Participant Recruitment: Expenses related to recruiting participants, including advertising and incentive payments, can add to the overall budget.
- Regulatory Fees: Some countries may require fees for regulatory submissions and ethics committee reviews.
- Monitoring and Auditing: Allocating budget for monitoring visits and potential audits is essential to ensure compliance and quality.
- Data Management: The cost of data collection, storage, and analysis needs to be factored into the budget.
- Insurance and Liability: Consideration for insurance coverage for research participants and liability protection for the research team is crucial.
Grasping these cost elements is essential for researchers, as they also emphasize the extensive management services provided by bioaccess®, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Furthermore, these experiments contribute significantly to local economies by creating jobs—over 1,000 jobs in recent studies—promoting economic growth with an estimated increase of 5% in local healthcare spending, improving healthcare access, and fostering international collaboration. By comprehensively understanding these factors, researchers can better plan their budgets and seek appropriate funding sources.
Identifying Funding Opportunities for Clinical Trials
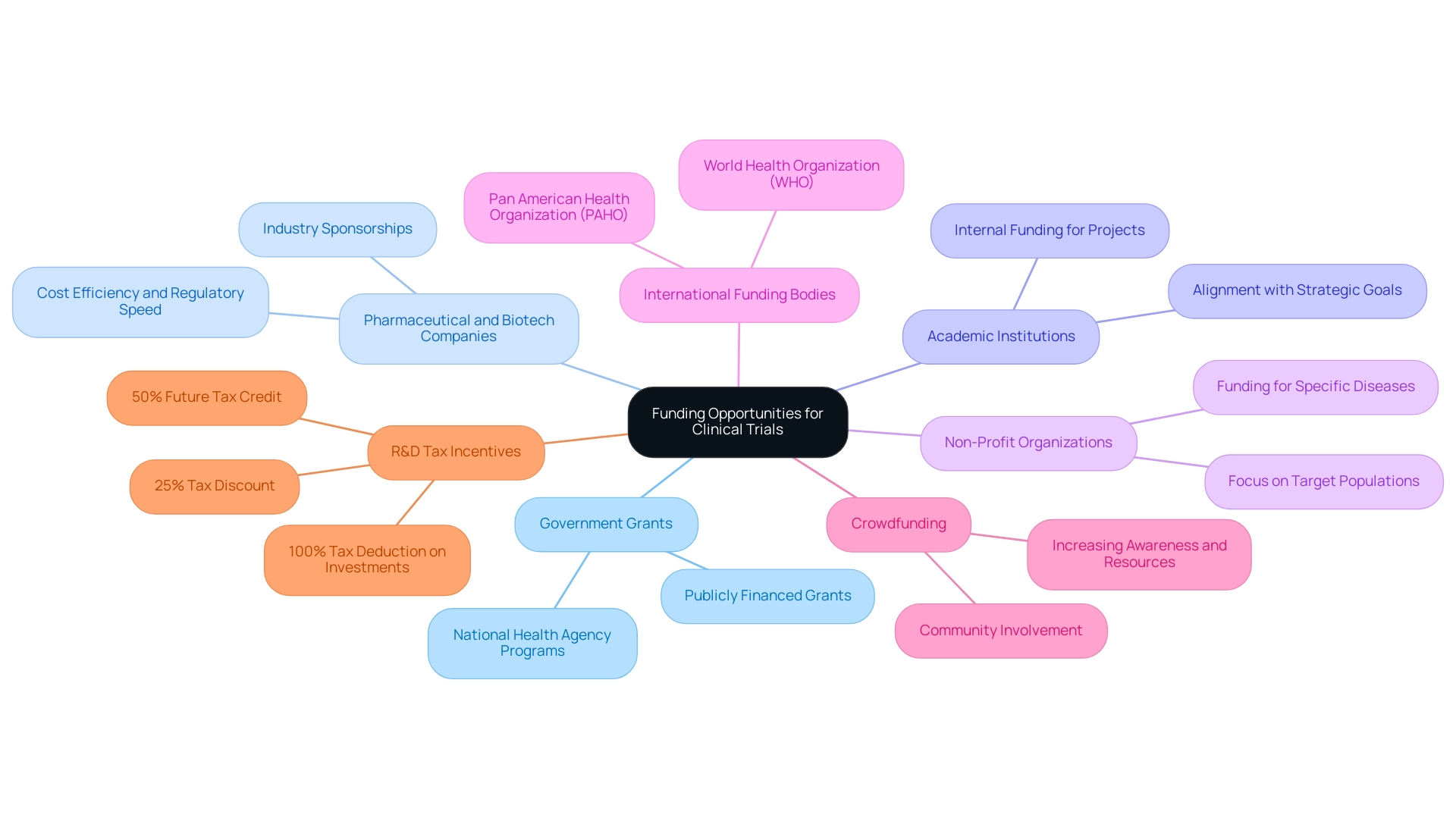
Researchers can investigate various funding options for medical studies in Latin America, especially in Colombia, which is notable for its competitive benefits:
- Government Grants: Numerous nations, including Colombia, offer publicly financed grants for health-related projects. National health agencies often run specific programs to support these initiatives.
- Pharmaceutical and Biotech Companies: Collaborating with industry sponsors can provide substantial financial support for clinical trials, leveraging Colombia's cost efficiency and regulatory speed.
- Academic Institutions: Universities frequently have internal funding for projects, especially those aligned with their strategic goals, enhancing local capabilities.
- Non-Profit Organizations: Non-profits concentrated on health-related studies often finance initiatives that tackle specific diseases or populations.
- International Funding Bodies: Organizations like the World Health Organization (WHO) and the Pan American Health Organization (PAHO) may offer grants for research initiatives in the region, further supporting international collaboration.
- Crowdfunding: Some researchers have effectively used crowdfunding platforms to increase awareness and resources for specific medical research, fostering community involvement.
- R&D Tax Incentives: Colombia offers significant R&D tax benefits, including a 100% tax deduction on investments in science and technology projects, a 25% tax discount, and a 50% future tax credit, which can greatly enhance funding opportunities for researchers.
By exploring these funding opportunities and understanding the comprehensive trial management services—including feasibility assessments, site selection, compliance reviews, and project management—researchers can enhance their chances of securing the necessary resources to conduct their trials while benefiting from Colombia's robust healthcare system and favorable economic conditions. Additionally, the impact of Medtech clinical studies on local economies is profound, creating jobs, promoting economic growth, and improving healthcare access, which can lead to better health outcomes for the population.
Conclusion
Latin America is positioned as a leading destination for clinical trials, driven by a combination of cost-effectiveness, diverse patient populations, and regulatory advantages. The region's lower operational costs, coupled with its rich ethnic diversity, enhance the generalizability of clinical trial results, making it an appealing choice for researchers. Furthermore, streamlined regulatory processes, exemplified by the efficiency of INVIMA in Colombia, facilitate quicker approvals, while the growing medical infrastructure supports high-quality research.
Despite these advantages, challenges persist, particularly in certain countries where clinical trial activity does not align with disease burden. Addressing these gaps is crucial for maximizing the potential of clinical research in the region. The strategic partnerships between local institutions and global organizations play a vital role in navigating these complexities, ensuring compliance and optimizing study execution.
Moreover, understanding the financial landscape and identifying funding opportunities are essential steps for stakeholders in the clinical trials process. By leveraging government grants, industry collaborations, and academic resources, researchers can secure the necessary funding to conduct impactful studies. Ultimately, the continuous investment in clinical trials not only enhances medical knowledge but also contributes to local economies, fostering job creation and improved healthcare access.
As the landscape evolves, the promise of Latin America as a hub for clinical trials remains strong, offering significant benefits for global health advancements.




