Introduction
Navigating Regulatory Environments in Latin America: Opportunities and Challenges
Conducting early-stage clinical trials in Latin American countries offers unique potential for researchers seeking diverse clinical data and cost-effective structures. However, navigating the regulatory landscapes of these countries requires a detailed understanding of the varied environments across Latin America. From the pivotal role of regulatory entities like ANVISA and MAPA to the significance of comprehensive oversight before market entry, this article delves into the regulatory complexities involved in successful and compliant clinical trials.
It also explores the growing need for an open science ecosystem and AI integration to enhance research capacity in addressing mental health challenges. Discover how Novineon, a key player in clinical research outsourcing, has successfully tackled these intricate regulatory processes in Latin America and learn valuable lessons for the sector. Get insights into the crucial role of Contract Research Organizations (CROs) as bridges in the development of innovative treatments.
Explore the significant impact that patient-centric clinical trials and electronic data capture technologies have on enhancing operational efficiency and patient outcomes. Furthermore, gain valuable knowledge from case studies of leading CROs like RP-CRO and PMC, highlighting the importance of localized understanding and partnerships in ensuring regulatory adherence and fulfillment of clinical trial objectives. Lastly, the article also touches upon the FDA's guidance on Institutional Review Boards and provides an overview of the EU Clinical Trials Regulation as it compares to Latin American regulatory requirements.
Navigating Regulatory Environments in Latin America
Conducting early-stage clinical trials, particularly first-in-human (FIH) and early-feasibility studies (EFS), in Latin American countries presents unique opportunities and challenges. The region's diverse population is a distinct advantage for researchers seeking wide-ranging clinical data. The cost structures in these countries are often seen as a benefit compared to other regions.
Moreover, there exists a drive to create supportive regulatory frameworks that are conducive to clinical research. However, successfully conducting clinical trials demands a granular understanding of these regulatory environments, which vary significantly across Latin America. Insights into the regulatory landscape reveal how entities like ANVISA, MAPA, and INMETRO are paramount, establishing standards and reviewing products and services to protect public health.
Additionally, the Law # 6360 underscores the necessity for drugs and medical products to be subjected to rigorous oversight before reaching the market. Furthermore, with mental health challenges being a significant public health concern in Latin America, as evidenced by the high prevalence of depression and anxiety, there's a growing need for developing an open science ecosystem to facilitate research and data sharing, as well as to integrate AI strategies, which in return could enhance the region's research capacity. These aspects underscore the importance of meticulous planning and strategic navigation in these regulatory territories to realize successful and compliant clinical trials.
Case Study: Novineon
At the forefront of clinical research outsourcing in Latin America, Novineon has carved a niche by adeptly handling the intricacies of regulatory processes across various Latin American jurisdictions. Their successful endeavors illustrate an in-depth knowledge of regional regulatory mandates for CROss and paint a picture of a strategic, compliant approach to clinical trial execution. Insights from Novineon's journey reveal the importance of localized understanding and offer strategic lessons for others in the sector.
Primarily, it underscores the significance of comprehensive research and the value of leveraging local expertise to streamline the complex regulatory pathways encountered in this diverse and evolving landscape. As noted by Kati, a health policy manager with deep knowledge of Latin American health systems, 'One stripe at a time: raising awareness of rare diseases in Latin America' underscores the need for such nuanced approaches in addressing health challenges within the region.
The Importance of CROs in Clinical Trials
Contract Research Organizations (CROs) serve as a crucial bridge in the development of innovative treatments, from the conception of the study design to executing the multi-faceted aspects of clinical trials. With their deep understanding of the varied regulatory landscapes, they are vital allies for sponsors aiming to drive their therapies from trial to treatment. CROs are instrumental in refining clinical trial designs, lending their expertise to enhance the balance between scientific rigor and operational efficiency.
KEN GETZ of PHARMAVOICE highlights the sector's ongoing innovation, emphasizing the need for 'great science with great execution' and the integration of technologies like electronic data capture, to bolster efficiency. Further, Treehill Partners evidence this trend, with insights gained over two decades in transaction advisory, underlining the significance of conscientious decision-making in study design — every link in the research process must reflect the strategic objectives and the exigencies faced by the company. The meticulous orchestration of clinical trials, which assess new medical interventions for safety and efficacy through phased approaches, is therefore not simply a matter of compliance, but a strategic imperative intimately connected to patient outcomes and the advancing frontier of medical care.
Case Study: RP-CRO
RP-CRO has established itself as an eminent Contract Research Organization in the dynamic and varied landscape of Latin America, a region recognized for its spectrum of economies from burgeoning markets like Brazil to lesser-developed regions. Their proficiency in the area reinforces the importance of bespoke strategies for clinical trials that adhere to intricate local regulations. Acknowledging the idiosyncrasies among Low- and Middle-Income Countries (LMICs), RP-CRO's adeptness lies in pinpointing key markets for clinical trials based on the disease's incidence and prevalence data.
This strategic specificity is crucial in optimizing voice of customer (VoC) research across divergent economies. Moreover, RP-CRO embraces the significance of cultivating viable partnerships with local entities. These alliances are indispensable to navigate the intricacies of regulatory landscapes and stakeholder engagements, which ultimately foster the fulfillment of clinical trial objectives.
Case Study: ScienceDirect
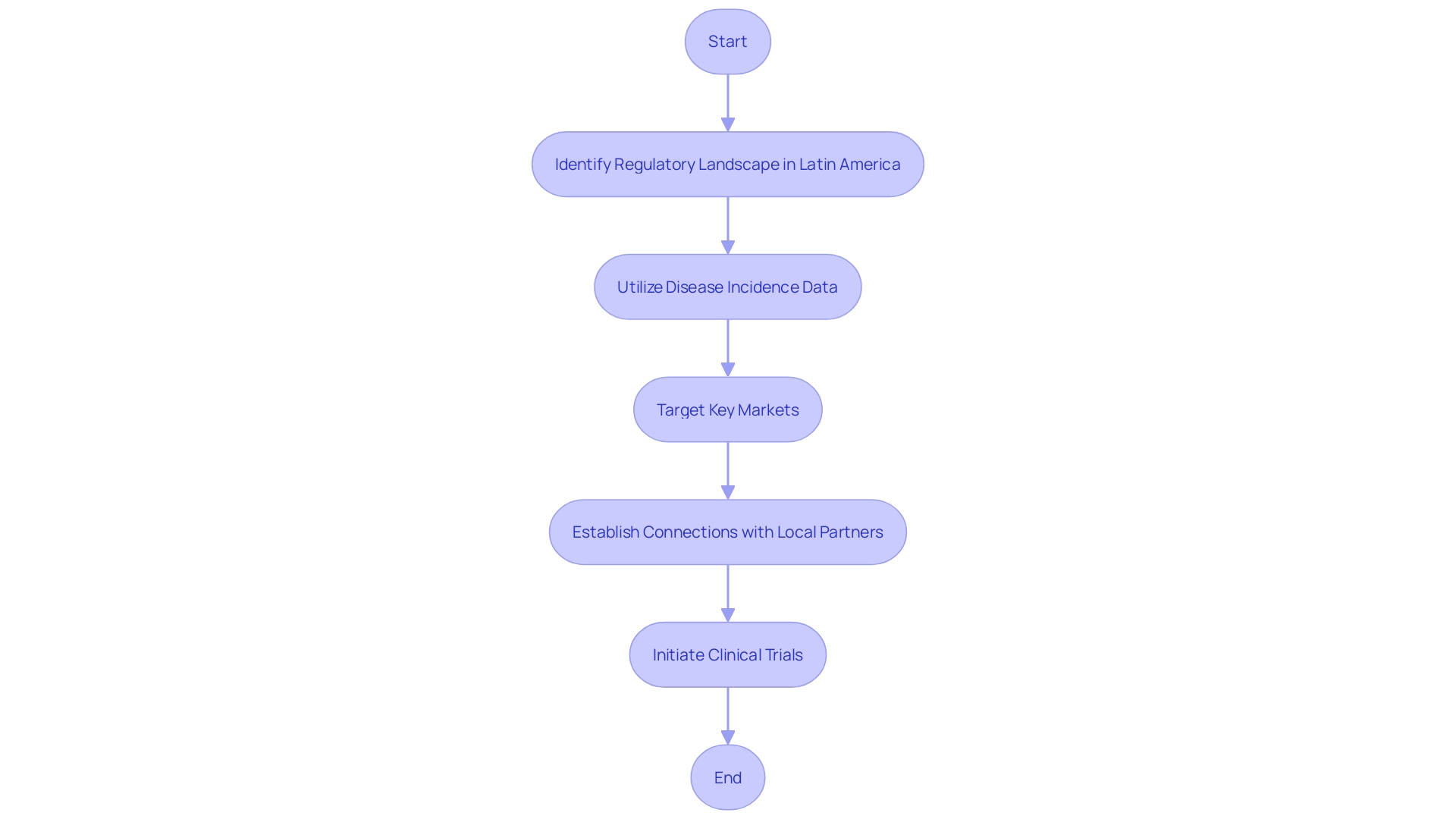
When initiating clinical trials in Latin America, identifying and understanding the distinctive regulatory landscapes across various nations is critical. As a region with emerging economies like Brazil and smaller, less developed markets, Latin America presents a tapestry of opportunities and challenges for clinical research. ScienceDirect’s foray into Latin American clinical trials underscores the importance of meticulous planning and local partnerships.
Utilizing disease incidence and prevalence data to pinpoint key target markets streamlines the process of selecting pivotal locations for conducting Voice of the Customer (VoC) research. This strategic approach is pivotal, recognizing that while no research can fully encompass all local variances, identifying significant regional commonalities can yield invaluable insights. Furthermore, establishing connections with reliable local partners is imperative for effective organization and engagement with local stakeholders, facilities, and regulatory entities.
ScienceDirect's experience thus sheds light on the pragmatic steps that may guide researchers and sponsors through the complexities of the regional regulatory environment.
Case Study: PMC
PMC, a distinguished Clinical Research Organization (CRO) operating on the global stage, has established a noteworthy track record of conducting clinical trials across the diverse landscape of Latin America. Their strategic approach to regulatory adherence has been pivotal in navigating the various regulatory environments found within the region. By focusing on the strength and resilience of their research teams, PMC mirrors the selection criteria seen in Regenera América's project funding processes, which emphasize the importance of a team's problem-solving capabilities and adaptability.
This emphasis on team dynamics, combined with in-depth regulatory knowledge, allows PMC to fulfill the stringent requirements set by Latin American health authorities, ensuring the successful execution of clinical studies in these emerging markets.
FDA Guidance on IRBs
Navigating the complex terrain of regulatory environments for clinical research in Latin America requires a firm grasp of the U.S. Food and Drug Administration's standards. As part of the U.S. Department of Health and Human Services, the FDA is integral in upholding the safety and efficacy of health-related products, including the stringent oversight of clinical trials through the roles defined for Institutional Review Boards (IRBs). The guidance provided by the FDA on IRBs is pivotal for any sponsor or Contract Research Organization (CRO) aiming to conduct trials within this jurisdiction, ensuring public health protection aligns with rigorous scientific research protocols.
EU Clinical Trials Regulation
In addition to Latin America, sponsors and CROs may also be involved in conducting clinical trials in the European Union (EU). The EU Clinical Trials Regulation sets out the regulatory framework for clinical trials and aims to streamline the authorization process. This section provides an overview of the EU Clinical Trials Regulation and highlights the key differences and similarities compared to Latin American regulatory requirements.
Blended Solutions by ICON
As regulatory landscapes evolve, particularly in the burgeoning Latin American market, a comprehensive understanding of new legislations such as Law 21,521 becomes essential. Effective as of February 3, 2023, the FinTech Law is an exemplary model, fostering competitive innovation and financial inclusion. This regulation notably introduces General Rules no.
493 and 494, guiding the registration and authorization for financial service providers, blazing a trail for an open finance system and categorizing crypto assets uniquely under its framework.
The practical implications of aligning with such evolving regulations are significant. For instance, a case study presented by ICON – a pioneer in drug development solutions – highlights the critical nature of local knowledge married with global resources and advanced technologies. ICON's foray into meeting the intricate demands of the FinTech Law underscores the necessity of such a blended approach for successfully navigating the minutiae of compliance—especially pertinent when clinical trials span multiple geographies and regulatory bodies.
The concept of patient-centric clinical trials is expanding, with discussions around real-world data (RWD) and patient access gaining traction. The challenges in translating research into meaningful outcomes are echoed in the reflections from a hypothetical situation involving a patient in rural Pennsylvania and their journey to participate in a clinical trial in Turkey. This example, coupled with Flatiron's research into RWD quality frameworks, underscores the importance of reliable data that resonates with the specific research question—a principle that informs ICON's methodology in maintaining compliance while enhancing the efficiency of clinical trials across various jurisdictions like Latin America.
Linical Resource Center
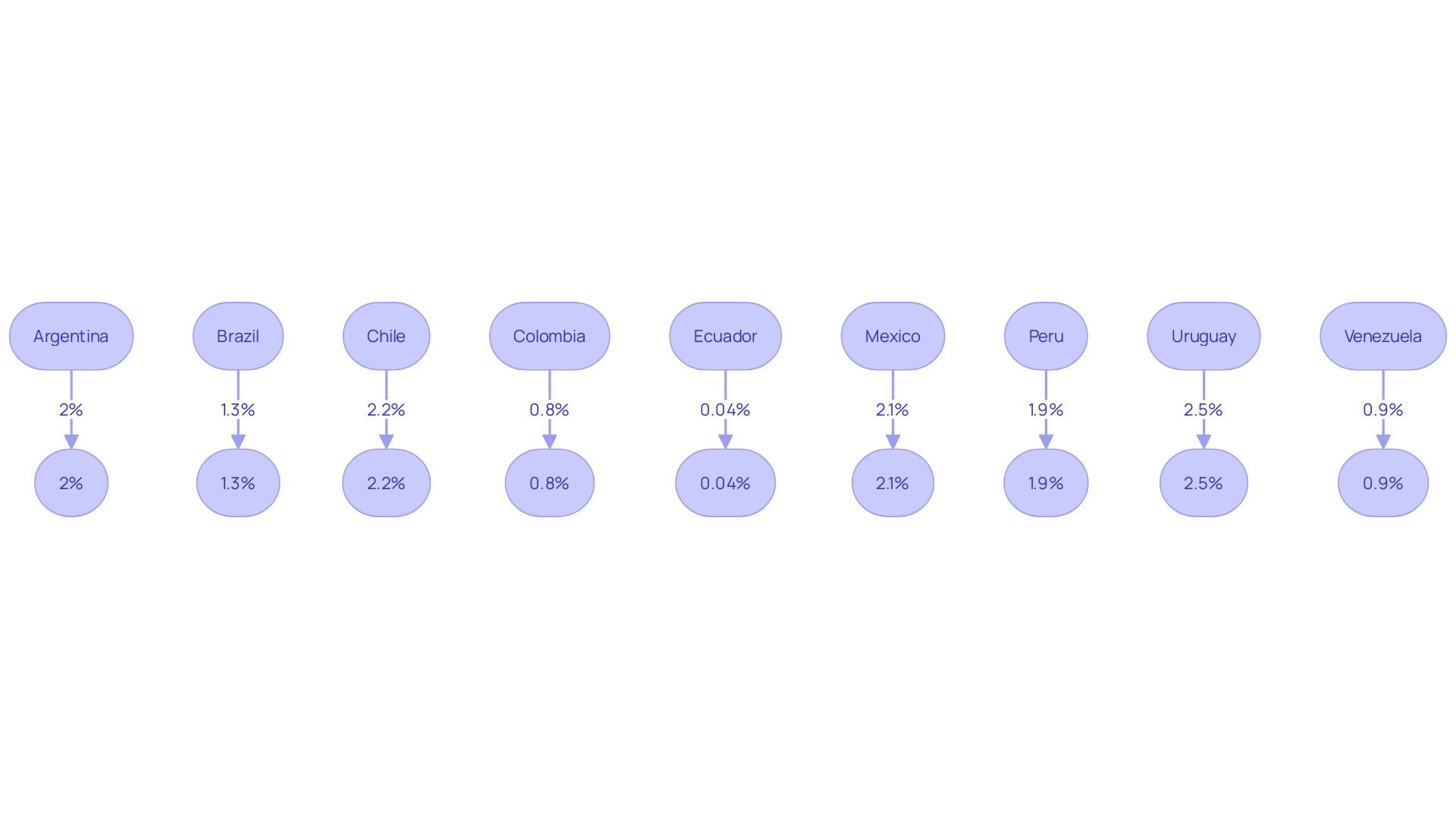
Vida Plena, an advocate for mental health in Latin America, reports staggering figures of mental health disorders in the region, notes the disproportionately high burden borne by low- and middle-income countries (LMICs). Latin America leads globally in depression and anxiety rates, with mental health and substance disorders accounting for significant percentages of total disease burden in these nations. Yet, public investment in mental health remains shockingly low.
This is evidenced by Ecuador dedicating a mere 0.04% of its national healthcare budget to mental health care, significantly less than other countries in the region. The repercussions of this neglect are far-reaching: economic loss due to reduced productivity and absenteeism, and dire impacts on areas as broad as cognitive function, education, and financial stability. This context mirrors the complications faced in clinical research, where regulations and bureaucratic hurdles in Latin America can pose additional barriers for participants, akin to the challenges faced by a rural Pennsylvania patient needing to travel to Turkey for a clinical trial.
Managing logistics in unfamiliar regulatory environments poses daunting hurdles. In light of this, Linical's Resource Center emerges as a critical lifeline, providing thorough guidance and tools to help sponsors and CROs navigate these complex regulatory landscapes, ensuring that clinical trials can proceed smoothly, and ultimately, contribute to better treatment outcomes and improved mental health in the region.
MDCG 2021-6
The issuance of MDCG 2021-6, a guidance document from the Medical Device Coordination Group, signifies a fundamental step in the application of the EU Medical Devices Regulation. For clinical trials positioned in Latin America, it provides a crucial framework, particularly when dealing with medical devices that encompass drug-device combinations, products that include devices as part of their packaging, or devices that incorporate ancillary medicinal substances. This guidance comes at a time when the emerging mental health crisis in Latin America presents a potent opportunity for medical innovation and the imperative integration of medical devices into treatment regimens.
Studies like the Wellcome Global Monitor on Mental Health indicate that Latin America bears the highest global rates of depression and anxiety, deepening interest in medical devices that can ameliorate this burden. As noted by industry experts, the proliferation of novel medical device applications, sometimes in software form such as Sand, is driving regulatory bodies to evolve and adapt at a fast pace to ensure safety and efficacy while navigating a burgeoning public health challenge. The strategic application of MDCG 2021-6 within Latin American contexts reflects a conscientious approach to tackling this healthcare segment, marrying robust regulatory oversight with a pressing healthcare need.
Introduction of new safety measures that ease the establishment of product defect further underscores the importance of navigating the unique regulatory terrain that balances innovation with patient safety.
Tufts CSDD Articles
The phenomenon of 'helicopter research,' wherein well-resourced scientists from developed areas conduct studies in less affluent regions with minimal local involvement, has been a longstanding concern in global health circles. It paints a familiar picture: large, privileged institutions embark on projects such as comprehensive cancer research grants, bringing technologies and methods often seen as avant-garde. Yet, despite the promising collaborative facade, these interactions may inadvertently perpetuate inequities.
Local researchers find themselves relegated to peripheral roles and the local community's voice—especially in regions of Latin America—is often marginalized or even overlooked. The engagement is short-lived, akin to a military foray than a sustained partnership, and the distribution of funds frequently benefits already well-compensated faculty and ignores the need for a diverse leadership team, including adequate representation for underprivileged backgrounds. This lack of cultural sensitivity and equity within research collaborations not only occurs in international settings but also domestically, reflecting a broader issue of disparity within the field.
Addressing these challenges is essential to build truly collaborative and sustainable research endeavors in Latin America and beyond. Economist Impact's Health Policy Practice manager, Kati, who holds a Master in Science in Global Health from Georgetown University, emphasizes the necessity to raise awareness of such issues in Latin America.
Conclusion
In conclusion, conducting early-stage clinical trials in Latin America offers unique opportunities for researchers but requires a detailed understanding of the varied regulatory landscapes. Regulatory entities like ANVISA and MAPA play a pivotal role in establishing standards and ensuring product safety. Novineon, a leading clinical research outsourcing company, has successfully navigated these regulatory processes by leveraging local expertise and forming strategic partnerships.
Contract Research Organizations (CROs) serve as crucial bridges in developing innovative treatments, refining trial designs, and enhancing operational efficiency. Patient-centric trials and electronic data capture technologies further contribute to improved outcomes.
Case studies of RP-CRO and PMC highlight the importance of localized understanding and partnerships in navigating regulatory landscapes and achieving trial objectives. Researchers and sponsors should also be familiar with the FDA's guidance on Institutional Review Boards (IRBs) and the EU Clinical Trials Regulation when conducting trials in Latin America.
The region's mental health challenges necessitate the development of an open science ecosystem and the integration of AI strategies to enhance research capacity. By following these principles and adopting a patient-centric approach, researchers can navigate the regulatory environments successfully and contribute to improved healthcare outcomes in Latin America.




