Introduction
The De Novo pathway is a crucial classification process that enables the introduction of novel medical devices into the market. This pathway is specifically designed for devices that do not have a comparable predecessor and have a low to moderate risk profile. By providing a clear and comprehensive submission to the U.S. Food and Drug Administration (FDA), manufacturers can navigate this pathway and showcase the safety and effectiveness of their innovative devices.
In this article, we will explore the eligibility criteria, key differences between the De Novo and 510(k) pathways, submission pathways, components of a De Novo submission, the review process, risk assessment and mitigation, as well as the benefits and challenges of the De Novo pathway. We will also discuss best practices for a successful De Novo classification, highlighting the importance of thorough research, documentation, proactive engagement with the FDA, and strategic risk mitigation. Join us as we delve into the intricacies of the De Novo pathway and its role in driving medical innovation.
What is the De Novo Pathway?
The De Novo classification process offers a pathway for instruments that lack a comparable predecessor on the market, providing a vital avenue for novel tools to demonstrate their safety and effectiveness. This pathway is crucial for gadgets that are the initial ones in their category, thereby not meeting the existing predicate criteria. The procedure includes a thorough assessment by the U.S. Food and Drug Administration (FDA), an organization within the Department of Health and Human Services, dedicated to protecting public health by ensuring the safety and effectiveness of human and veterinary drugs, biological products, and equipment.
One significant case demonstrating the significance of the De pathway is the progress of technologies for managing chronic diseases like diabetes, which impacts over 11% of the American population. Innovations such as automated insulin delivery systems have emerged, offering new possibilities for treatment that can reduce the burden of manual blood glucose regulation and insulin administration. These technologies demonstrate the process's ability to support the introduction of innovative healthcare equipment that has a significant effect on patient care.
The FDA's categorization of medical equipment into three tiers, each corresponding to different risk profiles, determines the pathway manufacturers must pursue for market entry. Devices deemed to have the highest level of patient risk, such as certain Software as a Medical Device (SaMD) applications, may fall under Class III. The FDA's comprehensive classification database and regulatory framework guide manufacturers in identifying the appropriate classification and subsequent registration pathway, whether it be Premarket Notification (510(k)), Pre-Market Approval (PMA), or another classification procedure. In order to sell a product in the United States, it must receive FDA Clearance, Approval, or, in the case of De Novo, be Granted.
The De Novo submission requires detailed information about the equipment, including its intended use, patient population, specifications, and functional components. This information must be presented coherently with pictorial representations, gadget specifications, and engineering drawings, if applicable. Furthermore, the submission should acknowledge alternative practices and procedures for the condition that the apparatus aims to address. A clear understanding of these elements is not only critical for FDA assessment but also ensures transparency and informed decision-making for healthcare providers and patients.
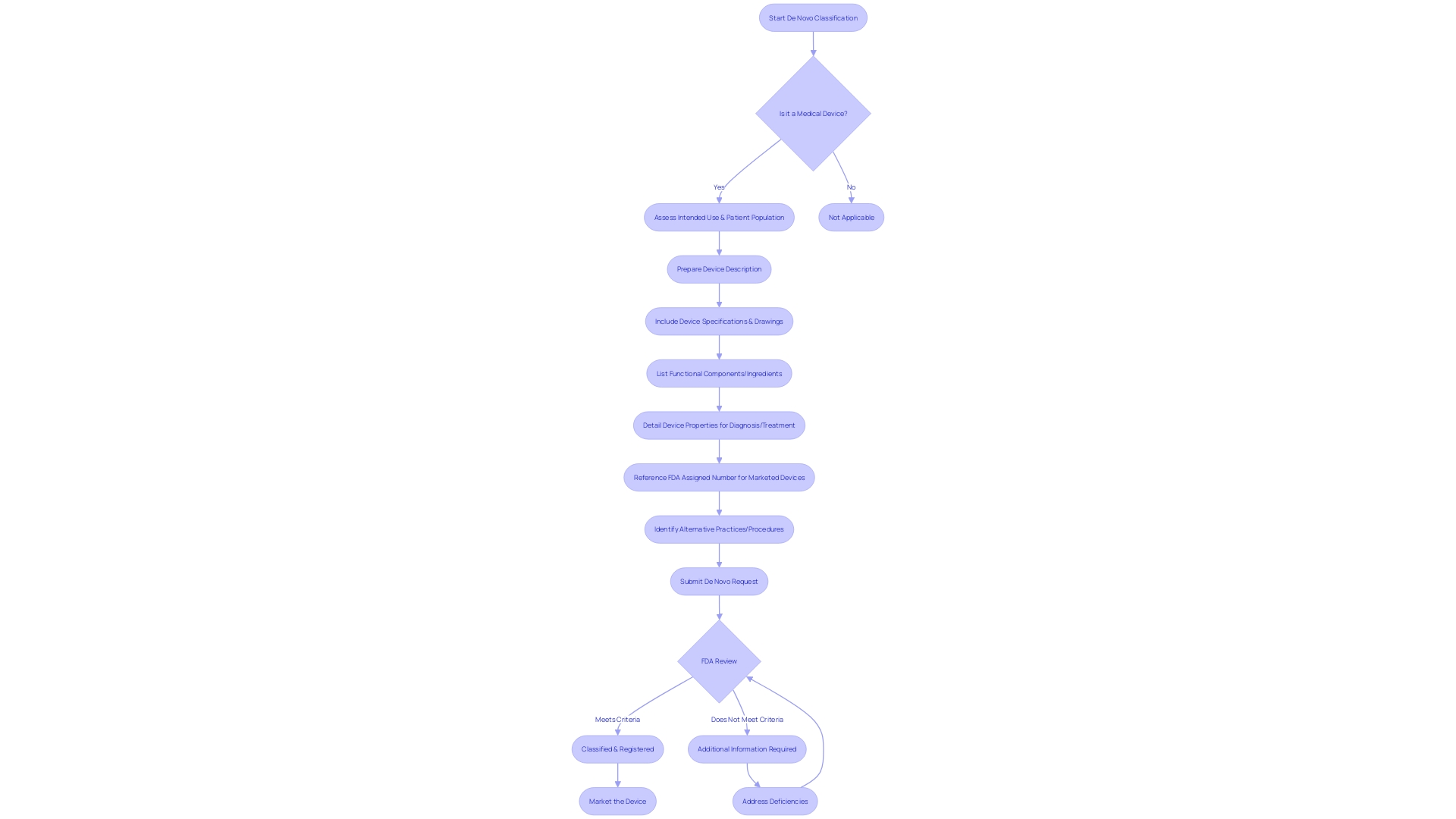
Eligibility Criteria for De Novo Classification
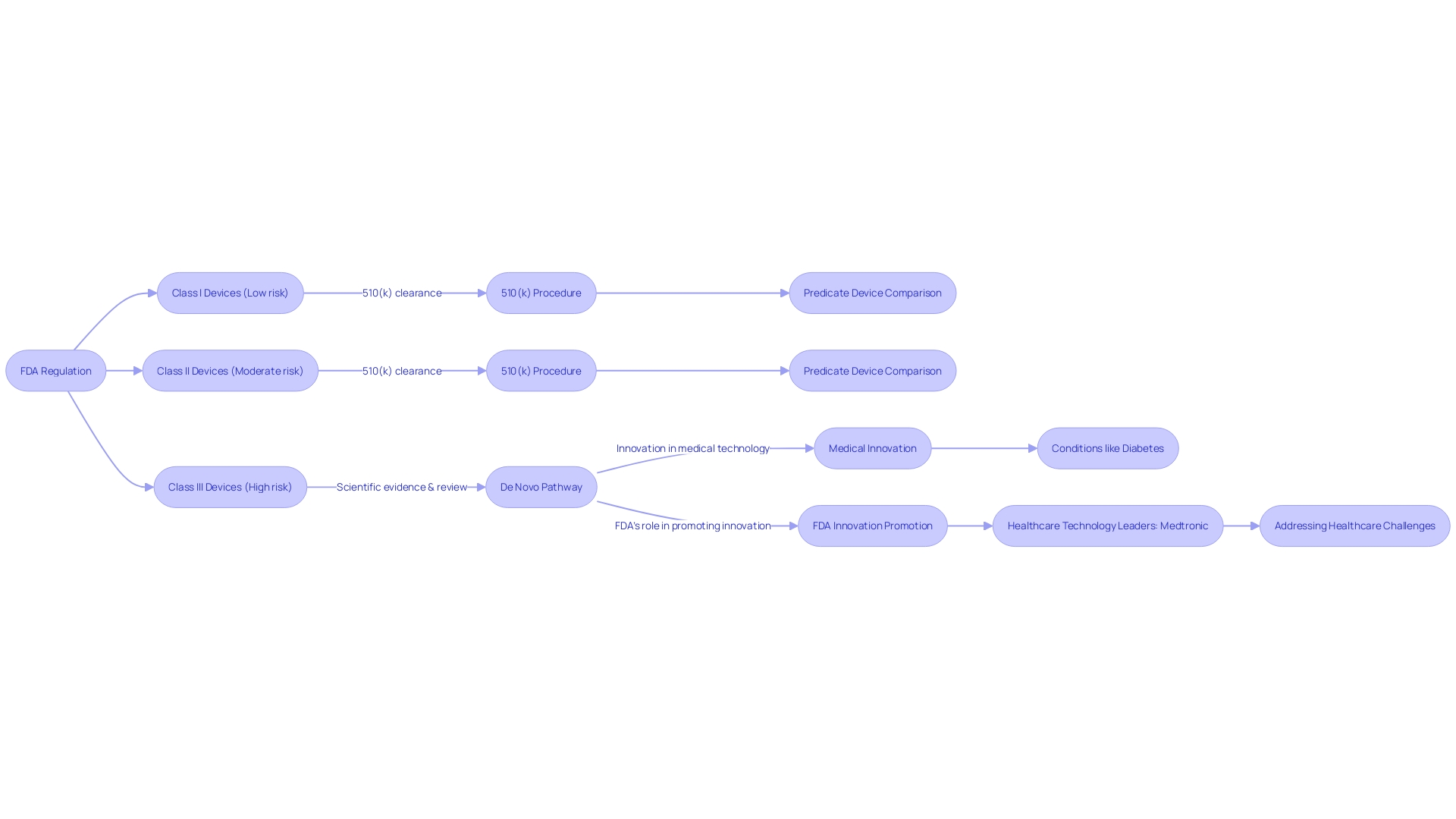
In order for a healthcare instrument to meet the requirements for classification, it must be unique, indicating that there is no currently available or lawfully marketed precedent. Additionally, it must have a risk profile that the FDA deems to be low to moderate. The evidence to support its safety and efficacy should be robust and scientifically sound. An excellent illustration of an object that may undergo the De Novo pathway is a Software as a Medical Device (SaMD) that meets the FDA's criteria for a Class III object. The categorization of a tool can be established by comparing the tool's description against the FDA's classification database or the Code of Federal Regulations (CFR), Title 21, Parts 862-892, which depict more than 1,700 tool types by area of expertise.
To navigate the regulatory pathways for medical equipment, which are determined by their risk classification and the necessary controls to mitigate health risks, one must understand the FDA's categorization into Class I (low risk), Class II (moderate risk), or Class III (high risk). For instance, the majority of neurological apparatuses are categorized as Class II or Class III. These classifications are important when considering the De Novo process, as they impact the level of evidence and documentation required for approval. The FDA's guidance and webinars, such as those concerning Implanted Brain-Computer Interface (BCI) Devices, offer stakeholders with insights into the regulatory considerations and pathways for introducing healthcare instruments to the market.
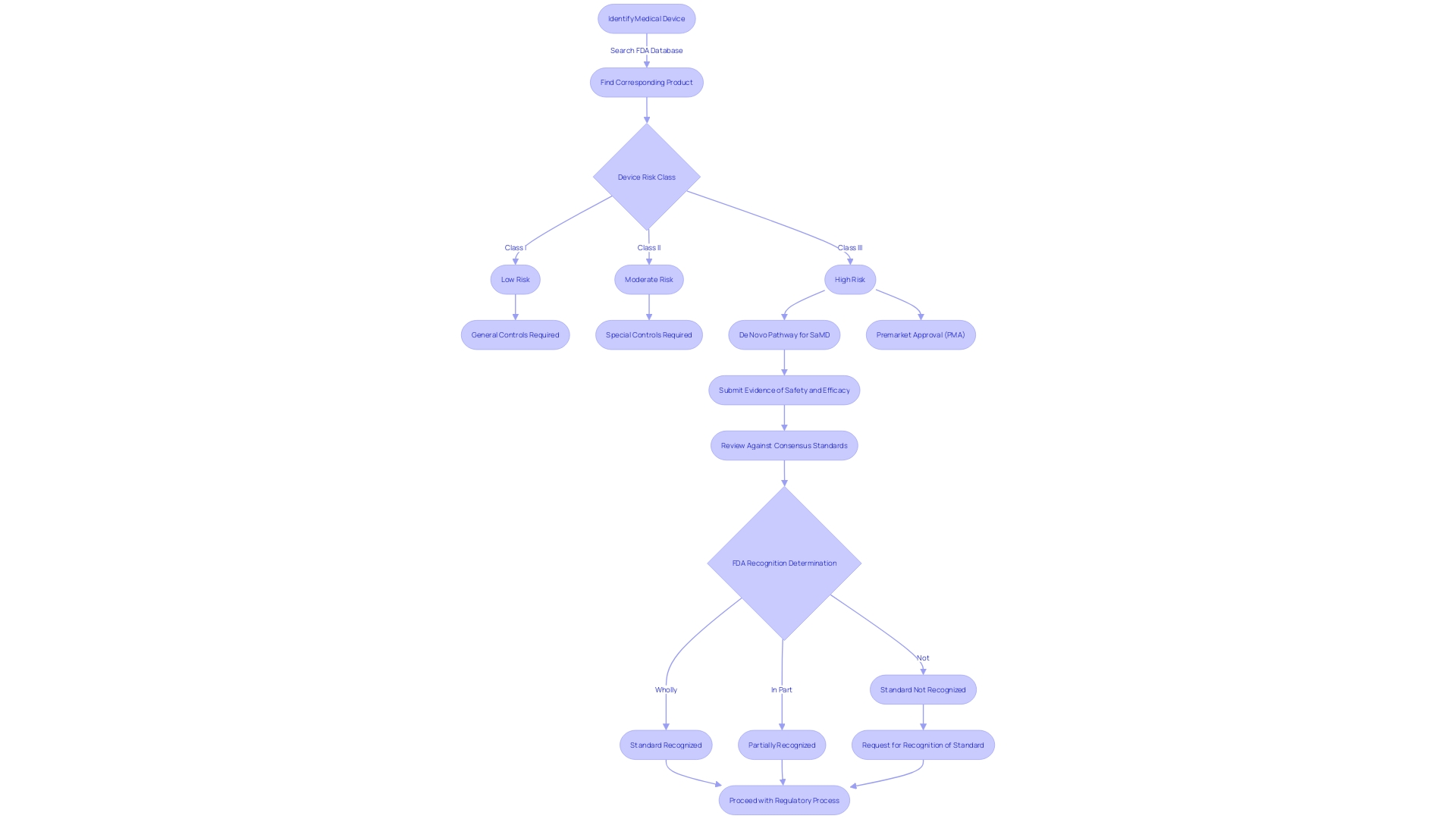
Key Differences Between De Novo and 510(k) Pathways
Understanding the different classifications and processes involved is necessary when navigating the regulatory pathways of the FDA for medical products. Medical instruments are categorized into three classes according to the level of risk to the patient, ranging from least to most: Class I, II, and III. The pathway designed for new products that lack a comparable existing device and are considered to have low to moderate risk, usually falling under Class I or II.
In contrast to the De Novo approach, the 510(k) pathway enables devices of any risk level to achieve clearance by demonstrating substantial equivalence to an existing, legally marketed device, commonly referred to as a predicate. This pathway is often pursued for Class II products, although it can be applicable to some Class I and III items as well.
The process of starting from scratch is crucial for advancing medical innovation, as it offers a pathway for groundbreaking equipment that lacks a predecessor but still presents a low to moderate risk profile. As part of the De Novo submission, detailed information regarding the apparatus's intended use, specifications, and its components or ingredients is provided. This includes a general description of the medical equipment or apparatus is designed to address, as well as the patient population it serves.
In the broader context of public health and safety, the FDA ensures that such information is presented in a manner that is clear and easily understandable to consumers, emphasizing the importance of transparency and accessibility in the regulatory process. This approach is in line with recent FDA standards for the clear and neutral presentation of information in direct-to-consumer advertising.
Furthermore, the organization's dedication to utilizing private sector knowledge through Voluntary Consensus Standards reflects the advantages observed in the healthcare equipment industry. These standards can streamline the development process and focus the FDA's review on the application's results, rather than the minutiae of protocol design.
In general, the new pathway facilitates the introduction of innovative healthcare tools into the market, ensuring patient safety while promoting technological progress and providing new treatment options that can have a significant impact on healthcare outcomes.
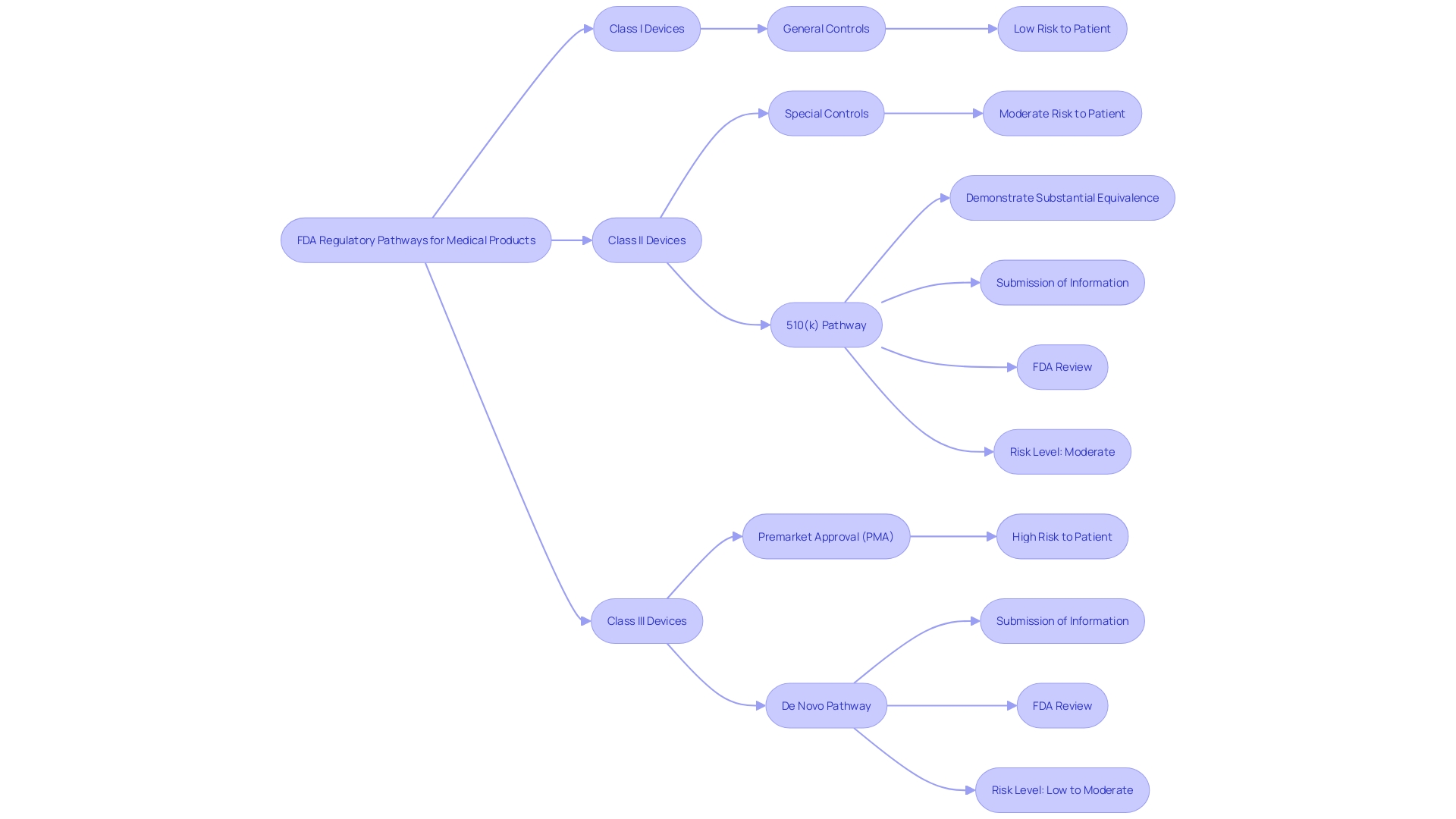
Submission Pathways for De Novo Requests
The FDA's classification process provides two separate submission routes for medical manufacturers: the direct pathway and the indirect pathway via a 510(k) premarket notification. Using the direct route, manufacturers can submit a request without a prior classification decision from the FDA. This path is usually chosen when there is no pre existing predicate object to refer to, which is often the situation with innovative technologies that may introduce new ethical, legal, or social considerations. Conversely, the indirect pathway involves initially submitting a 510(k) notification. If the FDA determines that the product does not have a substantially equivalent predicate, it may then advise the manufacturer to proceed with a De Novo request.
In either scenario, a comprehensive submission must be prepared. This includes a detailed description of the equipment's intended use, such as the condition it diagnoses, treats, prevents, cures, or mitigates, and the patient population it serves. Manufacturers need to provide specifications, engineering drawings, and details about each component or ingredient if the item is not singular in its construction. The submission should also explain the properties of the apparatus and how they relate to its intended medical function. Moreover, any FDA-assigned reference numbers for lawfully sold items meant for utilization with the new product must be included. An understanding of alternative practices and the international regulatory context adds depth to the submission, ensuring that the apparatus aligns with existing treatments and considers international standards for ethics and governance.
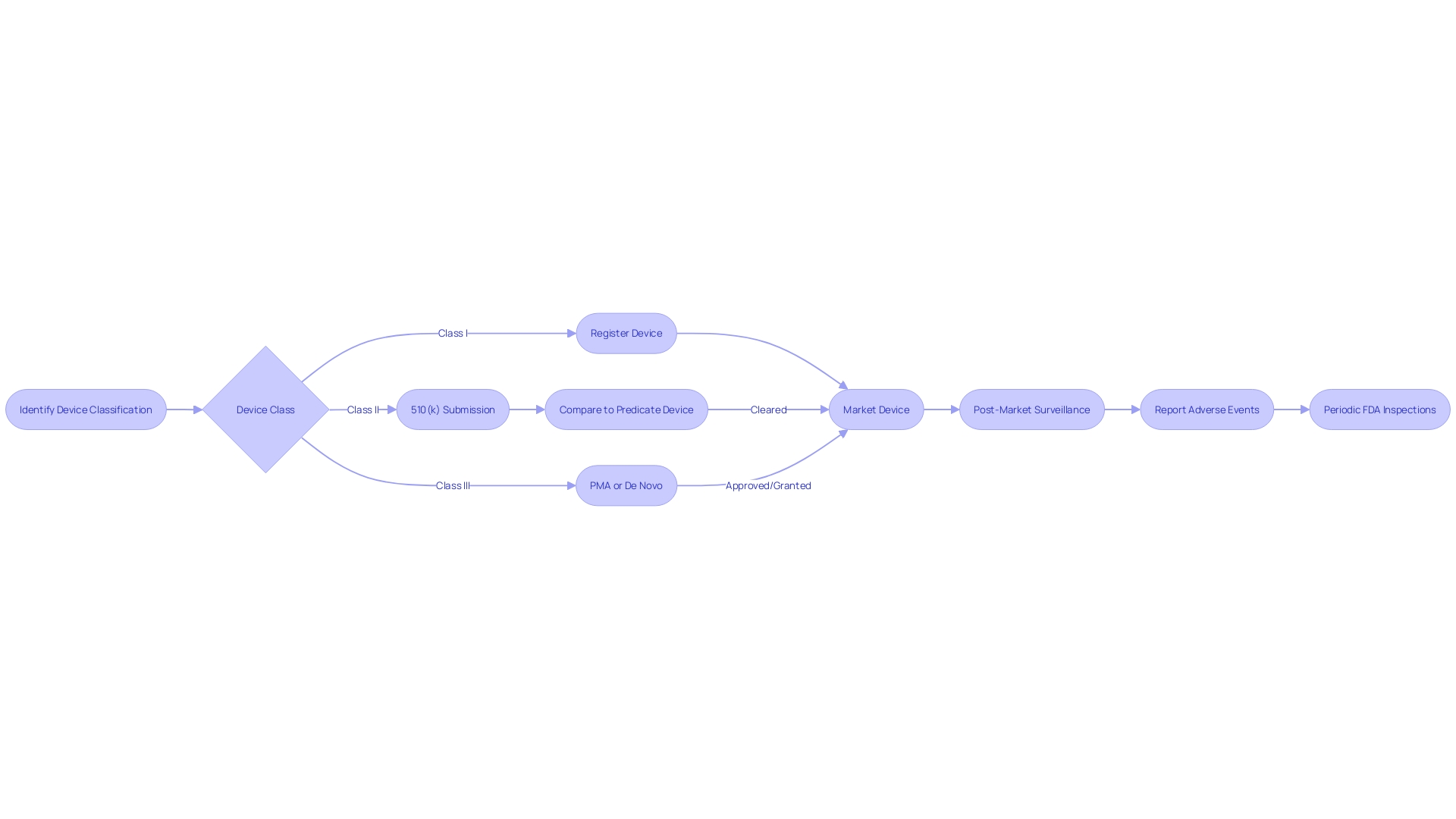
Components of a De Novo Submission
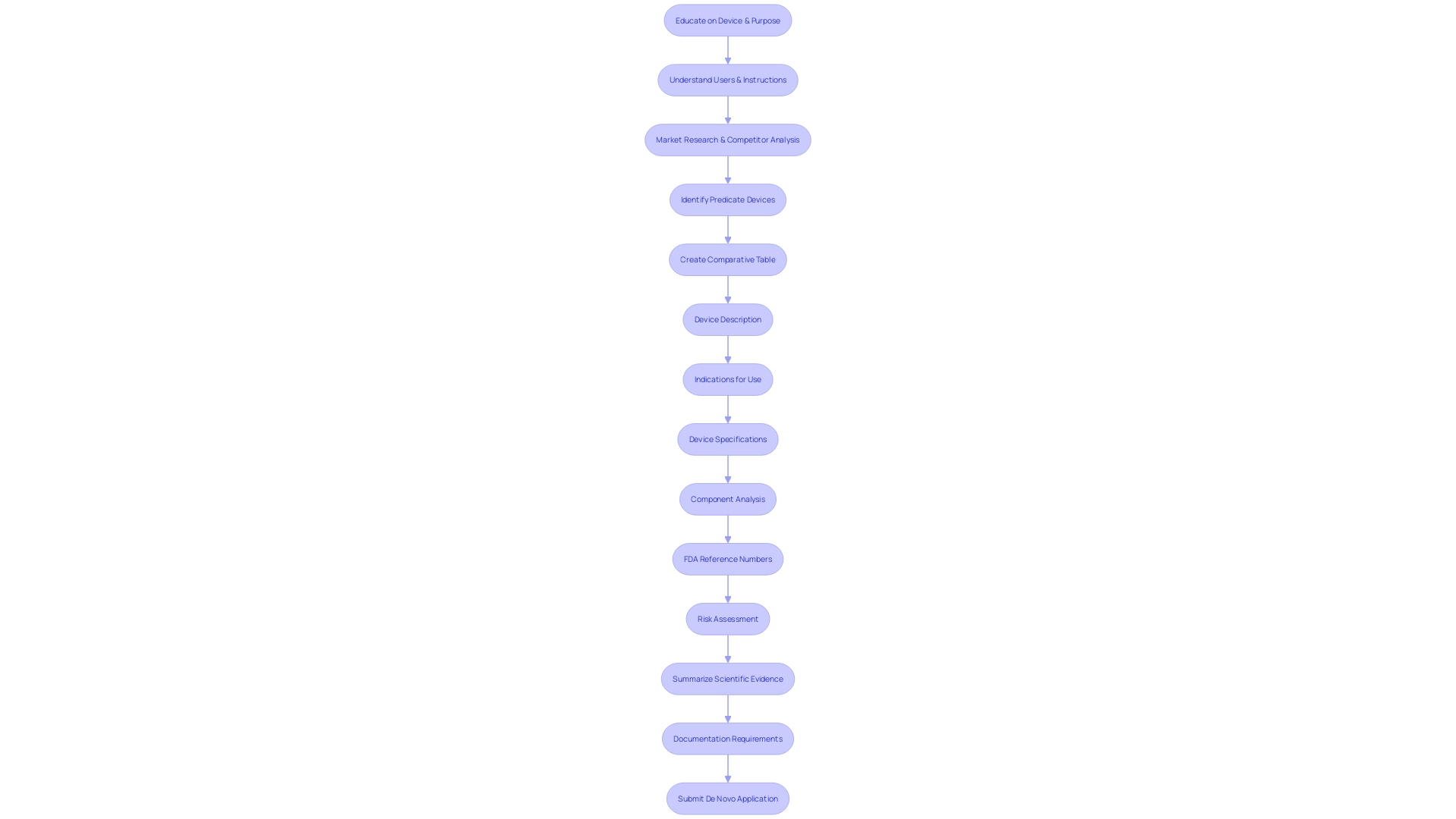
When preparing a De Novo submission for a medical product, it is crucial to provide comprehensive information that enables the FDA to conduct a thorough evaluation of the product's safety and efficacy. The submission should include a comprehensive description of the apparatus, featuring pictorial representations, specifications, and engineering drawings when applicable. It is crucial to outline the intended use of the apparatus, including the patient population it serves and all labeled uses, whether prescription or over-the-counter.
A risk assessment must be conducted to ensure the design and manufacturing processes of the equipment are sound and under control throughout their lifecycle. This assessment should include process validation studies that are representative of the production controls for the equipment. To ensure the safety and effectiveness of the equipment, summaries of scientific evidence must be provided, along with labeling information that clearly states the equipment's generic and proprietary names, indications for use, and the properties relevant to its intended role in healthcare.
Furthermore, the submission must include information on each of the apparatus's functional components or ingredients, particularly if the apparatus consists of multiple physical elements. It is also necessary to include the FDA assigned reference number(s) for any legally marketed instruments intended for use with the new equipment. The documentation should address any alternative practices and procedures known to the requester and provide a rationale for the introduction of the product to the market.
Recent FDA guidelines stress the importance of clear, conspicuous, and neutral presentation of information, including risks and side effects, in consumer-friendly language. This applies to both audio and visual components of advertisements, ensuring the information is easily understandable and accessible. As emphasized by the FDA's requirement, every stage in the procedure, from conception to end result, must prioritize the safety, effectiveness, and security of medical instruments to safeguard public health.
Review Process for De Novo Classification
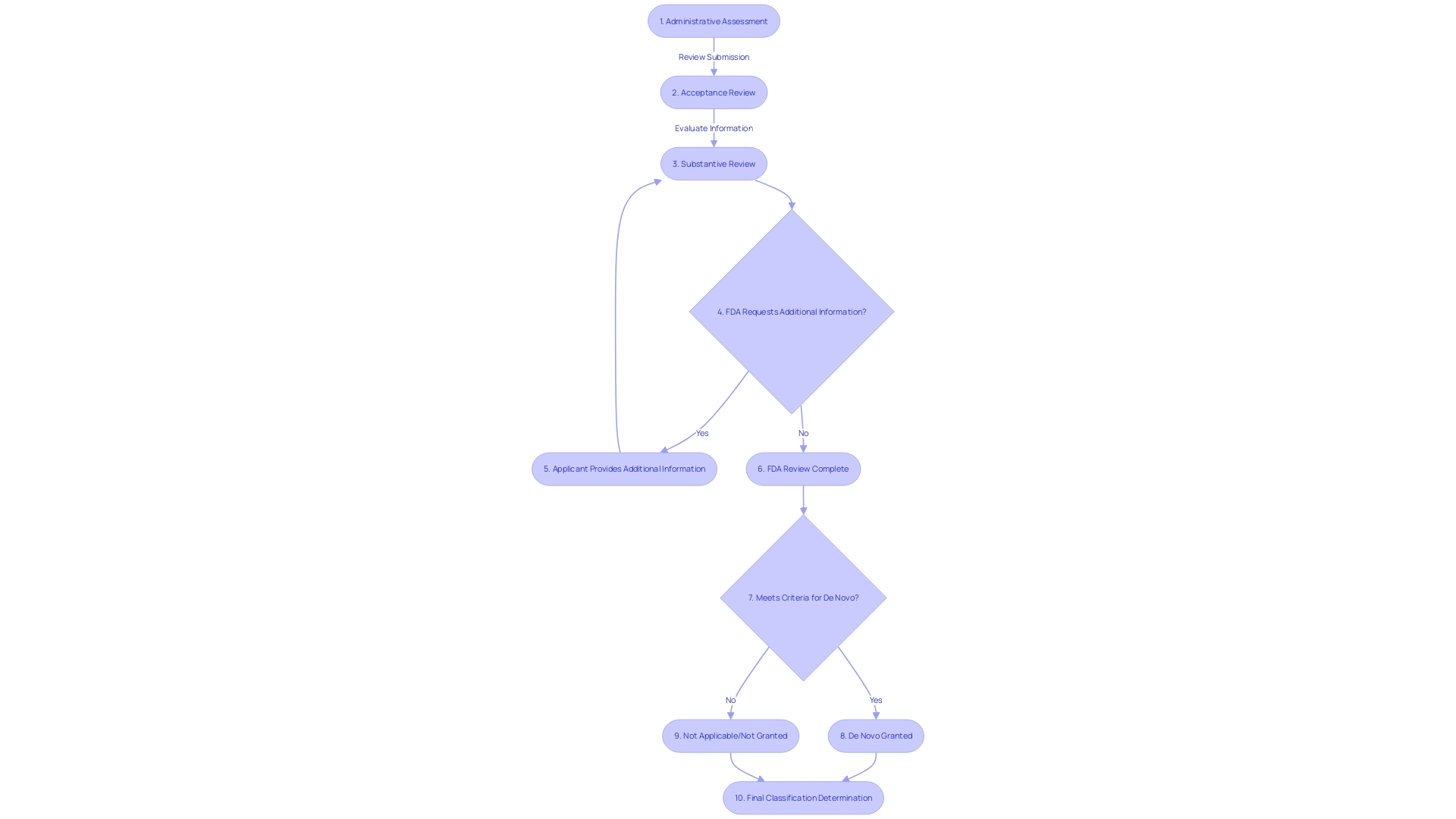
The De Novo classification review process by the FDA unfolds through a structured multi-stage approach. Initially, the FDA conducts a thorough administrative assessment to verify that the submission is comprehensive and adheres to the required standards. Afterwards, the agency thoroughly assesses the safety and efficacy of the product, relying on the scientific evidence provided. This crucial step may entail the examination of clinical data, bench testing, and other pertinent information. Upon finishing this evaluation, the FDA reaches a classification determination, establishing the essential regulatory controls for the product. These evaluations are crucial to ensuring that products meet the necessary criteria for safety and effectiveness before being introduced to the market. This methodical process is exemplified by the development of adaptive trial designs for establishing bioequivalence in highly variable drugs, which underscores the FDA's commitment to rigorous scientific inquiry and public health safeguards.
Risk Assessment and Mitigation in De Novo Submissions
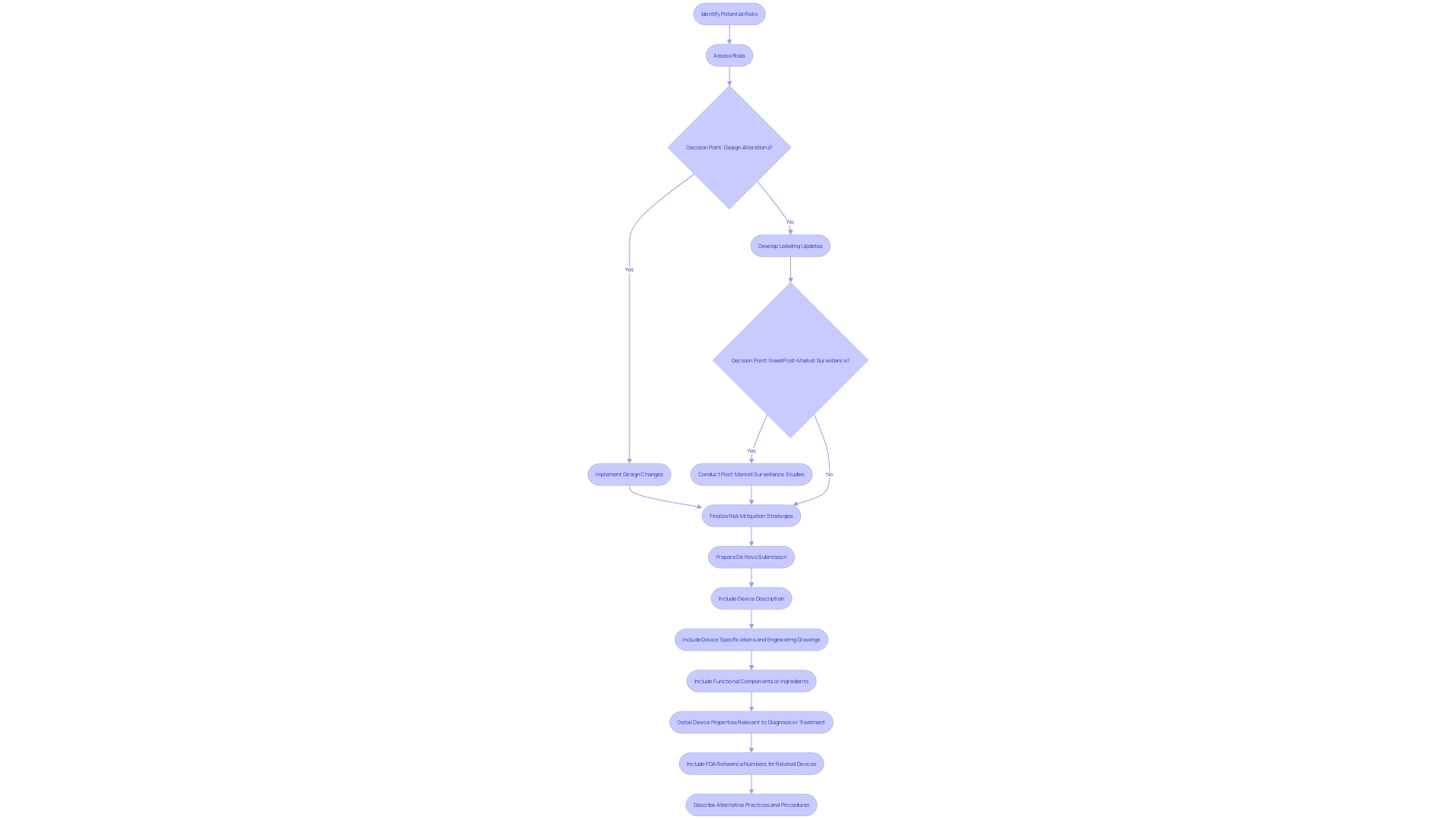
Risk management is a fundamental component of the De Novo submission process. Manufacturers of medical equipment are assigned with the meticulous job of identifying potential risks associated with their products. More than just identifying, they must assess these risks and develop robust mitigation strategies. These strategies may encompass a spectrum of actions, including but not limited to, design alterations, updates to labeling, and the initiation of post-market surveillance studies to continually monitor the tool's performance.
The FDA's role is to thoroughly review these risk mitigation proposals. The evaluation is to ensure that the strategies are not only appropriate but also that they comprehensively address all potential risks, safeguarding the instrument's safety and efficacy. For example, recent FDA guidance on the presentation of major side effects in direct-to-consumer advertisements underscores the importance of clarity and neutrality, principles that are equally relevant in the context of De Novo submissions, where the communication of risks must be unambiguous and balanced.
Manufacturers must provide a detailed description of the product, its components, and its intended use, including the patient population it serves. This information should be supported by pictorial representations, specifications, and engineering drawings where applicable. It is crucial for manufacturers to also include the characteristics of the product relevant to its intended therapeutic effect or its interaction with the body's structure or function. Furthermore, reference numbers for associated FDA-approved equipment should be included to streamline the review process.
The FDA's responsibility to ensure the safety, effectiveness, and security of healthcare equipment is a fundamental aspect of its mission, as demonstrated in the agency's recent activities and communications. Producers participating in the process must align their risk assessment procedures with the FDA's standards, ensuring that their products meet the strict requirements set forth to safeguard public health.
Benefits and Challenges of the De Novo Pathway
Understanding the classifications and pathways established by the FDA is essential when navigating the regulatory environment for healthcare equipment. The pathway serves as an important route for new healthcare instruments that do not have a similar precursor. It stands out as a mechanism that facilitates the introduction of groundbreaking technologies to the healthcare market, which are often essential for addressing unmet medical needs. While Class I and II products may be subject to less strict regulations and are usually approved through a 510(k) procedure, the pathway called De Novo is especially important for certain Class I and all Class III items that pose a moderate to high risk.
This pathway requires significant scientific evidence to authenticate the safety and effectiveness of an instrument. It is a more involved process than the 510(k) pathway and may result in longer review times. Despite these challenges, the De Novo pathway is instrumental in the advancement of medical innovation, especially for conditions requiring continuous management, such as diabetes. For example, the FDA's approval of new insulin delivery systems that automate manual tasks demonstrates the potential of De Novo-approved technology to greatly reduce the burden on patients with chronic diseases.
The FDA's dedication to promoting innovation in healthcare technology corresponds with the efforts of healthcare technology leaders like Medtronic, who strive to address some of healthcare's most pressing challenges. The all-encompassing approach to safety and effectiveness of equipment ensures that patients receive the most advanced health care possible. As the field of healthcare technology progresses, the unique pathway will continue to have a crucial role in providing innovative solutions to patients worldwide.
Best Practices for Successful De Novo Classification
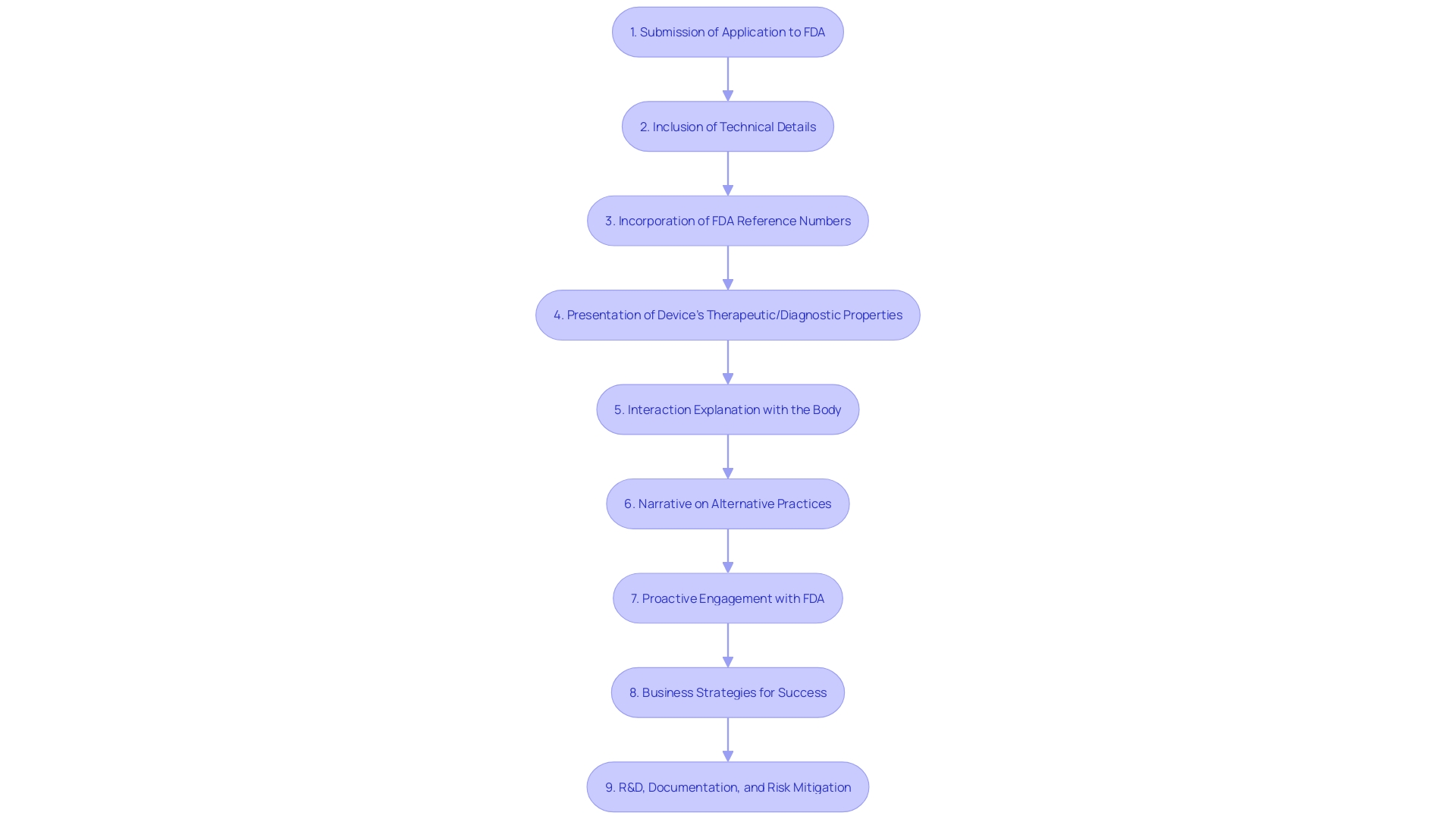
For manufacturers aiming for De Novo classification of their medical products, it is crucial to ensure a comprehensive and well-structured application to the FDA. This requires a careful approach starting with a precise expression of the tool's intended purpose, including a thorough explanation of the condition it targets and the demographic of patients it caters to. The submission should encompass the specifications of the equipment, engineering drawings, and, if applicable, a depiction of each of its components. Equally significant is the incorporation of any applicable FDA reference numbers for lawfully marketed accessories or components intended for use with the equipment.
Apart from these technical details, manufacturers must also present the properties of the equipment relevant to its therapeutic or diagnostic role. This should involve an explanation of how the apparatus interacts with the body to achieve its intended purpose. A narrative on alternative practices and procedures for the condition in question should be included to provide context on the instrument's place within the current healthcare landscape.
To enhance the strength of the submission, companies should proactively engage with the FDA. This can lead to valuable insights and guidance on regulatory requirements, helping to refine the submission process. Medtronic's approach exemplifies this proactive stance, with their commitment to confronting significant health challenges and providing insight-driven care as illustrated by their wide array of healthcare technologies that serve diverse health conditions.
Furthermore, successful medical instrument companies, as pointed out by an experienced business development leader, frequently share traits like resilience and confidence, but also follow business strategies that lead to profitable ventures through acquisitions, IPOs, or strategic alliances. This combination of business expertise and regulatory knowledge is crucial for companies looking for a new classification.
In conclusion, the pathway, while offering a route to market for novel devices, requires a thorough and strategic approach. Comprehensive research and development, detailed documentation, and proactive risk mitigation are key factors that, when combined with early FDA engagement, can significantly increase the likelihood of a successful De Novo classification.
Conclusion
The De Novo pathway is a crucial classification process that enables the introduction of novel medical devices into the market. It provides a pathway for devices that lack a comparable predecessor and have a low to moderate risk profile. Manufacturers must provide a clear and comprehensive submission to the FDA to showcase the safety and effectiveness of their innovative devices.
To qualify for De Novo classification, a medical device must be novel and have a low to moderate risk profile. Understanding the FDA's classification system and the necessary controls for different risk levels is essential for navigating the De Novo process.
The De Novo pathway differs from the 510(k) pathway, as it is tailored for devices without a comparable predecessor and with a low to moderate risk profile. The De Novo pathway is critical for advancing medical innovation and offering new treatment options that can significantly impact healthcare outcomes.
Manufacturers must provide a comprehensive submission that includes detailed information about the device's intended use, specifications, components, and properties relevant to its medical role. Understanding alternative practices and the international regulatory context is crucial for a successful submission.
In conclusion, the De Novo pathway is a critical classification process for innovative medical devices. Thorough research, documentation, proactive engagement with the FDA, and strategic risk mitigation are key factors for a successful De Novo classification. By following these best practices, manufacturers can navigate the regulatory landscape, drive medical innovation, and ensure patient safety.




