Introduction
Design controls are critical processes that guide the creation and management of medical devices, ensuring they meet the highest standards of safety, effectiveness, and quality. These controls encompass the entire lifecycle of a product, from conception to market release and beyond. Incorporating user-centered design is pivotal in this framework, focusing on the unique needs and experiences of all users, not just patients but also clinicians, support staff, and hospital maintenance personnel.
This holistic approach to design accounts for the diverse interactions individuals may have with medical devices, aiming to create seamless and meaningful end-to-end experiences. In this article, we will explore the purpose and phases of design controls, the standards and regulations governing them, the integration of risk management, and the benefits of effective design controls in the medical device industry. We will also highlight the advancements in testing methods and facilities that support innovation and ensure the safety and efficacy of medical devices.
What Are Design Controls?
Design controls are essential procedures that direct the creation and management of healthcare equipment, guaranteeing they adhere to the utmost standards of safety, effectiveness, and quality. These controls encompass the entire lifecycle of a product, from conception to market release and beyond. Incorporating user-centered approach is pivotal in this framework, focusing on the unique needs and experiences of all users, not just patients but also clinicians, support staff, and hospital maintenance personnel. This comprehensive approach to creating takes into consideration the varied interactions individuals may have with medical tools, with the goal of developing seamless and significant end-to-end experiences.
Service planning, in particular, plays a significant role in addressing the comprehensive requirements of various stakeholders within healthcare settings. It highlights the significance of comprehending behaviors, requirements, and motivations to create products that are not only influential but also connect with all individuals. Moreover, robust user research, usability testing, and iterative design processes are essential for refining devices in the medical field, ensuring they align with specific user touchpoints and needs effectively.
Aligned with the drive for advancement and safety in the sector, establishments such as UL Solutions' laboratory in Rochester Hills, Michigan, are enhancing testing techniques to meet the evolving needs of manufacturers in the healthcare industry. This progress promotes the sector's expansion by addressing concerns associated with excellence, security, and digital protection, thereby ensuring the welfare of beneficiaries and sustaining the pace of technological advancement.
Purpose of Design Controls
Design controls are a fundamental aspect in the development of medical products, addressing the complete range of individual requirements and ensuring compliance with strict regulations. These controls encapsulate a comprehensive approach that begins with the inception of a device and extends to its practical application within a healthcare environment. In this process, the approach centered on the needs and experiences of all individuals involved, from patients to the clinicians and hospital staff who are vital to patient care, plays a crucial role. It's not just about patient-centered solutions but also about service creation that caters to the ecosystem surrounding the patient, including nurses, doctors, and maintenance staff.
To design healthcare equipment that offers meaningful and applicable interactions, it is crucial to examine the behaviors, requirements, and motivations of these individuals. This involves research, usability testing, and iterative design processes. The objective is to enhance how users engage with healthcare instruments at each interaction, guaranteeing the product adequately fulfills their requirements. When it comes to software development for these gadgets, understanding the device's features, intended use, and potential risks becomes absolutely crucial. Safety, specifically, is an essential factor; all communications must be secure and encrypted, frequently through established protocols like Transport Layer Security (TLS), to comply with regulations related to healthcare equipment.
Additionally, the digitization of healthcare equipment is not just about transforming records into digital formatâit's about intelligent digitization. This process begins with discerning which data is critical and how to deconstruct the work product into actionable data. Effective digitalization serves as a catalyst, propelling not only toward desired goals but also toward necessary outcomes. As emphasized by Mary Joyce, vice-president and general manager of UL Solutions' mobility and critical systems group, the thriving technology sector for healthcare instruments in Michigan showcases the need for advanced safety, security, usability, and interoperability in instruments. UL Solutions' Rochester Hills laboratory is at the forefront of this, offering extensive testing capabilities that adapt to manufacturers' needs and processes aimed at reducing contaminants, thereby addressing potential risks and maintaining the pace of innovation.
Standards and Regulations Governing Design Controls
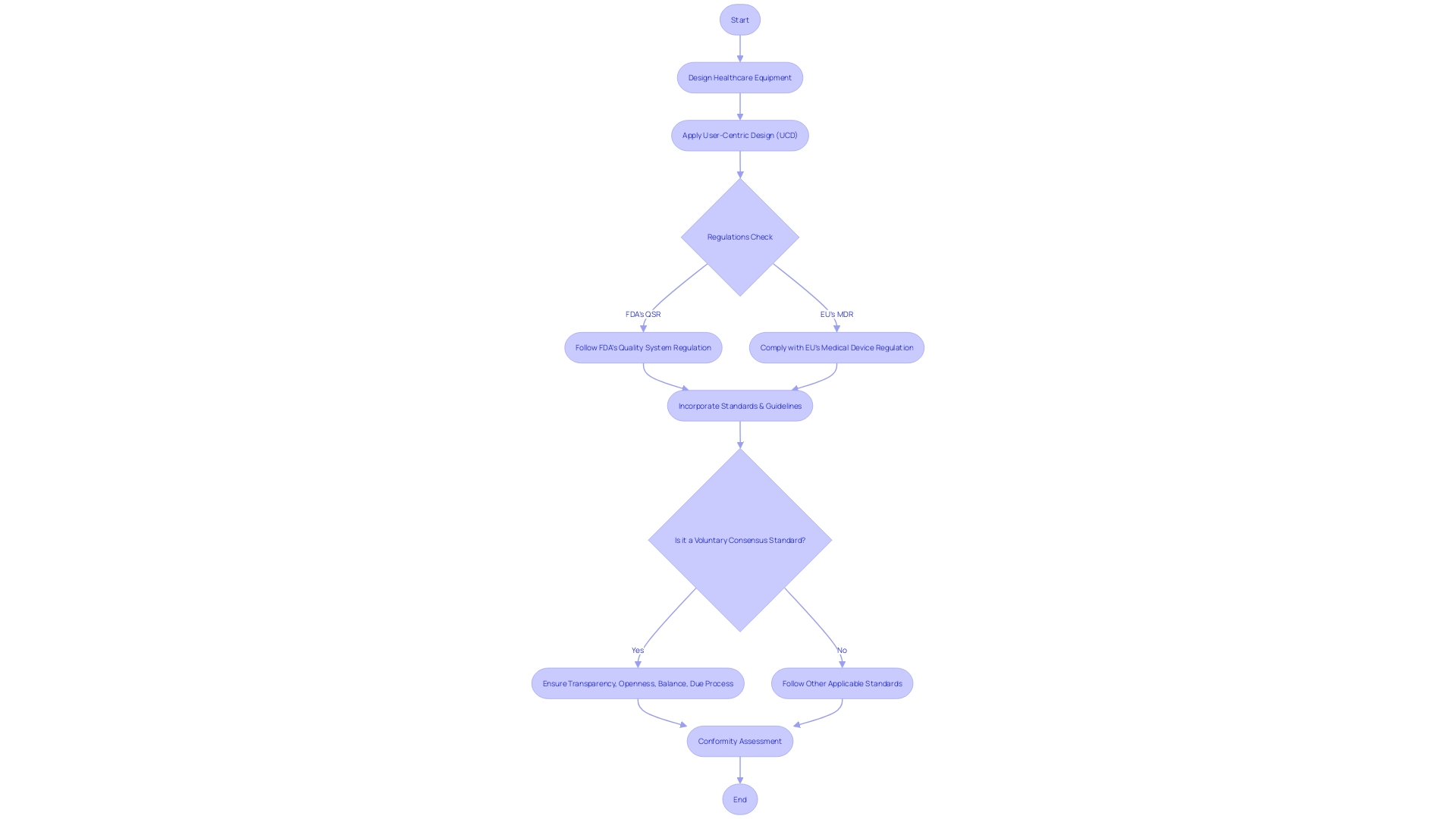
The industry for healthcare equipment follows processes of control, supported by regulations like the FDA's Quality System Regulation (QSR) and the European Union's Medical Device Regulation (MDR). These frameworks mandate precise control over the design process and offer guidance for documenting and executing design-related activities. In this domain, user-centric design (UCD) plays a crucial part, guaranteeing that medical instruments fulfill the requirements of all individuals, including clinicians, patients, and support personnel. UCD focuses on developing tools that improve individual encounters and engagements. This approach is not just about the end user but encompasses the entire spectrum of individuals who come into contact with the tool, including caregivers, maintenance staff, and laboratory technicians. The FDA upholds public health by ensuring the safety and effectiveness of healthcare equipment, and this includes overseeing the design control processes that contribute to high-quality healthcare outcomes. Adherence to OECD guidelines, such as the Conflict Minerals policy, and voluntary consensus standards developed by Standards Development Organizations (SDOs) are essential for maintaining regulatory quality and ensuring that products are safe, effective, and in compliance with federal regulations. These standards and guidelines assist manufacturers in navigating the intricate terrain of product compliance, guaranteeing that their products are responsibly sourced and meet the demands of a dynamic global market. By incorporating user-centered principles and adhering to rigorous standards, the industry aims to improve patient care and support the healthcare ecosystem.
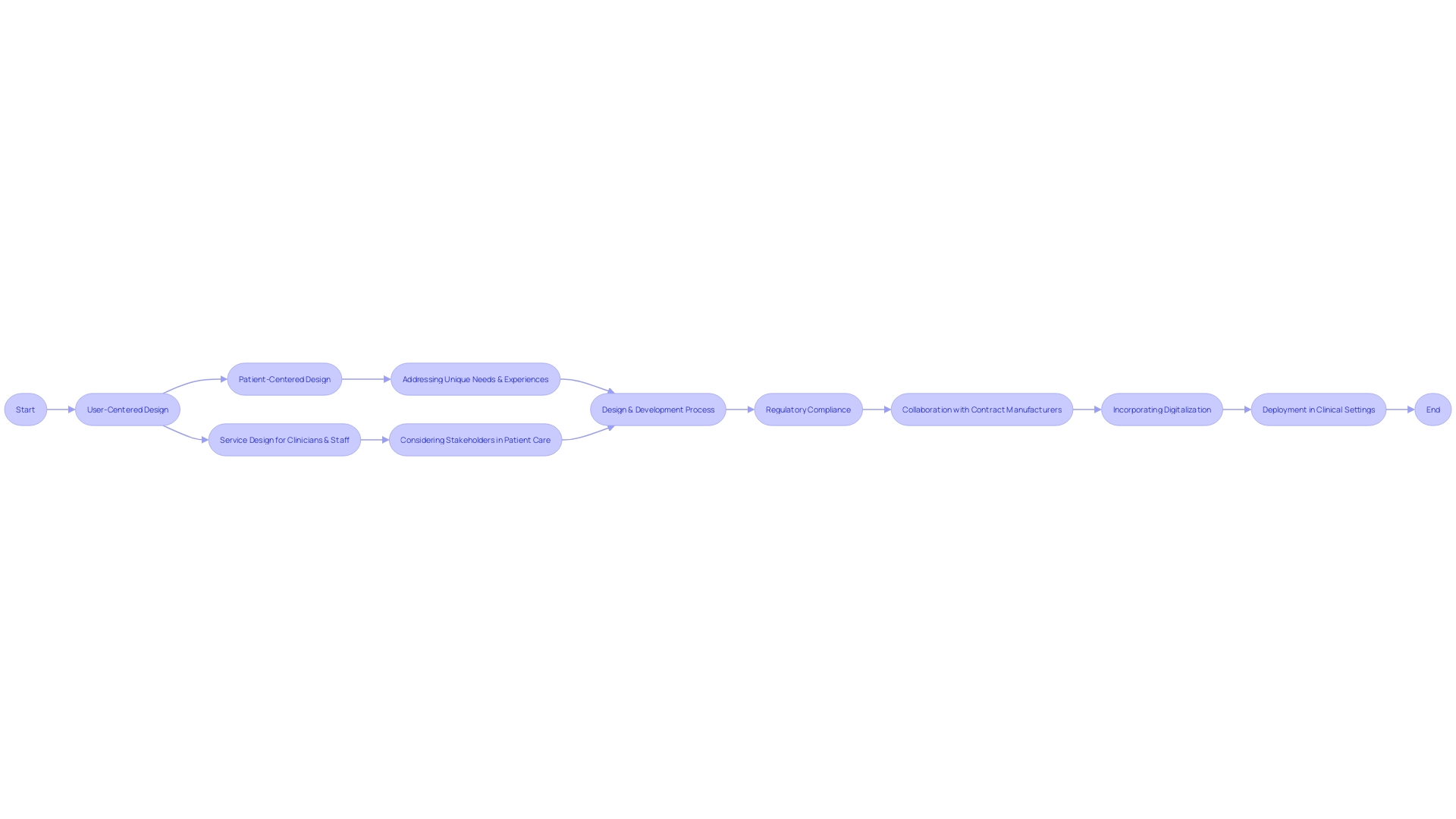
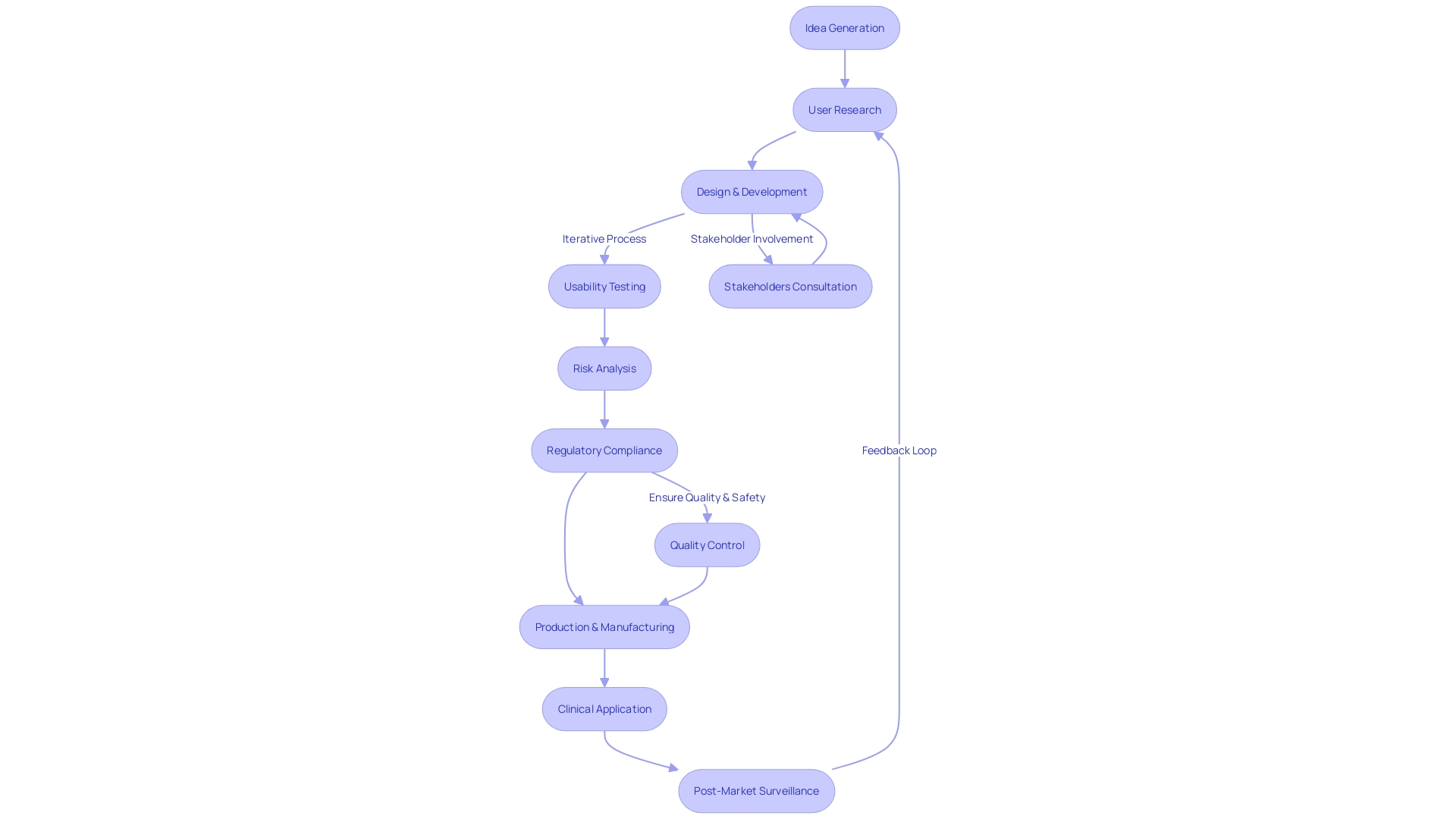
Phases of Design Control
The control process for medical instruments is a comprehensive journey that starts with the inception of the product and continues through to its deployment in clinical settings. This multi-phase approach incorporates service principles to accommodate the needs of all stakeholders, including clinicians, nurses, and hospital staff, ensuring that the care delivered to patients by these individuals is optimal.
User-centered approach (UCD) is crucial throughout this process, emphasizing the interactions between the apparatus and its usersâclinicians, patients, caregivers, and even cleaning or sterilization staff. By focusing on the individual user experience, UCD aims to enhance how users interact with healthcare equipment, encompassing usability testing and iterative development to refine the product effectively to meet users' requirements.
Innovation in this field is exemplified by the Diality system. Although not yet approved for sale, this patient-inspired haemodialysis system has been engineered to afford healthcare professionals the flexibility to customize haemodialysis prescriptions across care environments. Its user-friendly interface is designed to be accessible to a wide range of clinicians, showcasing how UCD can improve the usability of healthcare equipment.
Adherence to regulations is a crucial aspect of control. As the OECD's Conflict Minerals policy highlights, understanding the market reach of a medical device and the applicable regulations is fundamental. Companies often aim for global regulatory compliance to navigate the evolving market strategies and regulations smoothly.
Furthermore, the shift from creation to production can be challenging. Working with Contract Manufacturers (CM) requires meticulous effort to accurately replicate the specifications at scale. An evaluation carried out by the manufacturer can determine the preparedness of the product for large-scale production, guaranteeing that the manufacturing procedure corresponds with the initial planning.
Ultimately, the digitalization of control processes is not only about converting documents into digital format but comprehending the crucial data and how to employ it efficiently. Smart digitalization enables companies to achieve not only their desired outcomes but also the necessary compliance and business objectives.
User Needs Phase
At the start of the control process for medical products, recognizing and recording the requirements of the individuals and the intended application of the item is crucial. This foundational information directs and oversees the development phases, guaranteeing that the final product aligns with its purpose and meets user expectations. The approach to design must be holistic, considering all stakeholders, including patients, clinicians, and hospital staff, as their interaction with the equipment can profoundly impact patient care and outcomes.
The significance of emotional interaction with a healthcare apparatus cannot be emphasized enough, as initial responses to the apparatus's aesthetics can influence perceptions of excellence and effectiveness, potentially impacting usability and clinical outcomes. Hence, comprehending the requirements and aspirations of individuals is the initial phase in promoting favorable emotional connections with the equipment.
Design that focuses on the needs and motivations of individuals in the medical field requires thorough investigation into their behaviors, requirements, and aspirations, with the goal of developing products that provide valuable and applicable encounters. While patients and clinicians are the main individuals who use it, it is important to acknowledge that anyone who engages with the equipment, including caregivers, support staff, and technicians, is also a user. The emphasis on individual experiences, enabled by research, usability testing, and iterative development, guarantees that the product effectively fulfills these diverse requirements.
Michigan, a hub for talent and manufacturing in the industry, has shown clear progress in medical device technology and a strong emphasis on user-centered development. This progress is supported by facilities like UL Solutions' Rochester Hills laboratory, which provides comprehensive testing services tailored to manufacturers' needs, addressing quality, safety, and cybersecurity risks, thereby safeguarding individuals and fostering innovation.
Design Input Phase
During the crucial phase of design input, the emphasis is on carefully converting the needs of individuals into specific design specifications. This involves identifying the precise functional and performance standards anticipated for the healthcare equipment. It is a period of comprehensive user research, considering behaviors, needs, and motivations of all potential users to craft products that deliver meaningful and significant experiences. In the field of medical apparatus, this involves clinicians, patients, caregivers, support staff, and even cleaning and sterilization personnel. The process of user-focused development, particularly in the case of the Quality system, emphasizes creating a user-friendly interface that caters to both specialized and general clinicians. This method is supported by iterative development procedures to guarantee the apparatus fulfills its intended purpose effectively, with usability testing playing a critical role in improving the product to adequately cater to a wide range of users within the healthcare setting. The ultimate objective is to guarantee that every interaction with the healthcare equipment is intuitive and improves clinical outcomes.
Design Output Phase
The conversion from input specifications into output specifications is a crucial stage in the development of medical devices. This process encompasses the meticulous work of detailed planning, the creation of prototypes, and the generation of precise specifications. It's a phase where user-centered concepts take center stage, focusing on the unique needs, preferences, and experiences of not just patients but all stakeholders involved, including clinicians, nurses, and hospital staff. Service design becomes crucial in this case, ensuring that the equipment not only meets patient needs but also integrates seamlessly into the workflows of various healthcare professionals.
Highlighting the emotional interaction with the apparatus is equally crucial. The initial response of individuals upon encountering the device can shape their perception of its quality and functionality, which can have far-reaching implications for adoption, compliance, and ultimately, clinical outcomes. Establishing a favorable emotional interaction starts with a thorough comprehension of individuals' requirements and aspirations.
Furthermore, the output phase of the development process is not only concerned with the appearance and perception of the product; it also involves explainability and transparency. These principles guarantee that information affecting risks and patient results is communicated efficiently, taking into account the individuals and circumstances in which the equipment will be utilized. By conducting user research, usability testing, and iterative processes, the aim is to improve the tool to precisely fulfill the users' requirements.
Recent developments in the field, such as the Diality system, underscore the importance of user-friendly interfaces that cater to both specialists and non-specialists, allowing for the selection of appropriate haemodialysis prescriptions in any care setting. This focused approach to creation aims to enhance the safety and efficacy of healthcare tools, ultimately benefiting the healthcare system and patient care.
In the context of healthcare equipment development, it's crucial to choose the appropriate collaborators who can provide a wide array of knowledge and skills. The regulatory environment, with organizations such as the FDA and EMA, emphasizes the significance of comprehensive development procedures, particularly for high-risk class three products that necessitate more stringent examination. Therefore, the output phase of the development process is a convergence of technical accuracy, user focus, and compliance with regulations, all of which are necessary for delivering products that ensure safety, efficacy, and positive reception among all users in the healthcare setting.
Design Review Phase
Throughout the review stage, the development of medical equipment requires a thorough and organized examination of the documentation. This crucial process ensures that the outputs match exactly with the intended inputs and fulfill essential requirements. The principles of User-Centered Design (UCD) have a crucial impact on this process, concentrating on crafting solutions that meet the complex requirements of all individuals â including patients, clinicians, and hospital staff. UCD represents a holistic approach, engaging diverse stakeholders such as caregivers, support staff, and laboratory technicians throughout the design cycle. It ensures that every interaction with the equipment is intuitive, effective, and safe, thus enhancing the overall user experience and reliability of the medical product. Regulatory bodies like the FDA and EMA mandate stringent controls, especially for high-risk Class three products, to guarantee that they sustain life without compromising safety. The incorporation of UCD in controls not only aids in complying with these rigorous standards but also facilitates a smoother transition from concept to clinical application, ultimately improving patient care outcomes.
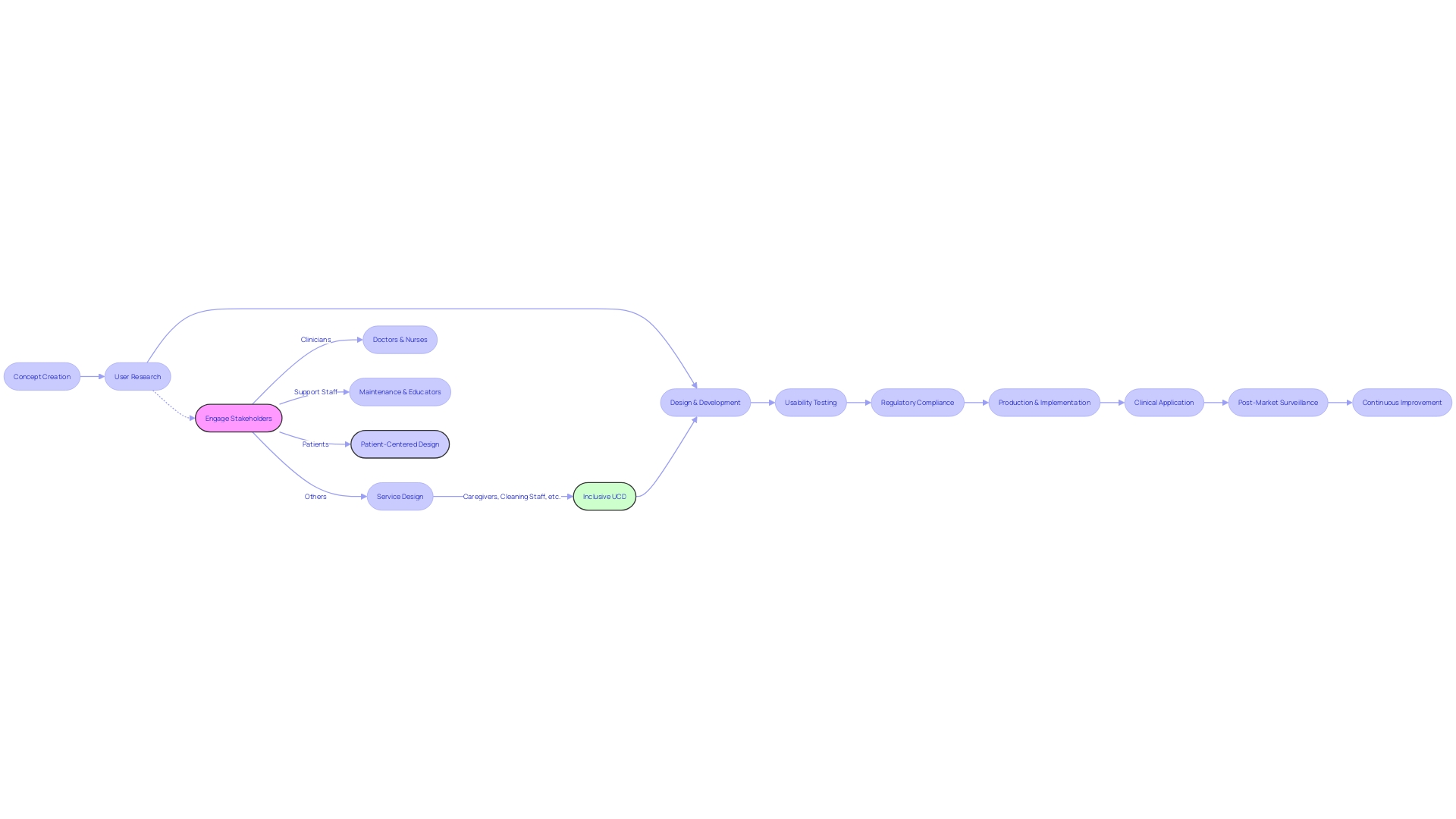
Design Verification Phase
Design verification is a crucial stage in the development of medical equipment, requiring a meticulous approach to testing and analysis. This process ensures that the end result is in line with the initial concept inputs. By carefully evaluating outputs against specified requirements, this phase seeks to validate that the device will perform safely and effectively when used as intended.
The importance of verification extends beyond mere compliance. It captures the core of service design, addressing the needs not only of patients but also taking into account the wider range of individuals such as clinicians, nurses, and hospital staff. This holistic approach recognizes that the efficacy of patient care is contingent upon all these stakeholders.
To promote a favorable emotional interaction with the apparatus, it is crucial to comprehend the requirements and wishes of the users. Emotional interaction often precedes functional experience, impacting perceptions of product quality and performance expectations. When usersâwhether they be clinicians, patients, or support staffâhave a favorable emotional response to an object, this can lead to enhanced adoption, compliance, and ultimately, clinical outcomes.
Moreover, safety considerations are of utmost importance in the development procedure, especially in software development for healthcare instruments. Ensuring encrypted and secure communication is crucial to adhere to regulations regarding healthcare equipment and safeguard individuals from potential hazards.
The synergy of rigorous testing, as demonstrated by UL Solutions' expanded capabilities in Michigan, with an empathetic approach to innovation that encompasses all interactions, is changing the landscape of instrument development. By utilizing user-centered design principles, developers can create products that not only comply with regulatory standards but also resonate with users, thereby promoting innovation and enhancing the standard of patient care.
Design Validation Phase
Validation of design in the industry of healthcare equipment is not just about ticking a box; it is about guaranteeing that every apparatus fulfills the complex requirements of real-world clinical settings. This process is a critical part of the medical tool lifecycle, starting from conception to deployment in healthcare settings. When validating the design, it is important to take into account the experiences of all individuals involved, including patients, clinicians, nurses, maintenance staff, and other individuals who interact with the equipment. This comprehensive approach ensures that the equipment performs reliably under varied conditions and aligns with the intricate workflows of a healthcare setting.
Person-Centered Design (UCD) plays a foundational role in this process by focusing on understanding and addressing the specific behaviors, needs, and motivations of each individual group. It's about creating a tool that not only enhances patient outcomes but also improves the experience for healthcare professionals. For example, the significance of service planning is emphasized when taking into account the numerous touchpoints and interactions that different hospital staff have with the apparatus. UCD includes thorough user research, iterative processes, and usability testing to enhance the product until it meets the elevated expectations in healthcare.
The significance of this approach is underscored by recent industry movements, such as Everly Health's efforts to enhance early detection and prevention of chronic diseases through advanced diagnostics. Similarly, companies like Cardiawave are preparing for a Series B financing round to support their non-invasive technologies, highlighting the importance of rigorous design validation processes ahead of market entry and FDA approval. The medical device landscape is complex and highly regulated, with the FDA categorizing devices based on risk and requiring rigorous processes for high-risk devices. As such, a comprehensive understanding of user needs and a track record of successful product launches are indispensable for companies navigating this challenging yet vital field.
Design Transfer Phase
The transition from design to manufacturing, commonly referred to as the design transfer phase, is a meticulous process that ensures the design intent is faithfully translated into a product that can be produced at scale. This includes not only establishing a robust manufacturing process but also the clear and comprehensive documentation that accompanies it. Activities central to this phase include process validation to confirm that the manufacturing process is capable of producing products that meet specifications consistently and training manufacturing personnel to execute the process accurately.
Thought must be given to the reality that the initial phases of a medical product's market presence are crucial. Initial product launches should strike a balance between meeting critical expectations and avoiding excessive complexity that may hinder scalability. This implies dedicating significant resources to tooling and part fabrication in order to establish a manufacturing process that is suitable for releasing a safe and effective product. Should the market response be favorable, production can then be scaled up accordingly.
Collaborating with Contract Manufacturers (CMs) is crucial as it brings different challenges. Perfecting the mass production process can be as laborious as the initial creation of the product. Therefore, acknowledging that completion of the concept does not equate to immediate production readiness is vital. Conducting a thorough gap analysis with a manufacturing partner is essential to evaluate the device's preparedness for production.
To connect the divide between creation and production, one must take into account the optimization of the creation to reflect production capabilities. As highlighted during the Smart Simulation Summit, creators often lack the necessary information to create models that are inherently manufacturable, relying instead on heuristics or legacy approaches. The repercussions of this disconnect become apparent during production, when making changes is both costly and challenging. To ensure the flexibility and responsiveness of the industry for manufacturing, it is crucial to incorporate early considerations into the development process.
Furthermore, the development of healthcare tools should not only prioritize the well-being of patients but also cater to the requirements of different stakeholders in the healthcare industry, such as doctors, nurses, and maintenance personnel. The structure must facilitate the workflow and adhere to hospital policies, ultimately influencing patient care outcomes. A thorough development partner for healthcare equipment will have the knowledge and comprehension of both the regulatory environment and market dynamics, guaranteeing a smooth progression from idea formation to market release, thereby saving time and improving the project's success.
When it comes to focusing on the needs of the users, it is vital to take into account all possible individuals who may use the equipment, going beyond just patients and healthcare providers to encompass those who provide care, offer support, and operate the machinery. The creation process should emphasize on generating significant user experiences through employing user research, usability testing, and iterative creation to meet users' needs effectively. By doing so, healthcare instruments can be developed to provide impactful and relevant experiences for all stakeholders involved in patient care.
Design Changes Phase
Maintaining the integrity of medical devices throughout the product lifecycle is crucial, particularly in managing and controlling alterations to the product's design. As new updates and features become necessary, or when integration with other systems is required, a robust quality system that accommodates regular product changes is essential. This method guarantees that any alterations to the design of the equipment are not only carefully assessed and recorded but also successfully executed to uphold the safety, effectiveness, and user contentment of the tool.
A philosophy that embraces continuous improvement and anticipates regular updates is especially crucial for objects that contain software or are part of larger systems. With the rapidly changing landscape of technology, the industry often sees the need for post-launch upgrades, making it crucial to have a system that adjusts to these changes while still meeting regulatory standards. Embracing smart digitalization strategies enables companies to efficiently manage their data and workflows, ensuring that they can keep pace with industry demands and innovate responsibly.
In the highly regulated sphere of manufacturing equipment for healthcare, it's important to partner with entities that possess a comprehensive understanding of the regulatory landscape. For example, the FDA classifies healthcare equipment based on risk levels, with class three apparatus requiring more rigorous approval processes due to their crucial role in patient care. Keeping up to date with filing trends and regulatory updates is an essential part of effective control processes, guaranteeing that companies in the healthcare technology industry can navigate the intricacies of the market.
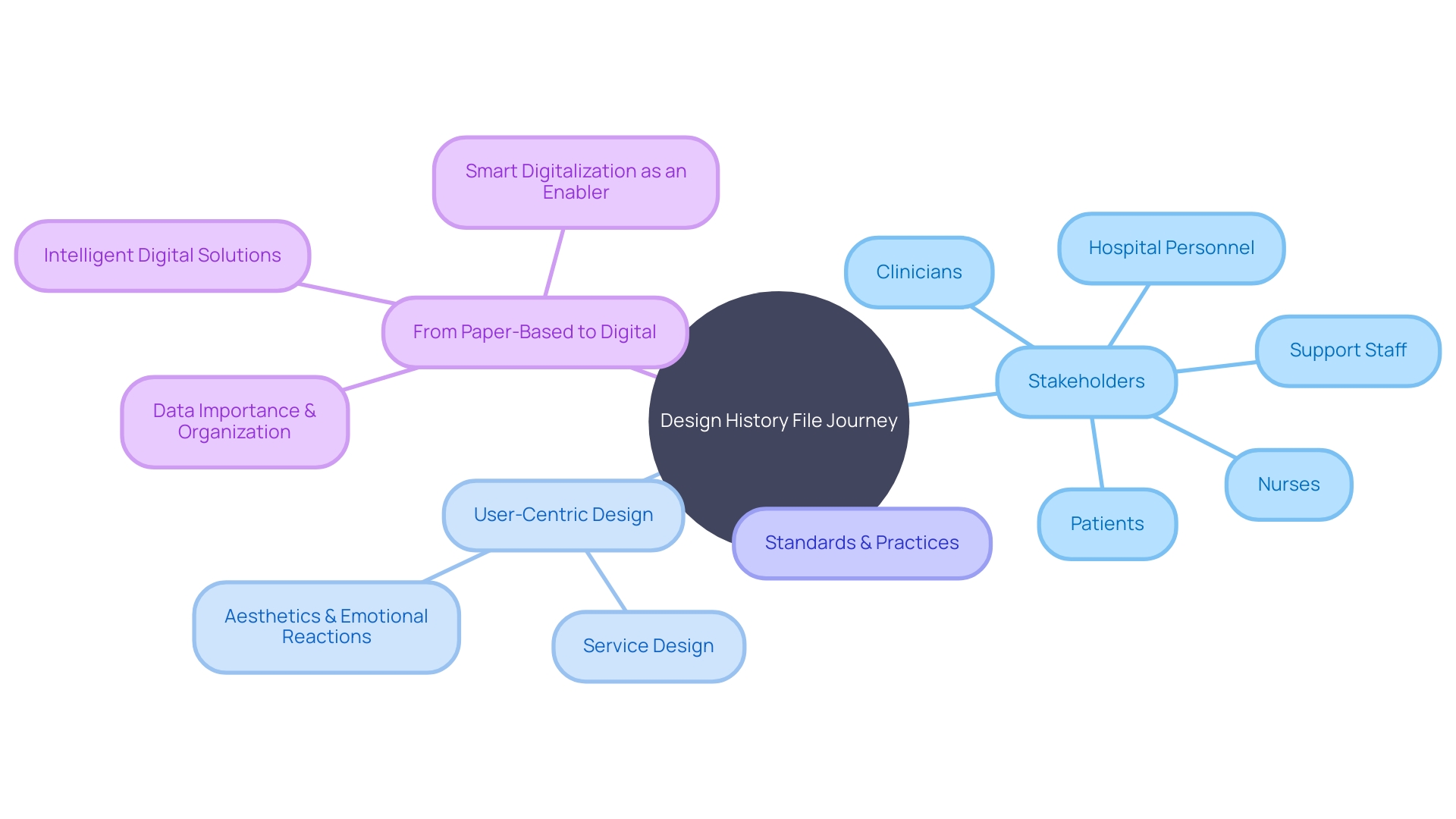
Design History File
A Design History File (DHF) encompasses much more than simply a repository of documents; it represents the culmination of thoughtful design principles and practices that are crucial in medical instrument creation. It encompasses the complete journey of an instrument, from conceptualization to deployment in healthcare settings, and importantly, it takes into account all stakeholders involved in patient care. This includes not only the patients themselves but also the clinicians, nurses, support staff, and hospital personnel who interact with the equipment in various capacities. The DHF is not just a static record; it is a dynamic reflection of user-centric and service design methodologies, which prioritize the needs, preferences, and experiences of every individual group. For example, the emotional interaction with a healthcare instrument - the user's immediate, unconscious reaction to seeing or handling it - can greatly impact their perception of the instrument's quality and performance. Understanding the importance of aesthetics in promoting positive emotional reactions is a crucial element in improving adoption, compliance, and ultimately, clinical results.
The FDA's continuous dedication to guaranteeing the safety, efficiency, and security of healthcare instruments is evidence of the significance of thorough design control activities documented in the DHF. With the recent FDA directive on clear and neutral presentation of prescription drug information in advertisements, it's evident that transparency and understanding are at the forefront of regulatory concerns. The DHF is the backbone that supports this transparency, demonstrating compliance with these stringent standards. Intelligent digitization, as an expansion of this procedure, entails identifying which information is most important and organizing it in a manner that promotes not only adherence, but also the overall objectives of the healthcare equipment sector. The transformation from paper-based records to intelligent digital solutions is not simply a matter of scanning documents; it entails a strategic approach to capturing and utilizing critical data to facilitate progress within the field. This method is crucial in navigating the intricacies of product design, guaranteeing that the ultimate results satisfy the diverse requirements of all users and adhere to stringent regulatory criteria.
Integrating Risk Management with Design Controls
Design controls and risk management are crucial in the development of these essential instruments, guaranteeing their safety and effectiveness. Incorporating risk management processes, such as hazard analysis and risk assessment, is essential for creating and manufacturing products that adhere to the utmost safety criteria.
Expert Bijan Elahi, who has an impressive 29-year tenure in safety risk management for devices, emphasizes the significance of comprehensive risk management, beyond the mere understanding of ISO14971. His approach includes practical advice and hands-on examples, assisting in navigating the complexities of risk management effectively. This is especially important as the healthcare instrument industry encounters a swiftly changing regulatory setting, with enhanced automation and digitalization.
The COVID-19 pandemic has underscored the need for robust risk management frameworks, as supply chain disruptions have led to a surge in automation within the healthcare sector. This has important implications for the assembly of healthcare instruments, where adherence to legal requirements is non-negotiable. Manufacturers must be equipped with the correct tools and knowledge to integrate these technologies seamlessly and remain compliant.
'UL Solutions' recent expansion of its testing facilities for healthcare equipment in Michigan, a hub for the manufacturing of healthcare equipment, illustrates the industry's dedication to addressing risks associated with quality, safety, and cybersecurity. The state-of-the-art laboratory offers comprehensive testing methods, underscoring the sector's focus on innovation and consumer safety.
Furthermore, with the increasing complexity of global regulations, as seen in the OECD's 'Conflict Minerals' policy and directives like RoHS, companies must stay informed and adapt to international compliance standards. This is essential not only for market access but also for ethical and financial accountability.
In the United States, the FDA's classification system for medical apparatus necessitates a nuanced understanding of the regulatory process, with class three devices, which are critical to life support, undergoing stringent reviews. Similarly, in Europe, healthcare instruments are regulated at the Member State level, with additional oversight by the EMA, emphasizing the significance of a comprehensive grasp of the varied regulatory frameworks.
These advancements in the healthcare equipment field emphasize the importance of incorporating risk mitigation into control measures, guaranteeing that instruments not only fulfill but surpass safety standards, thereby protecting patient welfare.
Benefits of Effective Design Controls
Design controls are a fundamental aspect of medical equipment development, ensuring that products are not only effective but also safe for both patients and healthcare providers. They cover a complete approach from the initial idea of a tool through to its clinical application, with a focus on user-centered development. This methodology places importance on the personal experiences of all potential individuals, including clinicians, support staff, and maintenance crews, in addition to patients. By focusing on the distinct needs and interactions these individuals have with healthcare equipment, organizations can enhance usability and foster positive emotional interactions, which can influence perceptions of quality and performance, ultimately leading to better clinical outcomes.
Furthermore, the execution of strong development controls goes beyond customer contentment. By conducting thorough user research, usability testing, and iterative processes, companies in the healthcare industry can successfully reduce risks associated with quality, safety, and cybersecurity. This comprehensive approach not only aids in maintaining regulatory compliance but also accelerates the time to market. For example, UL Solutions recently expanded its testing services in Michigan to address the growing demand for device testing, underscoring the industry's need for efficient and flexible testing capabilities that align with regulatory expectations and manufacturer requirements.
As technology advances, the importance of control measures becomes more and more critical. They provide a structured framework that guides the development process, ensuring that every touchpoint aligns with user needs and enhances the overall healthcare experience. Ultimately, effective design controls serve as a cornerstone for medical device companies, supporting innovation while upholding the highest standards of product quality and patient safety.
Conclusion
In conclusion, design controls are critical processes in the medical device industry that ensure devices meet the highest standards of safety, effectiveness, and quality. By incorporating user-centered design and adhering to regulations, companies can create seamless and meaningful experiences with medical devices.
Design controls encompass the entire lifecycle of a device and address the diverse needs of all users, including patients, clinicians, and support staff. User research, usability testing, and iterative design processes refine devices and improve user interactions.
Standards and regulations, such as the FDA's Quality System Regulation (QSR) and the European Union's Medical Device Regulation (MDR), guide design control processes and ensure compliance. Adhering to these standards is crucial for maintaining regulatory quality and device safety.
The design control process consists of various phases, from understanding user needs to transferring design to manufacturing. Integrating risk management procedures is essential to ensure device safety. Effective design controls offer benefits such as enhanced usability, positive emotional interactions, improved clinical outcomes, regulatory compliance, and accelerated time to market.
In conclusion, design controls are fundamental in the medical device industry, ensuring devices are safe and effective. By incorporating user-centered design and adhering to regulations, companies can create meaningful experiences with devices, ultimately enhancing patient care and supporting the healthcare ecosystem.




