Introduction
Medical devices play a vital role in modern healthcare, and ensuring their safety and efficacy is paramount. A Device History Record (DHR) serves as a comprehensive documentation tool that meticulously traces the life of a medical device from its initial design through production and into post-market surveillance. This article delves into the intricate details of what constitutes a DHR, the regulatory requirements set forth by bodies such as the FDA and the European Medicines Agency, and best practices for maintaining compliance.
By exploring key components and emphasizing the importance of thorough documentation, the aim is to highlight how DHRs contribute to the overall safety, quality, and regulatory adherence of medical devices.
What is a Device History Record (DHR)?
A Device History Record (DHR) meticulously documents the entire lifecycle of a medical instrument, from its design and manufacturing to quality control measures. This comprehensive compilation is crucial for ensuring that each item adheres to its specified requirements and complies with regulatory standards. Maintaining complete visibility into the Bill of Materials (BOM), including component serialization and lot traceability, is essential for risk management. This traceability becomes particularly important when regulations evolve, as it allows producers to quickly identify and address any components at risk. Regulatory authorities such as the FDA highlight the significance of a comprehensive benefit-risk evaluation during both the design and development stages, as well as in post-market monitoring, to verify the safety and effectiveness of the product throughout its lifecycle. 'In the intricate supply networks characteristic of healthcare equipment production, it is standard procedure for producers to seek compliance details from their upstream providers as a component of the certification process.'. This ensures a traceable history that can be reviewed during audits and inspections, safeguarding both the manufacturer and the end-users.
Key Components of a DHR
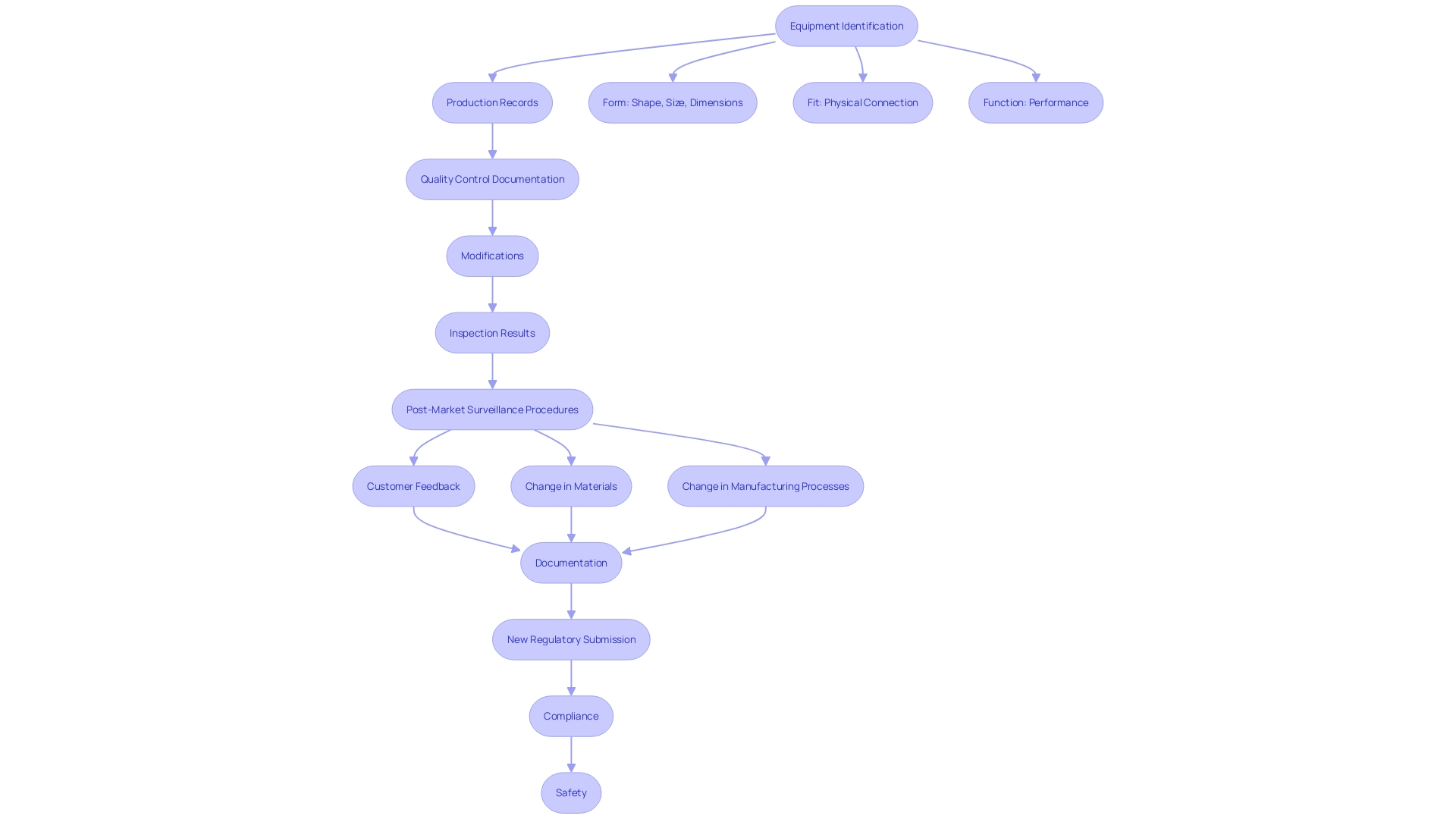
'A Product History Record (DHR) is a crucial element of medical equipment manufacturing, meticulously documenting the entire production process to ensure adherence to safety and efficacy standards.'. Key elements of a DHR include equipment identification, production records, and quality control documentation. Additionally, it records any modifications made during manufacturing, results from inspections and tests, and reports of complaints or non-conformances.
'This comprehensive documentation is crucial for internal audits and FDA inspections, providing a transparent trail of the product's journey from production to market.'. Effective post-market surveillance and monitoring are imperative, as emphasized by the Quality Management System Regulation (QMSR). Manufacturers must establish procedures to monitor and analyze post-market information, such as complaints and adverse events, to identify potential risks and take appropriate actions to mitigate them. 'These measures are vital for maintaining the safety and effectiveness of medical instruments throughout their lifecycle.'.
The recent recall of Philips' DreamStation for sleep apnea, which revealed that the company was aware of defects for over a decade before taking action, underscores the importance of rigorous post-market surveillance. This event emphasizes the importance for producers to actively handle risks and guarantee adherence to legal standards.
Integrating risk management principles into the design, production, and post-market processes can result in the creation of high-quality products that fulfill compliance standards and ensure optimal patient results. As the regulatory environment keeps changing, producers must remain alert and adjust to guarantee their products consistently meet the necessary standards.
Regulatory Requirements for DHR
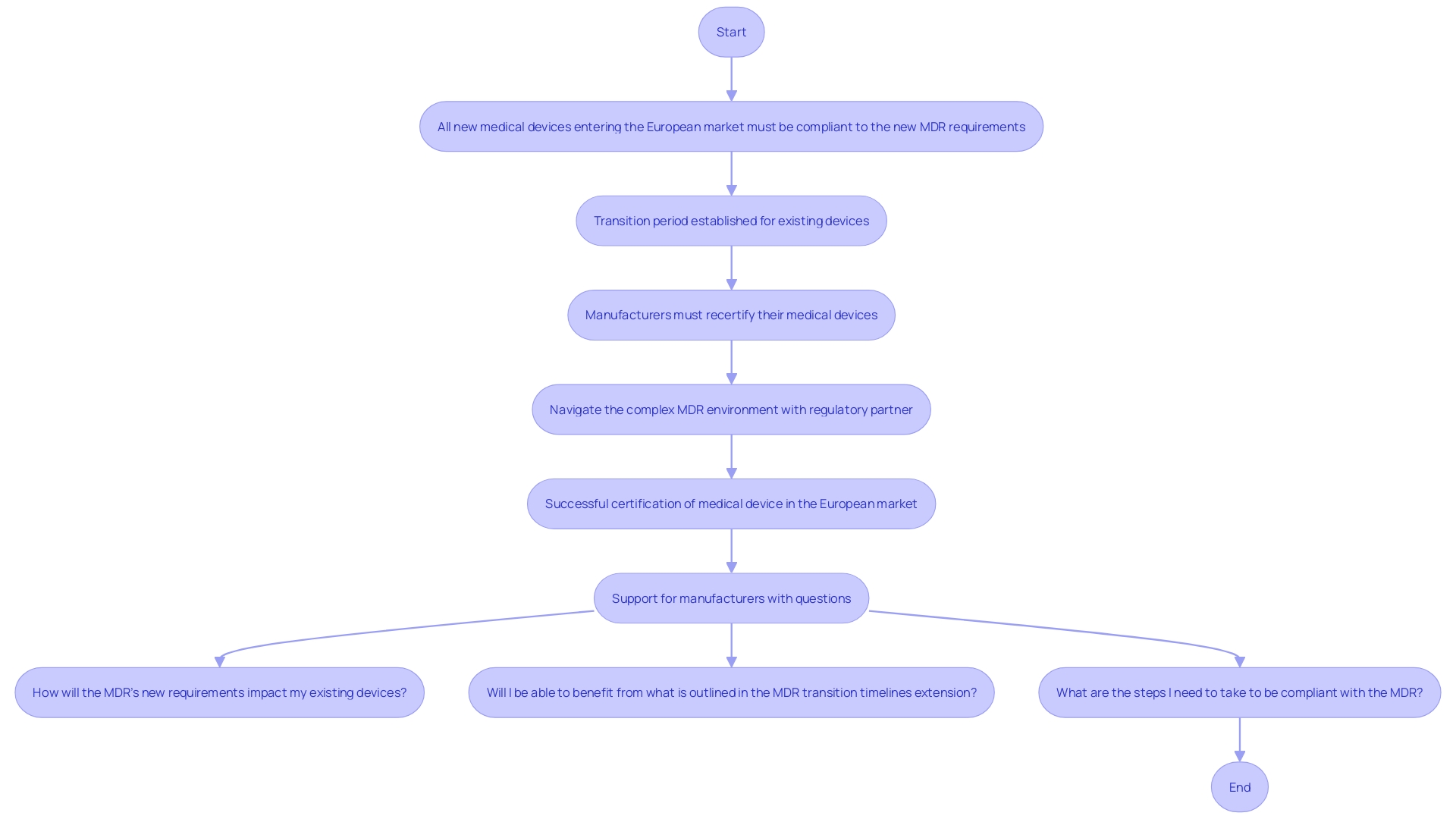
Regulatory organizations such as the FDA and the European Medicines Agency require that producers maintain Device History Records (DHRs) as part of their quality system regulations. This requirement ensures traceability and adherence to established guidelines, which is critical for demonstrating the safety and effectiveness of healthcare products. According to the new EU Medical Equipment Regulation (MDR), all new medical instruments entering the European market must be compliant with these stringent requirements, and manufacturers of existing products must recertify them accordingly.
The importance of maintaining comprehensive DHS is underscored by the need for robust clinical data, which serves as the backbone of regulatory submissions. As Article 62 of the EU MDR states, clinical investigations must ensure the protection of participants' rights, safety, and well-being while generating scientifically valid data. This data is pivotal for proving that a product is both safe and effective, a necessity for market approval. For instance, in the US, approximately 10-15% of successful 510(k) submissions for Class II products rely on clinical trial data, while all Class III products require extensive clinical trials to establish their safety and efficacy.
Additionally, the evolving environment of healthcare equipment rules necessitates producers to adjust constantly to new compliance requirements. Recent initiatives, such as the UK's refreshed Innovative Licensing and Access Pathway (ILAP), aim to expedite the marketing authorization process while ensuring patient safety. Dr. Laura Squire, Med Tech Regulatory Reform Lead, emphasized the importance of these advancements, stating that they offer significant opportunities for patient care improvements without compromising safety or quality.
In summary, adherence to DHR and other legal requirements is not only essential for avoiding penalties but also for ensuring the safety and effectiveness of medical devices. The changing oversight environment requires careful compliance with quality systems, strong clinical data management, and continuous adjustment to new governance frameworks.
Best Practices for DHR Compliance
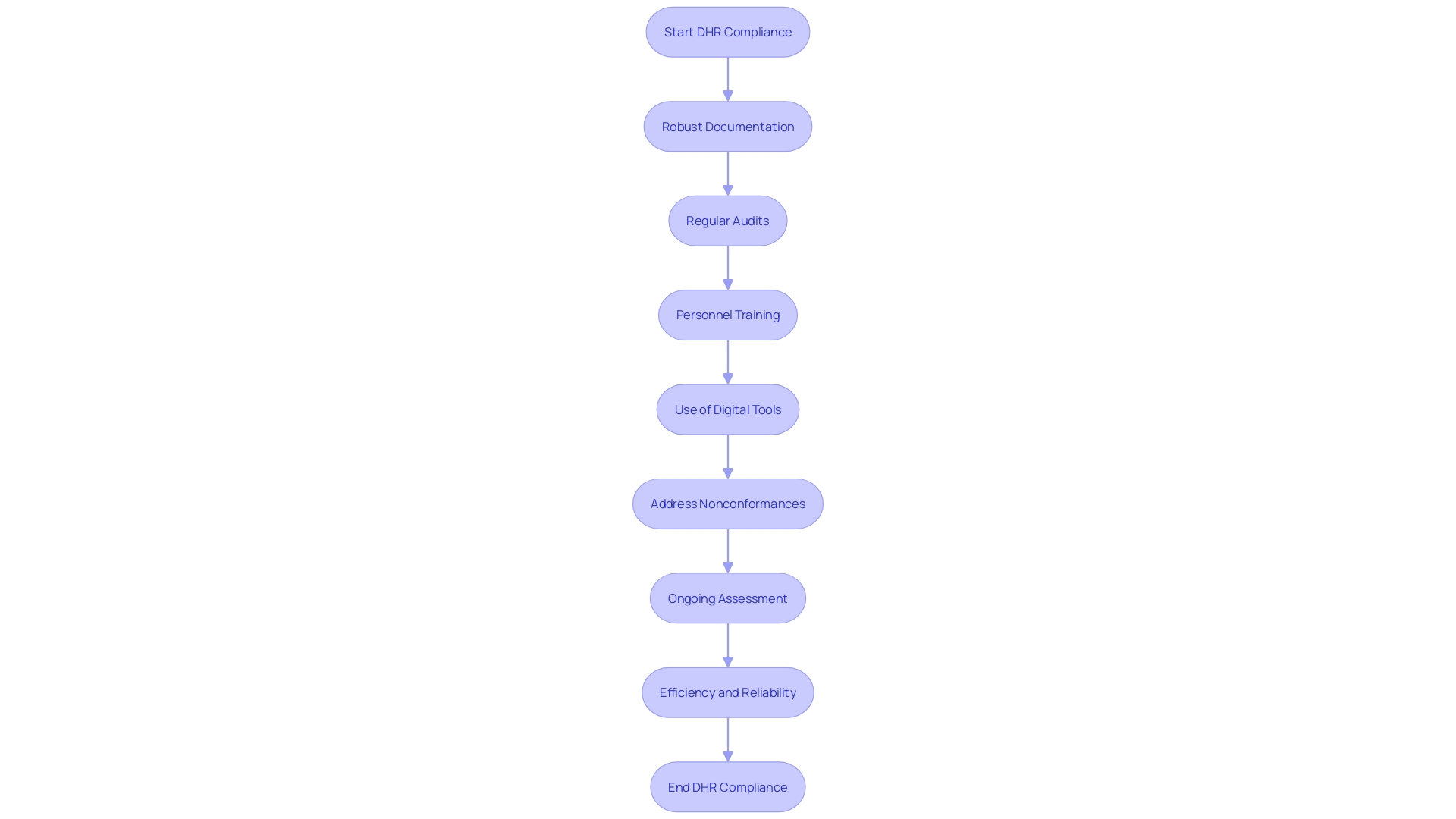
To ensure Device History Record (DHR) compliance under the MDR, manufacturers must adopt a comprehensive approach that includes best practices such as robust documentation procedures and regular audits. Utilizing electronic record-keeping systems can significantly reduce errors associated with manual handling, thereby enhancing accuracy and traceability. Training personnel on the criticality of maintaining accurate DHS and updating work instructions based on real-time data can further mitigate risks of nonconformance.
'Regular assessment and ongoing enhancement of DHR processes are essential to comply with compliance standards.'. Addressing nonconformances promptly is crucial, as errors can arise from various factors including supplier changes, manufacturing defects, and human error. Utilizing digital tools for data linkage and intelligent mock-ups ensures that every workstation maintains high standards of quality and accuracy. This multi-faceted strategy not only aids in regulatory compliance but also enhances the overall efficiency and reliability of the medical device manufacturing process.
Conclusion
The significance of Device History Records (DHRs) in the medical device industry cannot be overstated. DHRs serve as comprehensive documentation that tracks the entire lifecycle of medical devices, ensuring compliance with regulatory standards and enhancing safety and effectiveness. By meticulously recording each stage—from design and manufacturing to post-market surveillance—DHRs facilitate risk management and enable manufacturers to quickly identify and address potential issues.
Key components of a DHR include device identification, production records, quality control documentation, and records of any modifications or non-conformances. This thorough documentation not only supports internal audits and regulatory inspections but also plays a crucial role in post-market surveillance. The case of the Philips DreamStation recall underscores the necessity for proactive risk management and compliance, highlighting the consequences of inadequate monitoring.
Regulatory requirements established by bodies such as the FDA and the European Medicines Agency mandate the maintenance of DHRs as part of quality system regulations. These requirements emphasize the importance of robust clinical data for regulatory submissions, ensuring that medical devices meet safety and efficacy standards. As the regulatory landscape evolves, manufacturers must remain vigilant and adapt their practices to comply with new demands, which is essential for maintaining market approval and ensuring patient safety.
Best practices for DHR compliance involve adopting systematic documentation procedures, utilizing electronic record-keeping systems, and conducting regular audits. Continuous improvement and prompt addressing of nonconformances are critical to maintaining high standards of quality. By implementing these strategies, manufacturers can enhance the efficiency and reliability of their processes, ultimately contributing to better patient outcomes and regulatory adherence.




