Overview
The article focuses on the ethical challenges faced when conducting clinical research abroad, particularly in low- and middle-income countries, emphasizing the need for adherence to fundamental ethical principles such as respect for individuals, beneficence, and justice. It supports this by illustrating how cultural sensitivity, informed consent, and compliance with local regulations are essential to navigate these challenges effectively, ensuring that research is conducted ethically and benefits the local populations involved.
Introduction
In the realm of clinical research, ethical considerations are paramount, serving as the foundation upon which trust and integrity are built. As the landscape of medical inquiry evolves, the principles of respect for persons, beneficence, and justice emerge as guiding tenets that ensure the protection of participants and the validity of findings.
The complexities of conducting research, particularly in low- and middle-income countries, further underscore the necessity for a nuanced understanding of cultural sensitivities and regulatory frameworks. This article delves into the critical ethical principles that govern clinical research, the unique challenges faced in diverse settings, and the imperative of informed consent, highlighting the ongoing dialogue among industry leaders about the importance of ethical vigilance.
Through a detailed examination of case studies and expert insights, the discussion aims to illuminate the path toward ethical compliance and responsible research practices in an increasingly interconnected world.
Fundamental Ethical Principles in Clinical Research
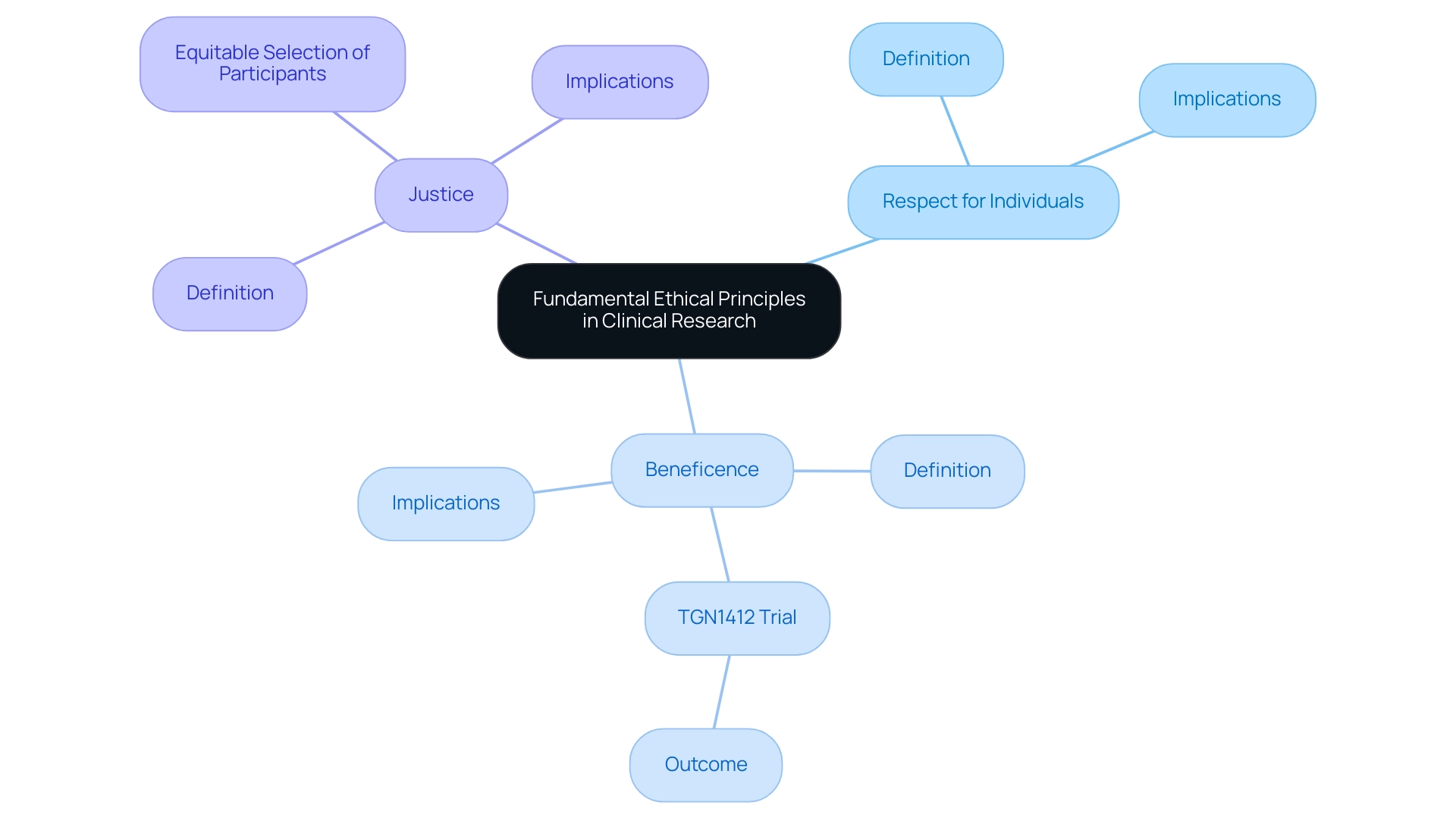
The basic moral principles that guide clinical studies are respect for individuals, beneficence, and justice. Respect for persons emphasizes the importance of recognizing participant autonomy, ensuring that individuals have the right to make informed decisions regarding their involvement in research. Beneficence mandates that researchers strive to maximize potential benefits while concurrently minimizing harm to participants.
A stark reminder of the consequences of failing to adhere to these principles is illustrated by the TGN1412 trial, where six healthy males became seriously ill within less than three hours of administration, underscoring the critical nature of moral oversight. Justice focuses on the equitable selection of participants, safeguarding vulnerable populations from exploitation. In the context of global research trials, the Ethical Challenges in Conducting Clinical Research Abroad make adhering to these principles even more critical due to varied cultural and regulatory landscapes.
Recent conversations among industry leaders highlight the importance of guidelines, with the recognition that strict compliance not only meets moral responsibilities but also positively impacts trial results. As Pliny the Elder wisely remarked, 'This only is certain, that there is nothing certain'; this emphasizes the crucial role of moral guidelines in navigating the uncertainties inherent in medical studies. Moreover, the case study on 'Avoidance of Misinterpretation' demonstrates the moral responsibilities of researchers, highlighting the risks linked to simplifying complex data for public comprehension and the potential for biased reporting in medical trials.
Ethical Challenges in Low- and Middle-Income Countries
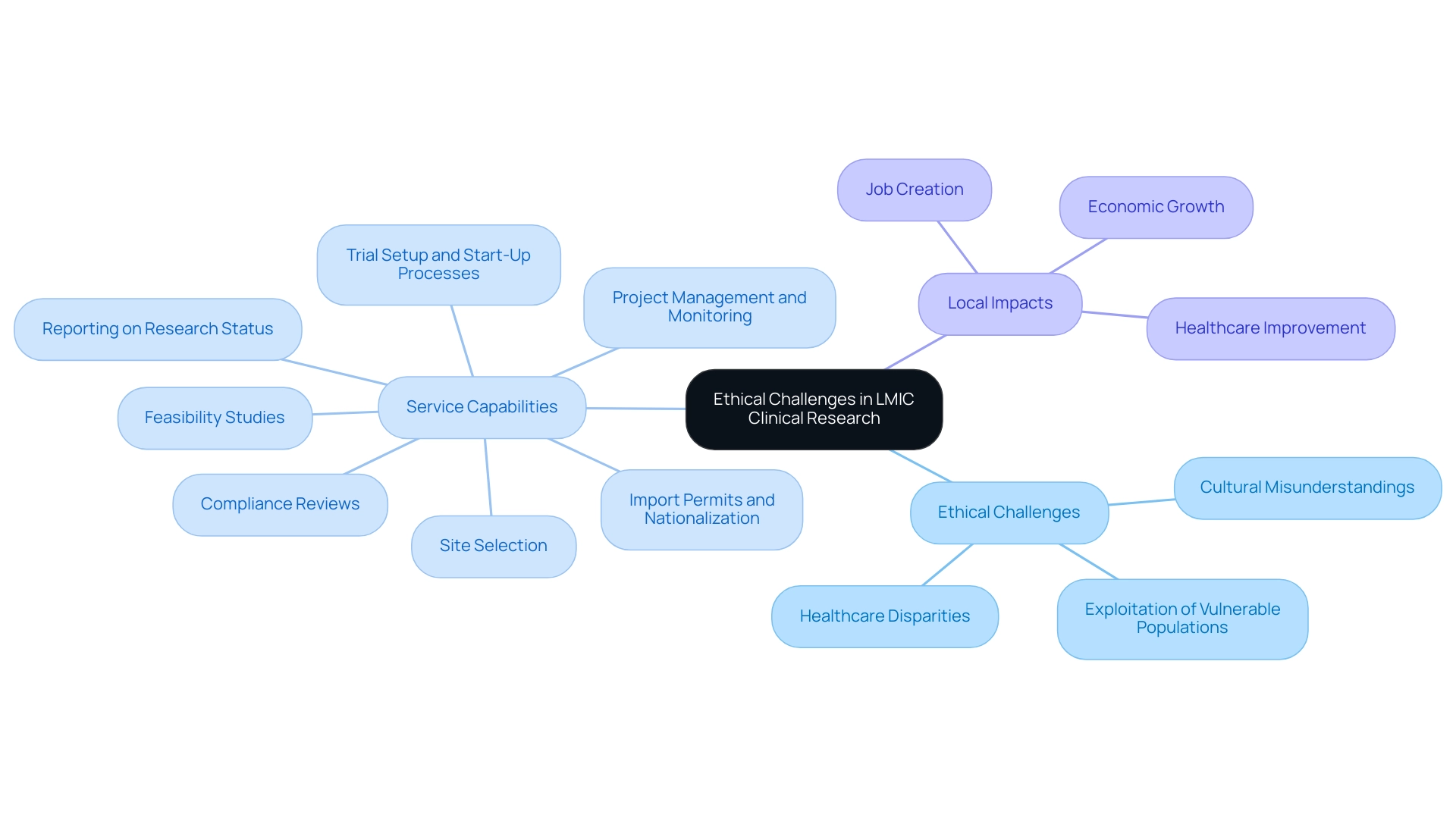
Carrying out clinical studies in low- and middle-income countries (LMIC) highlights the ethical challenges in conducting clinical research abroad that demand careful consideration. Disparities in healthcare access are profound, with many communities lacking necessary medical resources and interventions. Cultural misunderstandings can further complicate these trials and exemplify the ethical challenges in conducting clinical research abroad, potentially leading to the exploitation of vulnerable populations.
As noted by Alex John London, Professor of Philosophy and Director of The Center for Ethics and Policy at Carnegie Mellon University, 'When you ensure that the information is relevant to the local population, you build the foundation of knowledge necessary to generate beneficial interventions and policies, and this is a kind of benefit itself.' This viewpoint emphasizes the significance of customizing studies to meet regional health requirements, which not only improves moral acceptability but also guarantees that the advantages of the studies are fairly allocated. Our service capabilities include:
- Feasibility studies to assess the viability of conducting research in specific locations;
- Site selection to identify the most appropriate research sites;
- Compliance reviews to ensure adherence to local regulations and ethical standards;
- Trial setup and start-up processes, including obtaining necessary approvals from ethics committees and health ministries;
- Import permits and nationalization of investigational devices;
- Comprehensive project management and monitoring throughout the trial; and
- Reporting on research status, inventory, and adverse events.
These components are essential for navigating the complexities of medical trials in LMIC. Furthermore, the impact of Medtech research studies on local economies is significant, contributing to job creation, economic growth, and healthcare improvement through international collaboration. Media coverage, like the pieces by Clinical Leader, underscores the increasing acknowledgment of medical studies in Latin America and Colombia, stressing the importance of moral considerations and community engagement.
This coverage influences perceptions of clinical trials, reinforcing the importance of conducting research that is relevant and beneficial to local populations. A case analysis titled 'Moral Implications of Clinical Trials in Developing Countries' indicates that while conducting trials can provide necessary medical treatment, it highlights the ethical challenges in conducting clinical research abroad if not properly regulated. Trials should ideally focus on locally relevant diseases and offer Affordable Care.
Additionally, the recent PASTAL trial revealed that only fixed financial incentive interventions of $3 and $10 significantly improved outcomes, yet local policymakers remain hesitant to scale these programs. To foster trust and transparency, review boards should prioritize local expertise, enabling insights into community values and expectations. By doing so, researchers can navigate the complex landscape of healthcare access disparities, ensuring that their studies contribute positively to the communities they aim to serve.
Navigating Regulatory Frameworks for Ethical Compliance
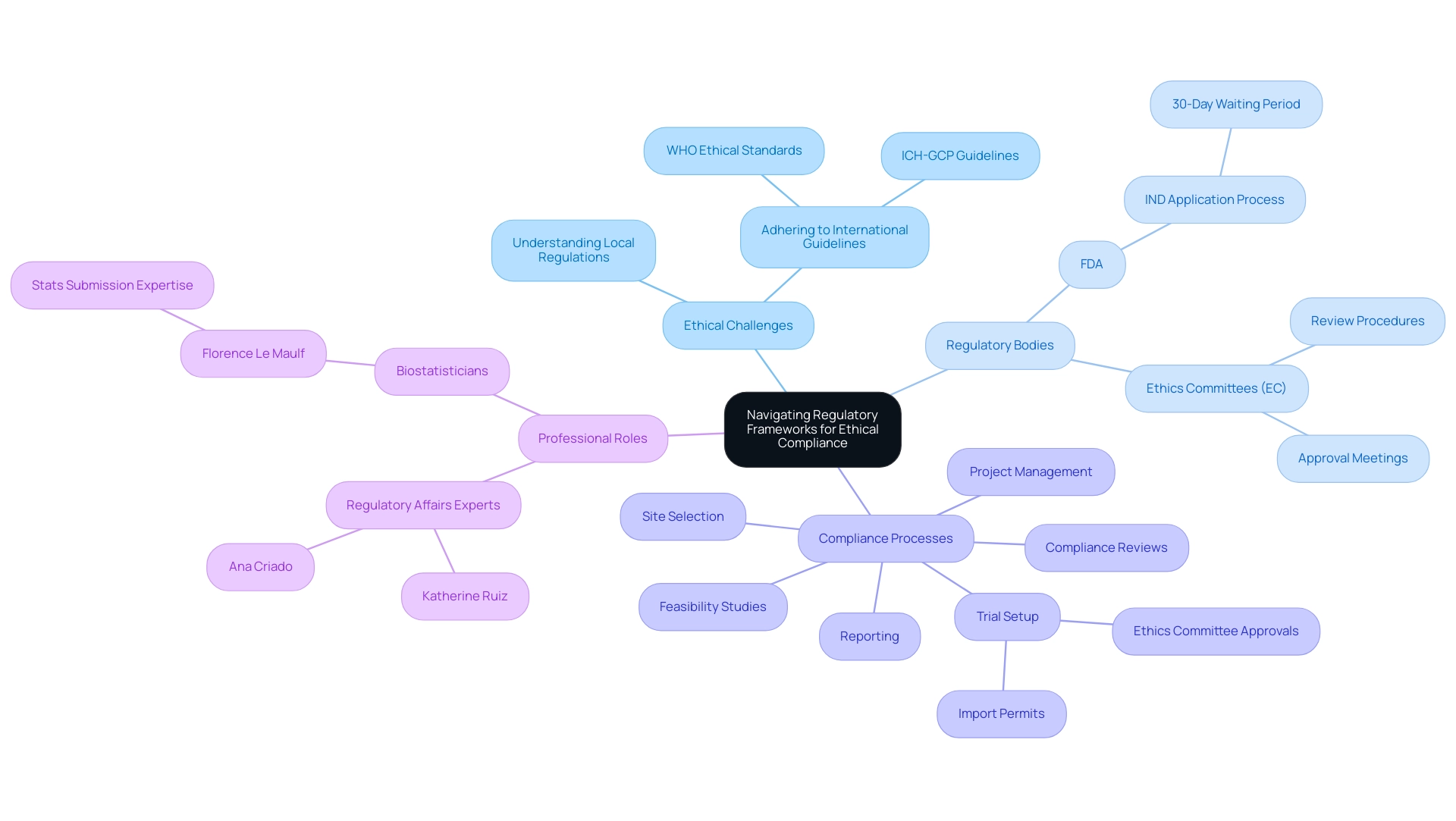
Navigating the intricate regulatory environment is vital for addressing the ethical challenges in conducting clinical research abroad to ensure moral compliance in research studies. Researchers are required to thoroughly understand the Ethical Challenges in Conducting Clinical Research Abroad, including local regulations and international guidelines such as the International Conference on Harmonization Good Clinical Practice (ICH-GCP) and the ethical standards established by the World Health Organization (WHO). Significantly, medical trials must not be started until 30 days after the FDA receives the Investigational New Drug (IND) application, unless earlier notification is given.
This waiting period underscores the importance of compliance with regulatory timelines. Each Ethics Committee (EC) has its own procedures for review, and there are no standardized regulatory requirements for clinical trial submission processes, adding to the complexity researchers face. Interacting with local regulatory bodies at the beginning of the inquiry process is vital to properly address the ethical challenges in conducting clinical research abroad and guarantee that the project complies with relevant laws.
This proactive approach not only fosters compliance but also enhances the integrity of the investigative process, ultimately helping to navigate the ethical challenges in conducting clinical research abroad and leading to more reliable outcomes. Our comprehensive trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup—including obtaining necessary import permits and navigating ethics committee approvals
- Project management
- Reporting
This ensures that researchers are supported every step of the way. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, and Ana Criado, a Director of Regulatory Affairs with expertise in biomedical engineering, health economics, and cannabis regulation, reinforce the importance of engaging with experienced professionals in navigating these regulatory complexities.
According to Florence Le Maulf, a Director of Biostatistics and subject matter expert in statistics submissions, her role in over 20 submission projects underscores the importance of adhering to regulatory frameworks for ethical compliance in research. Her insights emphasize how statistical rigor and adherence to guidelines are essential to the overall narrative of a drug's development. Moreover, statisticians and programmers play a vital role in submission projects by assisting research teams in deciding on the appropriate data pooling strategy that aligns with regulatory guidelines and drug-specific characteristics.
Cultural Sensitivity and Ethical Considerations in Research
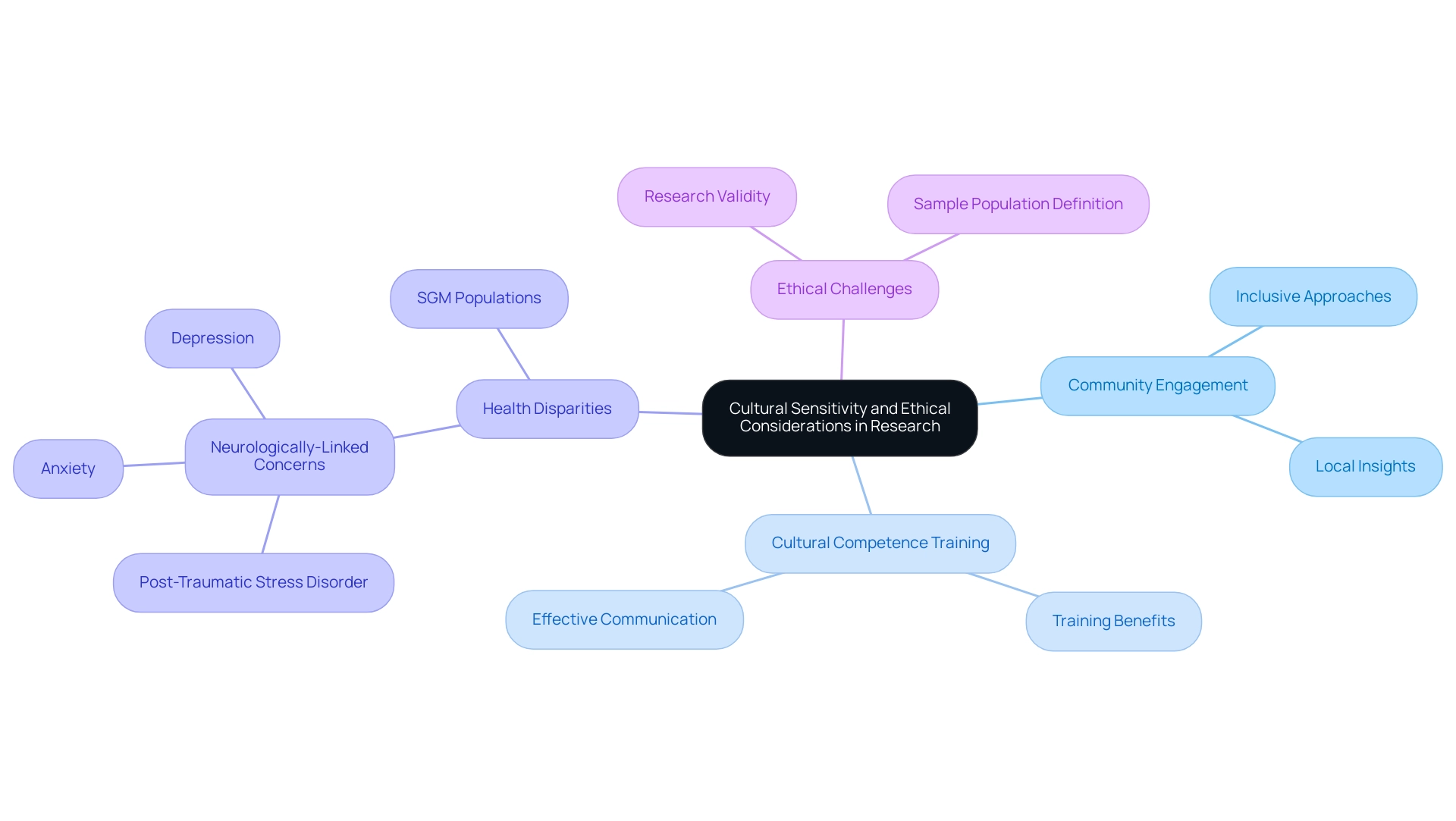
Cultural sensitivity is a critical aspect of successful clinical studies, particularly when considering the ethical challenges in conducting clinical research abroad, which require acknowledgment and respect for the diverse beliefs, practices, and values of the populations involved. Researchers must actively engage with local communities to gain insights into their perspectives, which allows for the incorporation of culturally relevant practices into study designs. This engagement is vital, as certain marginalized groups exhibit a significantly higher prevalence of neurologically-linked concerns, such as post-traumatic stress disorder, anxiety, and depression.
By fostering an inclusive approach, investigators can uncover rare side effects that may only manifest in specific populations, thus enhancing patient safety and overall study validity. Furthermore, training personnel in cultural competence is essential for effective communication and building trust with participants. This training not only reduces the ethical challenges in conducting clinical research abroad but also greatly enhances participant recruitment and retention, ensuring that the study is both ethical and impactful.
The National Institutes of Health (NIH) emphasizes this importance in their 2021–2025 strategic plan, stating that SGM populations are a health-disparities population and proposing support for new investigators to build a strong SGM workforce while increasing projects related to SGM health. As we enter 2024, the necessity for cultural awareness in healthcare studies is more pressing than ever, especially in addressing the Ethical Challenges in Conducting Clinical Research Abroad, requiring strategies that highlight community involvement and cooperation. For instance, the case analysis titled 'Data Collection and Reporting: Unmasking Hidden Truths' illustrates how data aggregation can obscure important health disparities among subpopulations, highlighting the necessity for researchers to carefully define their sample populations to ensure findings are applicable across diverse groups.
Informed Consent: Ethical Imperatives and Challenges
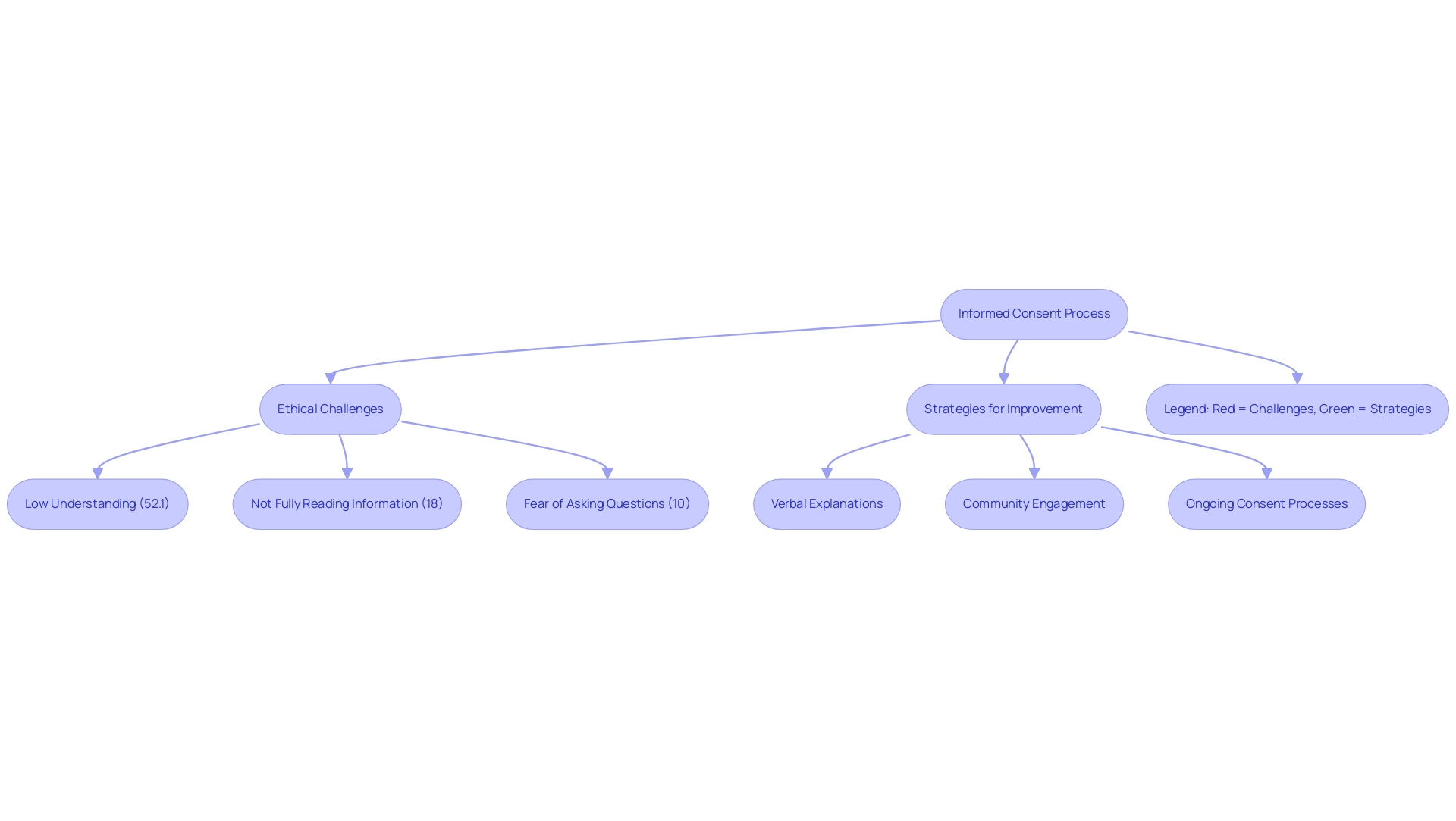
Obtaining informed consent is a cornerstone in addressing the ethical challenges in conducting clinical research abroad, safeguarding participants' rights and well-being. A crucial analysis highlighted that 52.1% of participants understood the concept of randomization, indicating a gap in comprehension that must be addressed. Researchers are assigned the responsibility of ensuring that consent forms are not only culturally sensitive but also linguistically accessible, enabling participants to comprehend the purpose, procedures, risks, and benefits fully.
In communities with diverse literacy levels or differing cultural perspectives on autonomy, ethical challenges in conducting clinical research abroad are particularly pronounced. Pope et al. noted that 18% of participants admitted to not fully reading the study information letter, while 10% expressed fear of asking questions regarding the study.
This highlights the ethical challenges in conducting clinical research abroad, particularly regarding the moral foundations of obtaining informed consent in clinical trials being undermined by patients' inadequate understanding, which may also extend to routine medical practices. To tackle these challenges effectively, researchers should employ strategies such as:
- Verbal explanations
- Community engagement
- Ongoing consent processes
Furthermore, recent discussions underscore the ethical challenges in conducting clinical research abroad, particularly emphasizing the moral imperatives surrounding informed consent and the necessity for a robust framework to guide responsible reporting and informed consent handling, especially in crisis situations where resources may be limited.
For instance, the case study titled 'Recommendations for Responsible Reporting in Crisis Situations' emphasizes the need for a framework to guide principled reporting of informed consent and data handling during crises. This approach not only fosters trust but also promotes ethical integrity in research practices across diverse communities, highlighting the urgent need for further research on the capacity assessment process and its reporting in clinical trials.
Conclusion
The exploration of ethical principles in clinical research underscores the critical importance of respect for persons, beneficence, and justice. These foundational tenets are essential in ensuring that participants are treated with dignity and their rights upheld, particularly in the complex environment of low- and middle-income countries. The challenges faced in these regions, including cultural sensitivities and healthcare disparities, highlight the necessity for researchers to adopt ethically sound practices that prioritize local needs and equitable benefits.
Navigating regulatory frameworks is another vital aspect of maintaining ethical compliance. By engaging with local authorities and adhering to international guidelines, researchers can foster an environment of integrity and trust. This collaboration not only ensures compliance but also enhances the reliability of clinical findings, ultimately contributing to better health outcomes.
Cultural sensitivity and informed consent emerge as pivotal elements in the ethical conduct of research. Engaging with communities to understand their unique perspectives allows for the design of studies that are both relevant and respectful. Furthermore, addressing the challenges surrounding informed consent—especially in diverse populations—ensures that participants are fully informed and empowered to make decisions about their involvement in research.
In conclusion, ethical vigilance in clinical research is not merely a regulatory requirement but a moral imperative that shapes the future of medical inquiry. By adhering to these principles, researchers not only protect participants but also enhance the validity and impact of their findings, paving the way for responsible and effective healthcare solutions globally. The ongoing dialogue among industry leaders emphasizes the need for a commitment to ethical practices that resonate with the diverse populations involved in clinical research, ultimately fostering trust and advancing public health.
Frequently Asked Questions
What are the basic moral principles that guide clinical studies?
The basic moral principles are respect for individuals, beneficence, and justice. Respect for persons emphasizes participant autonomy, beneficence focuses on maximizing benefits while minimizing harm, and justice ensures equitable selection of participants.
Why is respect for persons important in clinical research?
Respect for persons is important because it recognizes participant autonomy, ensuring that individuals have the right to make informed decisions regarding their involvement in research.
What does beneficence entail for researchers?
Beneficence mandates that researchers strive to maximize potential benefits of the research while simultaneously minimizing harm to participants.
Can you provide an example of the consequences of failing to adhere to ethical principles in clinical research?
A notable example is the TGN1412 trial, where six healthy males became seriously ill shortly after administration of the drug, highlighting the critical nature of moral oversight in clinical studies.
What does justice focus on in the context of clinical studies?
Justice focuses on the equitable selection of participants and safeguards vulnerable populations from exploitation.
Why are ethical principles particularly critical in global research trials?
Ethical principles are critical in global research trials due to varied cultural and regulatory landscapes that can complicate adherence to these principles.
How do guidelines impact clinical trial results?
Strict compliance with ethical guidelines not only meets moral responsibilities but also positively impacts the results of clinical trials.
What are some ethical challenges faced when conducting clinical research in low- and middle-income countries (LMIC)?
Ethical challenges include disparities in healthcare access, cultural misunderstandings, and the potential exploitation of vulnerable populations.
What is the significance of customizing studies to local populations?
Customizing studies to local populations ensures that the research is relevant and beneficial, building a foundation for knowledge that can lead to effective interventions and policies.
What service capabilities are essential for conducting clinical trials in LMIC?
Essential service capabilities include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting on research status.
How do clinical studies impact local economies in LMIC?
Clinical studies contribute to job creation, economic growth, and healthcare improvement through international collaboration.
What should clinical trials ideally focus on in developing countries?
Clinical trials should ideally focus on locally relevant diseases and provide affordable care to ensure ethical conduct and community benefit.
How can researchers foster trust and transparency in clinical trials?
Researchers can foster trust and transparency by prioritizing local expertise in review boards, allowing for insights into community values and expectations.

