Overview
The article addresses the critical ethical considerations for clinical trials in Colombia, underscoring the necessity of adhering to foundational principles such as:
- Respect for autonomy
- Beneficence
- Non-maleficence
- Justice
It elaborates on the regulatory framework established by INVIMA, which, in conjunction with the comprehensive management services provided by organizations like bioaccess®, ensures that trials are conducted with the utmost ethical standards. This framework not only protects participant rights but also fosters trust in the research process, which is essential for the integrity of clinical research in the region.
Introduction
In the realm of clinical trials, ethical considerations stand as essential pillars that uphold the integrity and trustworthiness of medical research. As Colombia positions itself as a key player in the global clinical trial landscape, the emphasis on ethical practices grows increasingly vital. With a diverse population and a regulatory framework governed by INVIMA, the nation navigates the complexities of informed consent, participant recruitment, and data privacy.
This article delves into the multifaceted ethical landscape of clinical trials in Colombia, exploring the guiding principles for researchers, the regulatory environment shaping their practices, and the ongoing challenges they face. Through this examination, the significance of ethical integrity in advancing medical research and protecting participant rights is underscored, highlighting the commitment to fostering a responsible and inclusive clinical research environment.
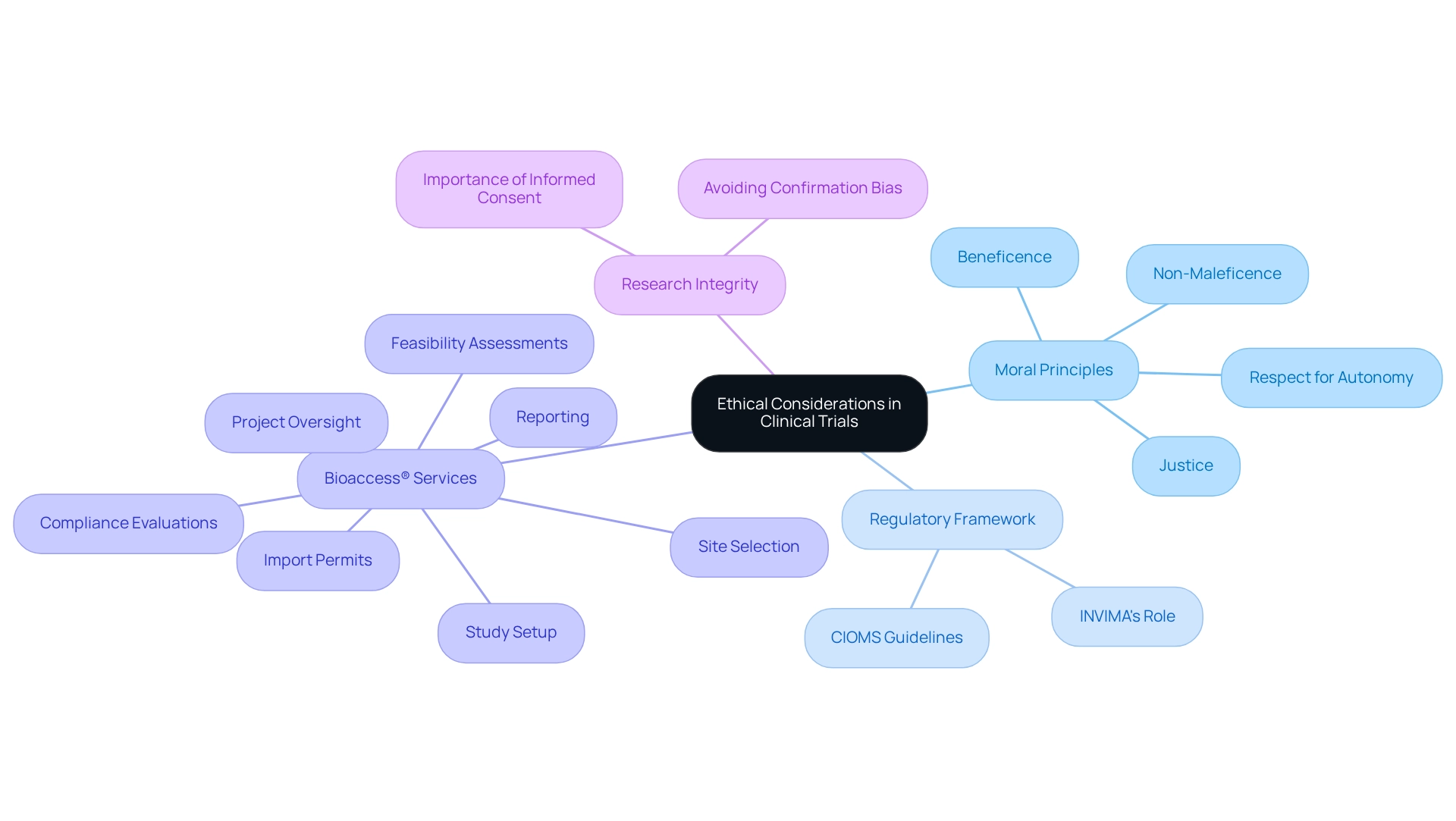
Defining Ethical Considerations in Clinical Trials
Ethical factors in research studies are crucial for safeguarding the rights, safety, and well-being of participants. Central to these considerations are the principles of respect for autonomy, beneficence, non-maleficence, and justice. These moral principles guide researchers in navigating the complexities of medical studies, ensuring compliance with established standards.
In Colombia, ethical considerations for trials are intensified by the nation's rich cultural and social diversity, necessitating a nuanced approach to oversight, particularly under the regulatory framework set forth by INVIMA (Colombia National Food and Drug Surveillance Institute).
INVIMA plays a pivotal role in overseeing the marketing and manufacturing of health products, including medical devices, ensuring adherence to health standards. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's rigorous supervision enhances the ethical considerations for trials in Colombia. This regulatory environment empowers bioaccess® to lead Medtech research in Latin America, focusing on innovation and regulatory excellence.
Bioaccess® offers comprehensive management services for research studies, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
These services are meticulously designed to ensure that medical trials are conducted efficiently and ethically, upholding the highest standards of regulatory compliance.
The American Statistical Association has established formal guidelines that underscore the significance of integrity in research, reinforcing the necessity of moral principles in statistical practice. Additionally, the CIOMS guidelines, released in 2002, provide a framework for appropriate conduct in clinical research, emphasizing the importance of informed consent and the obligation to communicate research results to participants. It is imperative for researchers to inform participants about study outcomes, and participants should feel empowered to request this information if it is not readily available.
As Pliny the Elder aptly noted, "This only is certain, that there is nothing certain," highlighting the inherent uncertainties in research. A pertinent case study titled "Avoiding Confirmation Bias in Research" cautions against the unconscious preference for anticipated results over contrary evidence. This bias can undermine analytical objectivity, emphasizing the necessity of maintaining rigorous standards in research practices.
By prioritizing moral principles, researchers can bolster the credibility of their findings and contribute to a more robust scientific inquiry.
As we approach 2025, the significance of these moral principles remains paramount. Upholding autonomy, beneficence, non-maleficence, and justice not only fosters trust between researchers and participants but also ensures that trials are conducted with the highest moral standards. This commitment to ethical integrity, supported by INVIMA's regulatory framework, is vital for advancing medical research and underscores the ethical considerations for trials in Colombia to protect participants.
Furthermore, the impact of Medtech research studies on local economies, including job creation and healthcare enhancement, underscores the greater significance of ethical considerations for trials in Colombia.
Overview of Colombian Regulations Governing Clinical Trials
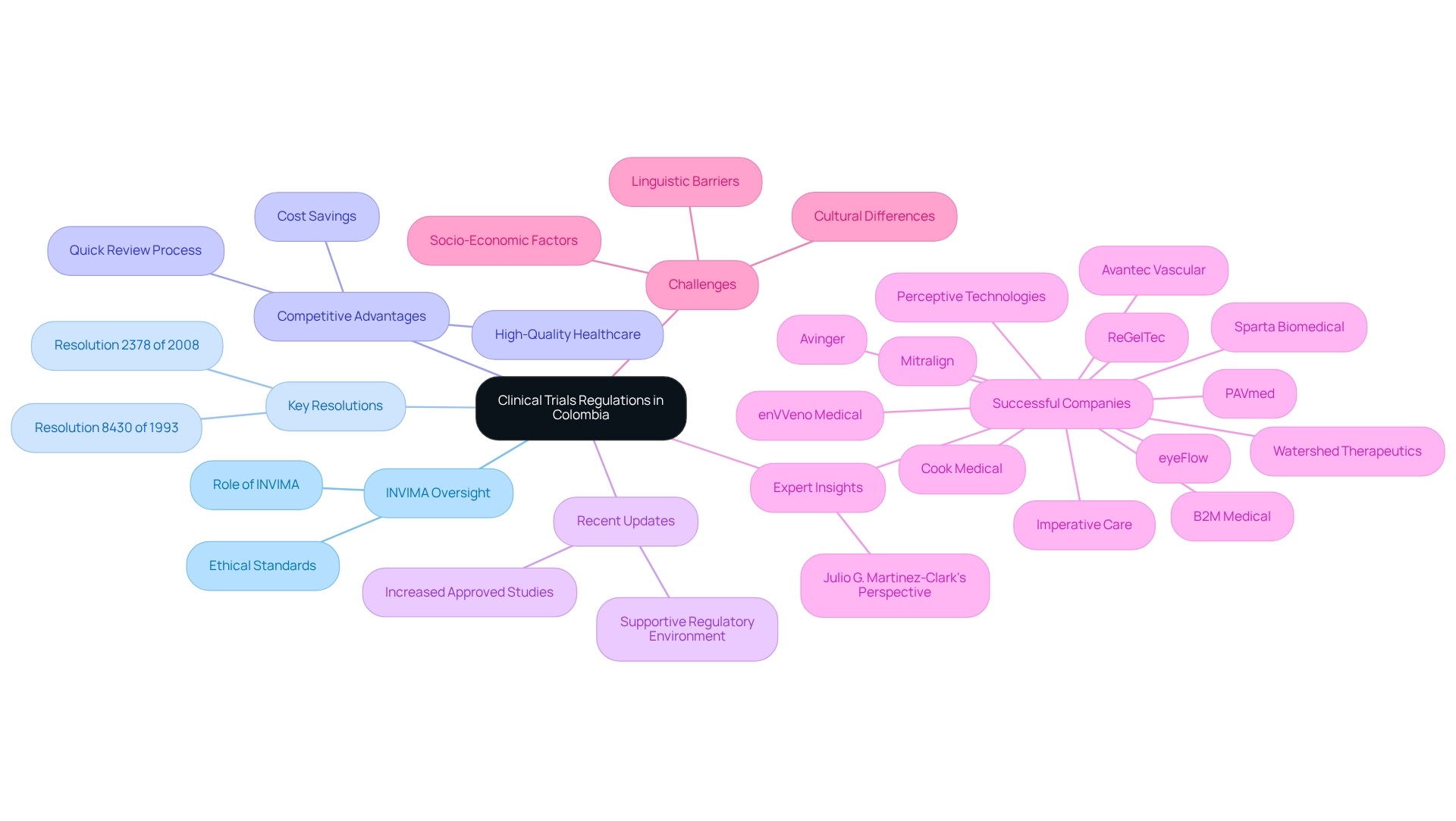
In Colombia, the oversight of medical studies is primarily managed by the National Food and Drug Surveillance Institute (INVIMA), which enforces several critical resolutions, notably Resolution 8430 of 1993 and Resolution 2378 of 2008. These regulations establish rigorous standards for ethical review procedures, informed consent methods, and the protection of rights, underscoring the ethical considerations for trials in Colombia to ensure the prioritization of individual well-being throughout the research process.
Adherence to INVIMA regulations transcends mere legal obligation; it is essential for maintaining the ethical integrity of studies, particularly in light of the ethical considerations for trials in Colombia. Researchers must navigate these regulations with care to uphold the rights and safety of participants, which is vital for fostering trust and credibility within the research community.
Colombia offers significant competitive advantages for first-in-human studies, including cost savings exceeding 30% compared to North America and Western Europe, alongside a regulatory review process that typically spans only 90-120 days. The nation boasts a high-quality healthcare system, ranked 22nd by the World Health Organization, and is home to hospitals recognized as some of the finest in Latin America. With a population exceeding 50 million and approximately 95% covered by universal healthcare, patient recruitment is robust, further enhancing the feasibility of research studies.
Recent updates to research regulations in Colombia reflect a commitment to fortifying the regulatory framework, rendering it more favorable for both local and global research initiatives. For instance, the Colombian research landscape has witnessed a surge in successful studies, with companies like Mitralign and Avinger capitalizing on the supportive regulatory environment to conduct innovative investigations.
Statistics indicate that Colombia's oversight of research has become increasingly efficient, with INVIMA reporting a significant increase in the number of approved studies in recent years. This trend highlights the effectiveness of the regulatory framework in promoting responsible research practices. Furthermore, the broader Latin American research market is projected to reach USD 506.1 million by 2030, emphasizing the region's growing significance in the global arena.
Expert insights underscore the importance of Resolution 8430 of 1993 and Resolution 2378 of 2008 in shaping the ethical considerations for trials in Colombia. Julio G. Martinez-Clark, CEO of bioaccess, notes that Colombia’s combination of a large and diverse population, experienced research sites, and efficient regulatory processes positions it as an attractive option for U.S. medical device firms aiming to conduct medical studies.
Moreover, the diverse disease landscape in Latin America, including the rising incidence of conditions such as cancer and heart disease, presents numerous opportunities for specialized research. However, addressing linguistic, cultural, and socio-economic challenges is crucial for the success of outsourced medical studies in the region.
As the research environment continues to evolve, staying informed about the ethical considerations for trials in Colombia and their implications is essential for investigators pursuing responsible and compliant research. bioaccess™ stands out as a vetted CRO and consulting partner for U.S. medical device firms, offering comprehensive trial management services that encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
The Importance of Informed Consent in Clinical Research
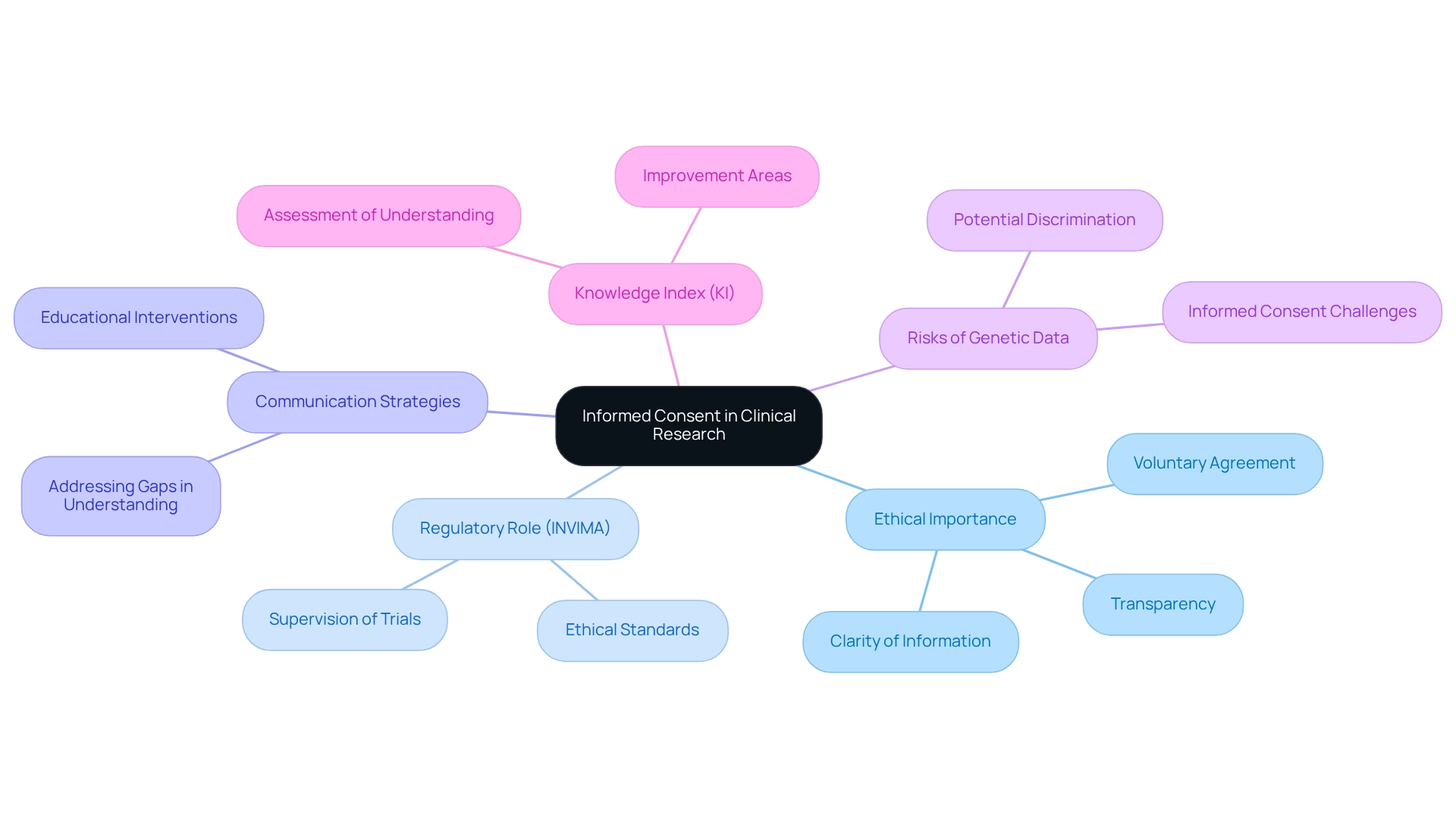
Informed consent serves as a cornerstone of ethical medical research, ensuring that individuals are thoroughly informed about the study's nature, potential risks, and benefits before agreeing to participate. In Colombia, the informed consent process reflects the ethical considerations for trials, functioning not only as a regulatory requirement but also as a moral obligation that must be meticulously documented. This documentation should encompass clear and comprehensible information tailored to the individual's level of understanding, ensuring transparency regarding all aspects of the study.
The importance of informed consent in research studies cannot be overstated, particularly in 2025, as it fosters trust between researchers and subjects. With over 15 years of experience in the Medtech sector, bioaccess® emphasizes the necessity of educational interventions to enhance understanding of informed consent, addressing existing gaps in both developed and developing countries. For instance, a recent discussion among ethics committee members highlighted the risks and benefits associated with broad consent for future use, underscoring the need for clear communication to prevent exploitation and ensure individuals are fully aware of how their data may be utilized.
Statistics indicate that many individuals involved in research studies may not fully comprehend the consequences of their agreement, which can lead to vulnerabilities, particularly concerning genetic information. As CEI-020, the President, noted, "These studies can generate pharmacogenetic profiles which makes communities easily identifiable later... these communities are susceptible to potential discrimination based on the genetic markers." Effective informed consent procedures in Colombian medical research must, therefore, incorporate ethical considerations for trials, ensuring strategies for documenting consent that prioritize understanding and voluntary agreement, free from coercion.
Instances of effective informed consent methods in Colombia illustrate the significance of ethical considerations for trials, demonstrating the potential for improved engagement of individuals and adherence to ethical standards, ultimately strengthening the integrity of research studies. Furthermore, the development of a Knowledge Index (KI) to assess individuals' knowledge emphasizes ongoing efforts to enhance comprehension of informed consent in research studies. The KI aims to evaluate how well individuals understand the information presented during the consent process, thereby identifying areas for improvement.
As the regulatory body, INVIMA plays a vital role in supervising these processes, ensuring that trials adhere to the highest standards of ethical conduct. Notably, INVIMA is designated as a Level 4 health authority by PAHO/WHO, which underscores its capability to oversee health products and guarantee safety for individuals. As the landscape of medical research evolves, the significance of informed consent remains a critical focus, ensuring that individuals are not only informed but also empowered in their decision-making processes.
Ethical Recruitment Practices in Colombian Clinical Trials
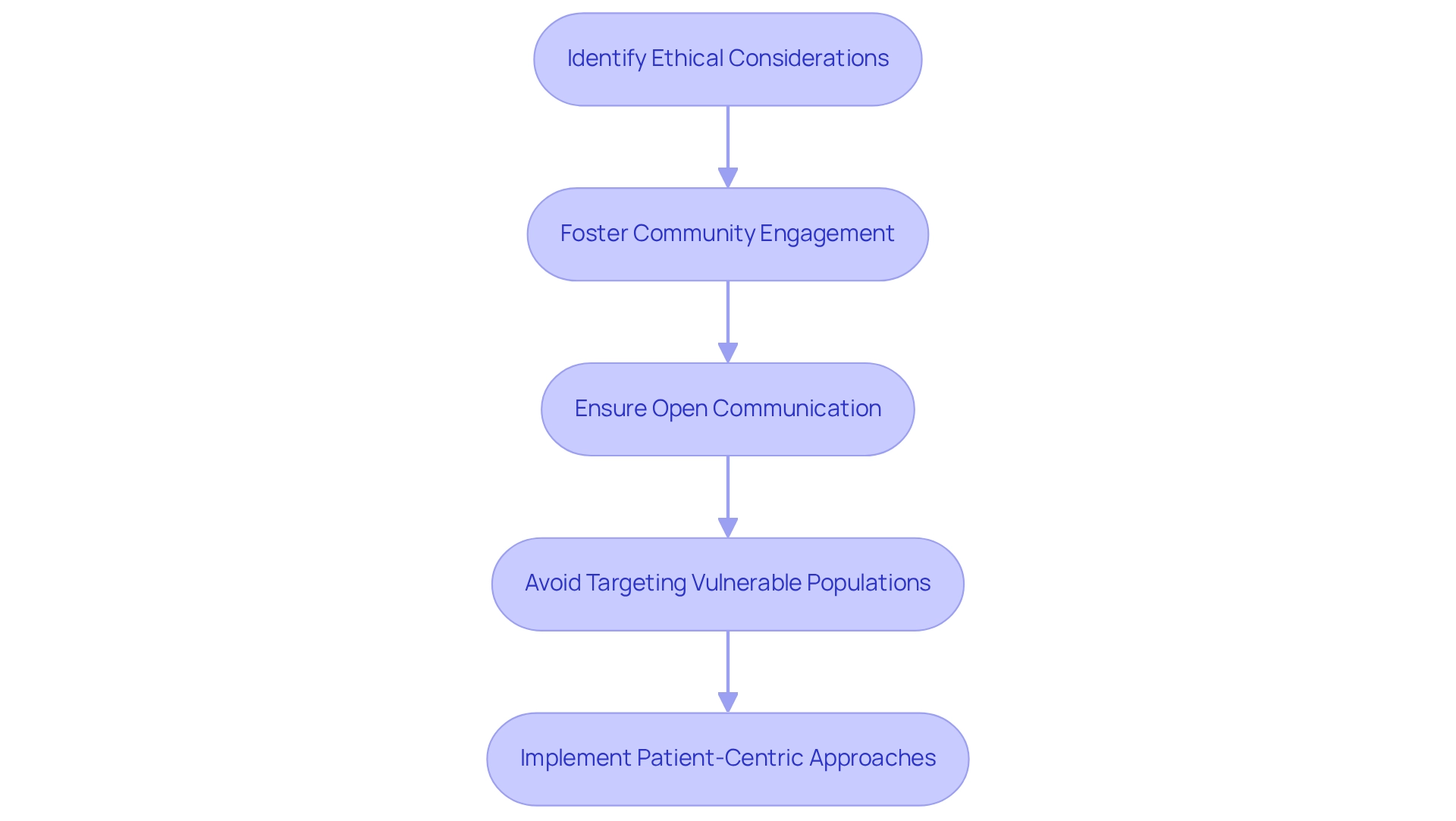
Ethical recruitment methods are crucial for guaranteeing that individuals in clinical trials are chosen equitably and without bias. In Colombia, ethical considerations for trials are significantly impacted by the socio-economic environment, as disparities in income and education can influence individuals' access to information and their willingness to participate in studies. To navigate these challenges, researchers must adopt culturally sensitive approaches that resonate with diverse communities.
Key strategies for ethical recruitment include fostering community engagement, which involves building trust and rapport with local populations. This can be accomplished through outreach programs that inform prospective individuals about the study's purpose and advantages. Open communication is essential; researchers should clearly express the study's goals and ensure that individuals fully comprehend their rights and the implications of their involvement.
Moreover, ethical considerations for trials in Colombia dictate that we must avoid targeting vulnerable populations unless there is a compelling justification and robust safeguards are in place. Ethical recruitment practices not only safeguard individuals but also improve the validity of the research by ensuring a representative sample. Statistics show that challenges in participant recruitment can result in delays spanning from one to six months for numerous studies, highlighting the significance of efficient strategies.
In 2025, ethical considerations for trials in Colombia will increasingly emphasize community involvement and cultural awareness in recruitment practices. Case studies, such as the collaboration between bioaccess™ and Caribbean Health Group, highlight the importance of understanding local contexts and addressing the unique needs of different populations. This collaboration seeks to establish Barranquilla as a prominent location for medical studies in Latin America, backed by Colombia's Minister of Health, which further underscores the necessity for patient-focused strategies.
Moreover, media attention from Clinical Leader has emphasized these initiatives, demonstrating the increasing interest in research studies in the area. By applying these strategies, researchers can enhance subject retention and the overall quality of trials, ultimately leading to more trustworthy and significant outcomes. As Natalie Yeadon noted, "The problem with clinical trials often lies in the recruitment process, which can significantly affect the outcomes and integrity of the research."
This reinforces the need for patient-centric approaches that prioritize participants' needs and perspectives. Furthermore, the partnership has accomplished more than a 50% decrease in recruitment time and 95% retention rates, showcasing the efficiency of these responsible recruitment practices.
The Role of Ethics Committees in Clinical Trials
Ethics committees, commonly referred to as Institutional Review Boards (IRBs), are pivotal in overseeing clinical studies, particularly in light of the ethical considerations for trials in Colombia. These committees bear the critical responsibility of reviewing study protocols to ensure adherence to ethical standards and the protection of participant rights. They meticulously assess the risk-benefit ratio of proposed studies, oversee ongoing experiments, and ensure compliance with both national and international regulatory standards.
In Colombia, the process for obtaining research study approval consists of several essential steps. Researchers must first secure approval from their site's IRB/ethics committee (EC), followed by obtaining study approval from Colombia's regulatory agency, INVIMA (National Food and Drug Surveillance Institute). Finally, an import permit from the Ministry of Industry and Commerce (MinCIT) is required to ship investigational devices to their site. This structured approach is crucial for safeguarding participants and enhancing the integrity of the research conducted.
Current data indicate that IRBs in Colombia are committed to upholding high standards, emphasizing ethical considerations for trials, with approval rates for clinical research protocols reflecting a thorough examination of moral factors. The IRB evaluates the research protocol to ensure clarity and focus on the research question, which is vital for the responsible execution of studies.
The role of ethics panels extends beyond initial protocol endorsement; they engage in ongoing oversight of studies to ensure that moral standards are consistently upheld. This includes systematic discussions during meetings, which are essential for fostering multidisciplinarity within the committees. Such discussions enhance the committees' ability to address complex ethical issues that may arise during legal proceedings.
As Glenn Begley noted, "These hypothesis generating trials should not, under any circumstances, initiate a research trial," underscoring the potential consequences of inadequate examination in research trials.
A notable case study exploring the impact of Clinical Ethics Committees (CECs) on institutional policy preparation illustrates the complexities involved in assessing how these policies affect clinical practice. The research found that while evaluating policy impact is challenging due to numerous influencing factors, the policy development process itself can significantly enhance awareness and discussion of ethical issues among clinicians. Moreover, it is important to recognize that REC approvals can facilitate the recruitment of subjects into morally ambiguous studies due to insufficient examination of research outcomes, highlighting the need for robust review processes.
As of 2025, the role of ethics committees in Colombian research studies remains crucial, ensuring that the rights and welfare of participants are prioritized. This oversight is particularly significant given recent discussions regarding the adequacy of examination in ethically questionable studies, which emphasizes the ethical considerations for trials in Colombia to prevent improper treatment of patients due to erroneous research results. Furthermore, bioaccess offers comprehensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
These services are designed to help overcome the regulatory challenges faced by medical device startups in Colombia, ensuring that ethical standards are upheld throughout the research process.
Data Privacy and Confidentiality in Clinical Trials
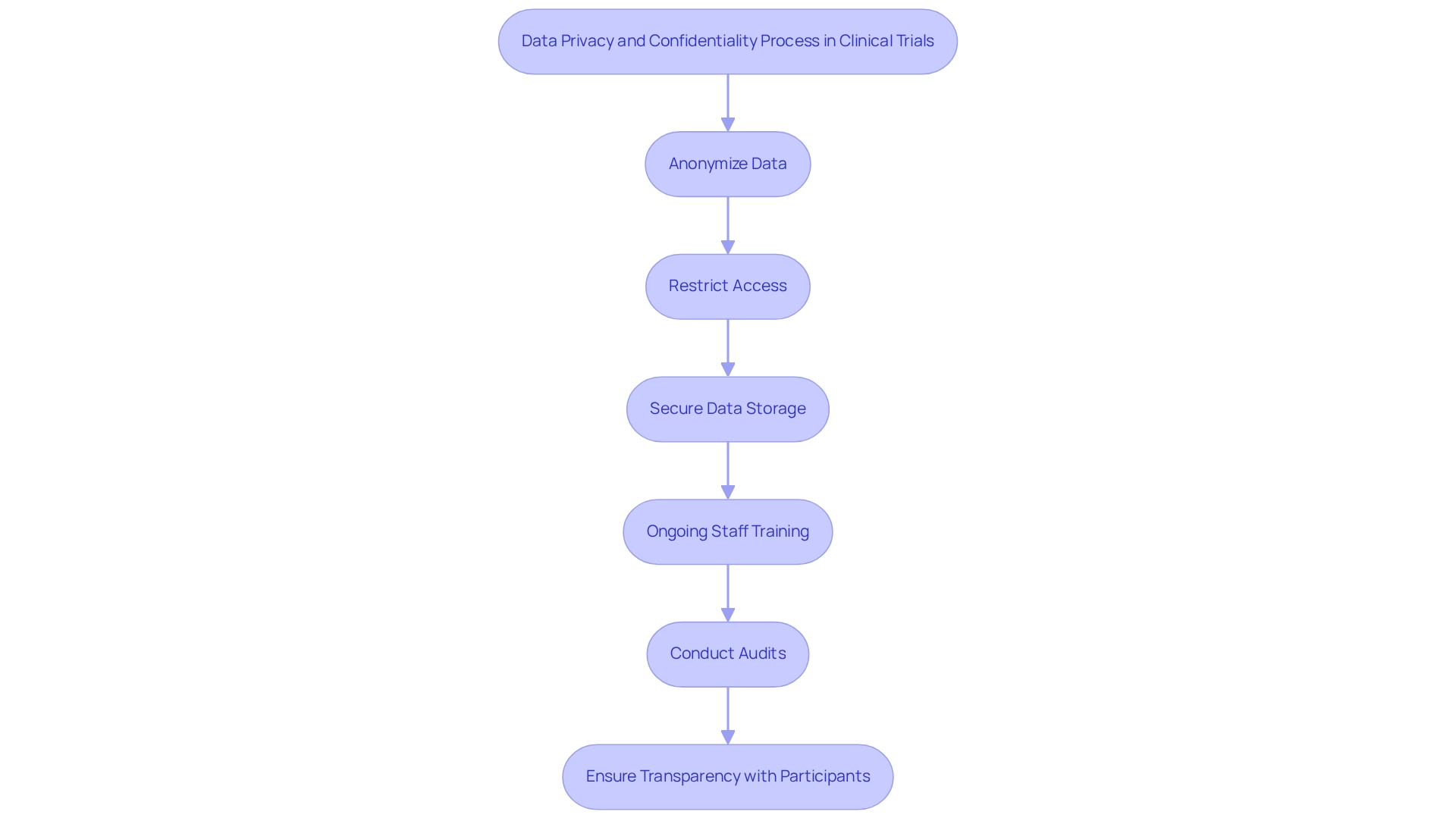
In Colombia, data privacy and confidentiality stand as critical components of clinical trials, governed by Law 1581 of 2012, which establishes stringent regulations for the protection of personal data. Researchers are mandated to adopt comprehensive data protection strategies to safeguard the information of those involved. This encompasses practices such as:
- Anonymizing data to prevent identification
- Restricting access to sensitive information to authorized personnel only
- Ensuring secure storage of data to mitigate risks of breaches
Furthermore, it is essential for researchers to provide ongoing training for staff on data protection protocols and conduct audits to confirm adherence to established practices. This commitment not only strengthens compliance but also enhances the overall integrity of the research process.
Moreover, researchers must uphold transparency with individuals regarding the use of their data. Participants should be clearly informed about how their information will be utilized and the specific measures implemented to protect their privacy. This approach not only fosters trust but also aligns with moral standards and regulatory obligations, ensuring that the rights of individuals are maintained throughout the research process.
As medical studies evolve in Colombia, adherence to these data privacy regulations is paramount. The implications of Law 1581 of 2012 extend beyond mere compliance; they signify a commitment to principled research practices that prioritize participant welfare and data integrity. Additionally, INVIMA, the Colombia National Food and Drug Surveillance Institute, has specific requirements for approving first-in-human medical device research studies, emphasizing safety, scientific validity, and ethical conduct that researchers must follow in their investigations.
In this context, Mintao Nie's observation regarding decentralized governmental structures underscores the challenges in adhering to international human rights standards. This highlights the ethical considerations for trials in Colombia and emphasizes the necessity of robust data protection measures. By implementing these strategies, researchers can enhance the credibility of their studies and contribute to the advancement of medical technology in a responsible manner, aligning with bioaccess®'s expertise and tailored approach to research. Furthermore, bioaccess® is dedicated to addressing any concerns related to data protection through established grievance procedures, ensuring that client concerns are managed with compliance and transparency.
Addressing Ethical Challenges in Clinical Trials
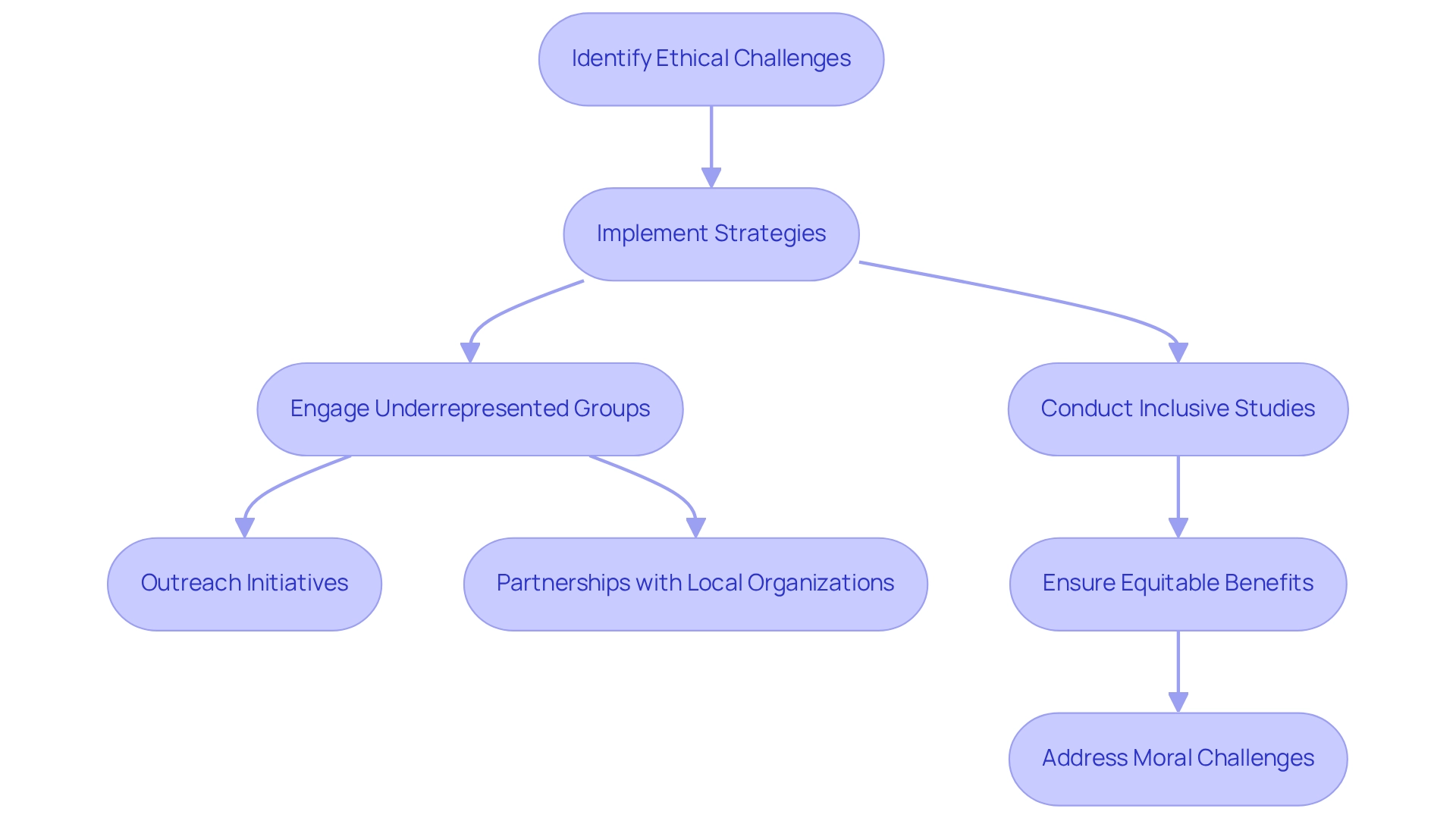
Ethical challenges in clinical trials frequently arise from disparities in access to treatment and the potential exploitation of vulnerable populations. In Colombia, ethical considerations for trials necessitate that researchers ensure equitable benefits for all contributors. This involves actively implementing strategies to engage underrepresented groups and designing studies that do not inadvertently disadvantage any demographic.
For example, a case study on adaptive randomization techniques illustrates how biases can skew treatment estimations, underscoring the need for meticulous study design to avert moral pitfalls.
Statistics indicate that involving too few individuals can lead to flawed outcomes and a lack of diversity within the study group, thereby exacerbating existing inequalities in access to treatment. This highlights the critical importance of principled research practices that prioritize inclusivity, particularly regarding ethical considerations for trials in Colombia. As one participant remarked, "If the AI is efficacious, it’s an accurate diagnostic, but is this cost-effective if it costs a million dollars to run?"
This perspective emphasizes the urgent need for cost-effective solutions in clinical studies, especially in light of ethical considerations.
To tackle these challenges, ongoing moral training and heightened awareness among researchers are imperative. Current strategies for ensuring equitable access to treatment in research reflect the ethical considerations for trials in Colombia, including outreach initiatives aimed at marginalized communities and partnerships with local organizations to enhance participation. Furthermore, bioaccess™ aims to expedite the development of medical devices through its expertise in overseeing Early-Feasibility Studies, First-In-Human Studies, and other essential assessments, which can significantly influence the resolution of these moral challenges.
The collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, represents a significant step forward in positioning Barranquilla as a leading hub for research studies in Latin America.
As Colombia navigates the complexities of medical research in 2025, ethical considerations for trials in Colombia remain vital in addressing these moral challenges. Recent discussions in ethics, medicine, and public health, such as those highlighted in Rouault-Plantaz's article, further emphasize the necessity of fostering an inclusive environment. By prioritizing equitable access, researchers can contribute to a more just and efficient research landscape.
Future Directions for Ethical Practices in Colombian Clinical Trials
As medical studies in Colombia continue to progress, there is a growing emphasis on ethical considerations for trials in Colombia to align with global standards. This evolution is marked by several key trends:
- A commitment to transparency in testing processes
- Active involvement of community stakeholders in research design
- The incorporation of technology to streamline informed consent procedures
For example, the concept of 'Quality by Design' highlights the significance of comprehensive planning in research, promoting a careful strategy that reduces reactive protocol changes and improves overall study efficiency.
This method aligns with new regulatory guidelines that prioritize efficiency and quality in research execution, as highlighted in the case study on 'Quality by Design in Research.' Furthermore, the future of principled practices in Colombian research studies is expected to be influenced by ongoing regulatory modifications that emphasize data protection and patient rights. In this context, bioaccess provides extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
These services are crucial for maintaining high ethical standards and ensuring that researchers remain vigilant and engaged in discussions surrounding these practices. The integration of sophisticated encryption and data anonymization methods will further strengthen the integrity of medical studies, promoting trust among contributors and stakeholders alike.
The significance of technology cannot be overstated. Innovations in digital platforms are transforming the informed consent process, making it more accessible and comprehensible for individuals. As Rahul Bafna observed, the application of healthcare technology at the consumer level has surged over the past decade, greatly affecting moral practices in medical studies.
As we approach 2025, the focus on ethical considerations for trials in Colombia will likely showcase a wider commitment to improving participant involvement and protecting their rights, ultimately resulting in more robust and ethically responsible research outcomes. However, it is essential to remain aware of the fundamental business model challenges in biotech, as warned by Max Baumann, especially as the clinical end-markets become increasingly crowded.
Conclusion
The ethical landscape of clinical trials in Colombia is intricately woven with the principles of respect for autonomy, beneficence, non-maleficence, and justice. These principles not only guide researchers in their conduct but also enhance the integrity and credibility of medical research. The role of INVIMA as the regulatory body is pivotal, providing a robust framework that ensures participant safety and ethical compliance throughout the research process. As Colombia continues to emerge as a significant player in the global clinical trial arena, the commitment to these ethical standards becomes increasingly crucial.
Informed consent, ethical recruitment practices, and data privacy are fundamental components that reinforce the ethical foundation of clinical trials. By prioritizing clear communication, community engagement, and stringent data protection measures, researchers can foster trust and empower participants, ensuring that their rights and welfare are protected. The active involvement of ethics committees further ensures that trials adhere to both national and international ethical standards, safeguarding participants against potential exploitation and bias.
Looking ahead to 2025, the ongoing evolution of ethical practices in Colombian clinical trials will likely be characterized by increased transparency, community engagement, and technological advancements. These developments not only promise to enhance participant understanding and involvement but also aim to address the socio-economic disparities that can affect trial accessibility. By embracing these ethical imperatives, Colombia can solidify its reputation as a leader in responsible and inclusive clinical research, ultimately contributing to better healthcare outcomes and advancements in medical technology.
Frequently Asked Questions
What are the main ethical principles guiding research studies?
The main ethical principles guiding research studies are respect for autonomy, beneficence, non-maleficence, and justice. These principles help ensure the rights, safety, and well-being of participants.
How does Colombia's cultural diversity affect ethical considerations in research trials?
Colombia's rich cultural and social diversity necessitates a nuanced approach to ethical oversight in research trials, which is particularly addressed under the regulatory framework established by INVIMA.
What role does INVIMA play in overseeing medical studies in Colombia?
INVIMA (Colombia National Food and Drug Surveillance Institute) oversees the marketing and manufacturing of health products, ensuring compliance with health standards and enhancing the ethical considerations for trials in Colombia.
What services does Bioaccess® provide for research studies?
Bioaccess® offers comprehensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting.
What guidelines emphasize the importance of integrity in research?
The American Statistical Association has established guidelines that highlight the significance of integrity in research, along with the CIOMS guidelines, which stress the importance of informed consent and communicating research results to participants.
Why is it important for researchers to inform participants about study outcomes?
It is important for researchers to inform participants about study outcomes to uphold ethical standards and ensure participants feel empowered to request this information if it is not readily available.
What competitive advantages does Colombia offer for first-in-human studies?
Colombia offers significant cost savings exceeding 30% compared to North America and Western Europe, a fast regulatory review process (typically 90-120 days), and a high-quality healthcare system with robust patient recruitment.
How have recent updates to research regulations in Colombia impacted the research landscape?
Recent updates have fortified the regulatory framework, making it more favorable for local and global research initiatives, leading to a surge in successful studies and increased efficiency in the approval of research.
What challenges must be addressed for successful outsourced medical studies in Latin America?
Challenges such as linguistic, cultural, and socio-economic factors must be addressed to ensure the success of outsourced medical studies in the region.
What is the projected growth for the broader Latin American research market by 2030?
The broader Latin American research market is projected to reach USD 506.1 million by 2030, indicating the region's growing significance in the global research arena.




