Introduction
Navigating the FDA's 510(k) submission process for medical devices requires a comprehensive understanding of the different pathways available. The Traditional, Special, and Abbreviated 510(k) routes each have distinct requirements and considerations. To ensure a successful submission, it is crucial to thoroughly analyze the device's intended user base, understand its usage instructions, and comprehend all warnings and precautions.
Collaborating with the marketing department can provide valuable insights into the competitive landscape and potential predicate devices. Additionally, consulting the FDA's 510(k) database for Summaries of Safety and Effectiveness Data (SSEDs) is essential for discerning the nuances among devices. By meticulously preparing the submission and aligning it with the specific requirements of the chosen pathway, medical device manufacturers can navigate the FDA approval process more effectively, bringing their products to market with confidence and compliance.
Types of 510(k) Submissions: Traditional, Special, and Abbreviated
Navigating the FDA's 510(k) submission process requires a comprehensive understanding of the three primary pathways: Traditional, Special, and Abbreviated. The Traditional 510(k) is the standard route for most devices, necessitating a thorough comparison with one or more similar legally marketed devices, known as predicates, to demonstrate substantial equivalence. For the Special 510(k), the focus is on devices that have undergone minor modifications and where the manufacturer can reference its own existing data.
The Abbreviated 510(k) leverages guidance documents, special controls, and consensus standards to streamline the review process.
To ensure a clear and successful submission, it is imperative to delve deeply into the device's intended user base, which may include clinicians, physicians, dentists, and patients, and to fully comprehend the device's usage instructions along with all warnings and precautions. Collaborating with the marketing department to grasp the competitive landscape can provide valuable insights into potential predicate devices that share the intended use and technological characteristics with the subject device. A comparative analysis, facilitated by the creation of a table to juxtapose these devices, is a strategic step in this process.
Moreover, it's essential to consult the Summaries of Safety and Effectiveness Data (SSEDs) from the FDA's 510(k) database to discern the nuances between your device and the identified predicates. Remember that any comments or data submitted to the FDA as part of this process will become public record; hence, confidential information must be carefully excluded or submitted via the specified written/paper methods to protect sensitive details.
By meticulously preparing your submission and ensuring it aligns with the specific requirements of the chosen 510(k) pathway, you can navigate the FDA approval process more effectively, thereby bringing your medical device to market with greater confidence and compliance.
Finding a Predicate Device
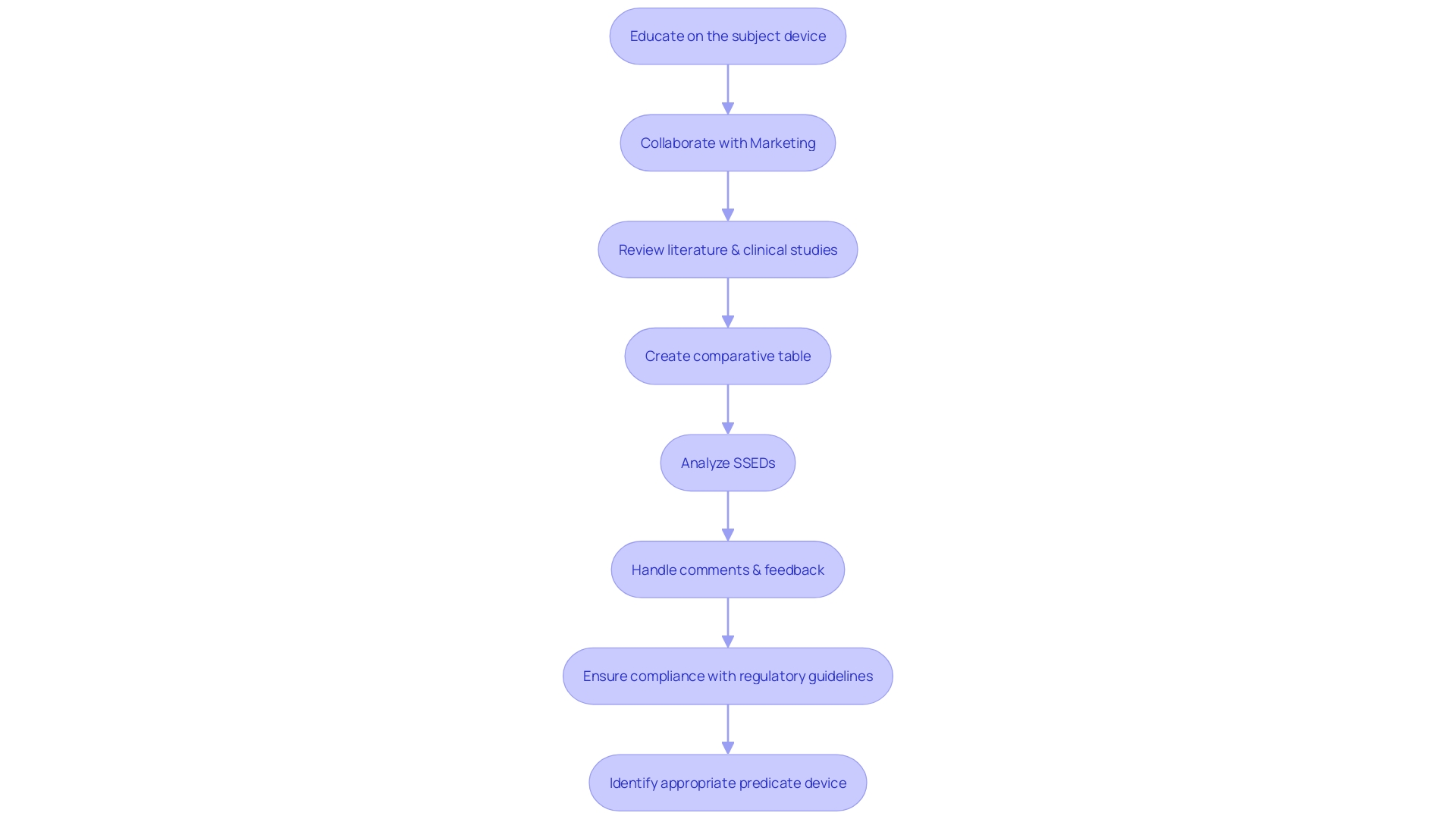
Identifying an appropriate predicate device is a pivotal step in the FDA 510(k) submission process for medical devices. The task involves a thorough investigation into existing devices that are similar in intended use and technological characteristics to the new device. A comprehensive understanding of the subject device, its users, and its operational instructions is essential.
Collaborating with the marketing department can provide valuable insights into the competitive landscape, which is beneficial when reviewing research literature, clinical studies, and other informational materials to determine a suitable predicate device.
A predicate device is essentially an already FDA-cleared device that serves as a benchmark for comparison. Creating a comparative table can streamline the process of contrasting your device against potential predicates, focusing on their similarities and differences. This comparison is crucial as it forms the basis of demonstrating substantial equivalence, a key FDA requirement.
In-depth analysis of the Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA’s 510(k) database is indispensable. These summaries provide detailed information on predicate devices' safety and effectiveness, which can guide developers in aligning their devices with established standards.
It’s imperative to handle comments and feedback during this process with discretion, ensuring confidentiality and compliance with regulatory guidelines when submitting any documentation to the FDA. The diligent evaluation of predicate devices not only supports a successful 510(k) submission but also upholds the FDA's mandate to protect public health by assuring the safety and effectiveness of medical devices.
Locating and Using FDA Guidance Documents
Navigating FDA guidance documents is a critical step for medical device manufacturers to comply with regulatory standards. The FDA, as a protector of public health, ensures the safety and efficacy of medical devices. It is paramount for manufacturers to be aware of and adhere to the guidelines set forth by the FDA to avoid the risks associated with non-compliance, such as legal consequences, fines, or recalls.
To leverage these comprehensive resources, manufacturers must first understand that guidance documents are not legally binding but offer clarity and recommendations on regulatory issues. They can be located through the FDA's website, which provides a searchable database for ease of access. When submitting comments or feedback on these documents, it's essential to follow the proper procedures outlined by the FDA to ensure that no confidential information is inadvertently disclosed, as comments become part of the public record.
Moreover, in our digital era, the FDA encourages the adoption of modernized compliance programs to match the pace of technological advancement. Resistance to these changes can hinder innovation and delay the delivery of life-saving devices to the market. As noted by industry experts, embracing new methods and tools is necessary to maintain compliance while expediting product development.
By thoroughly understanding and utilizing FDA guidance documents, medical device manufacturers can not only ensure regulatory compliance but also contribute to the overarching goal of safeguarding public health.
Content Requirements for a Traditional 510(k) Submission
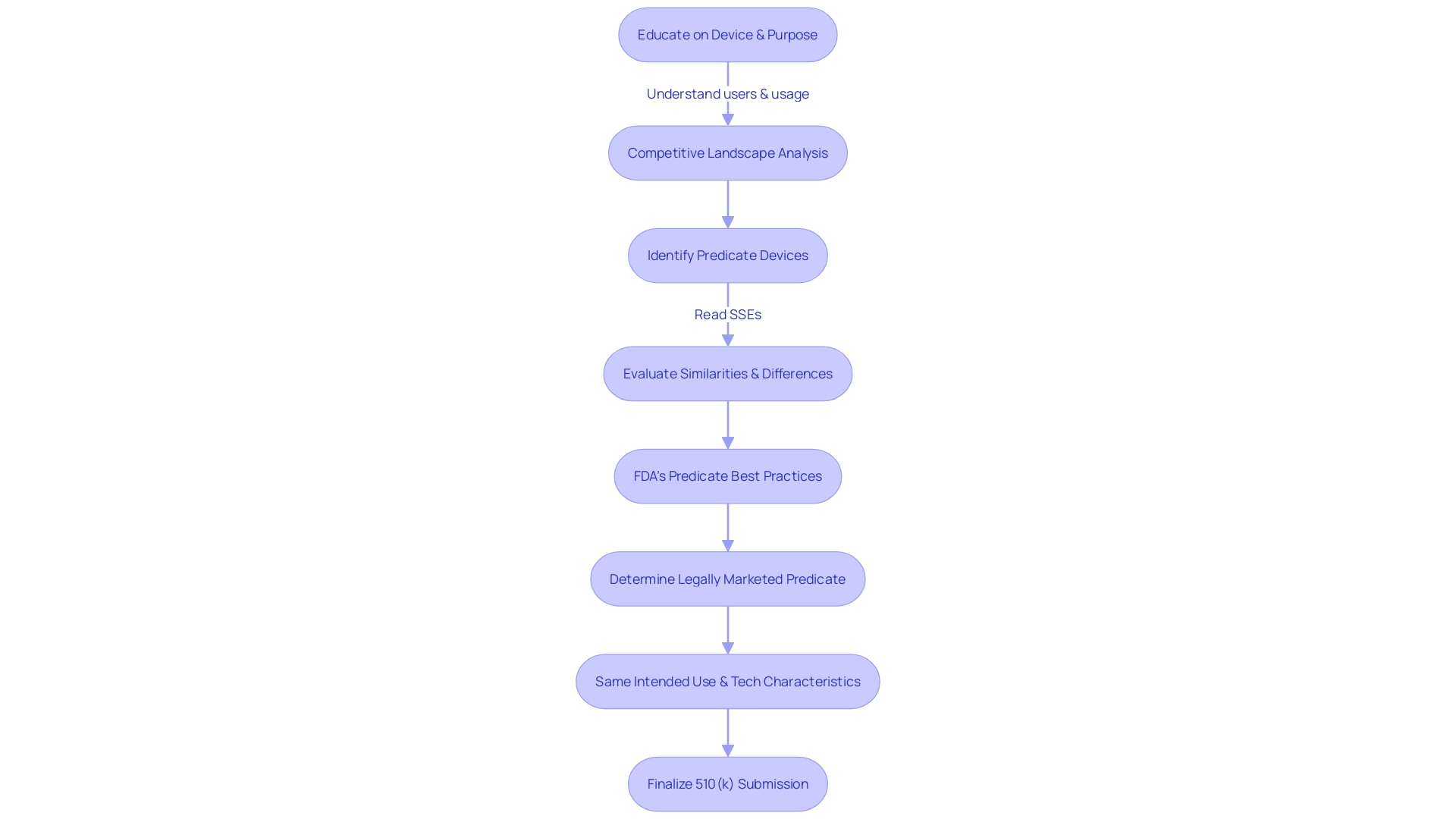
Preparing a traditional 510(k) submission involves a thorough understanding of the device and its intended use. Comprehensive knowledge of the device's users, such as clinicians, physicians, dentists, and patients, is crucial, along with a clear grasp of the instructions for use, which includes any warnings and precautions. Additionally, it is important to research the competitive landscape, seeking insights from marketing teams and reviewing materials like research literature, clinical studies, brochures, and labeling to identify potential predicate devices that share the same intended use and technological characteristics.
A comparative table becomes a pivotal tool in this process, highlighting the similarities and differences between your device and the selected predicate. This should include details that emerged from scrutinizing the Summaries of Safety and Effectiveness Data (Sets) available within the FDA's 510(k) database. When differences in indications for use or technological characteristics arise, clinical data may be necessary to demonstrate substantial equivalence.
This could involve a range of data, including results from clinical trials, which in the case of midomafetamine for PTSD treatment, showed statistically significant improvements in symptoms in the treatment group over the control group.
Statistical evidence from clinical trials, alongside non-clinical data such as bench testing, biocompatibility, and software testing, bolsters the submission. The FDA's draft guidance outlines scenarios when clinical data may be essential, such as when there's a difference in indications for use or technology between the new device and the predicate or when non-clinical testing alone cannot determine substantial equivalence.
Lastly, it's important to remember that all comments and data submitted to the FDA become part of the public docket. Confidential information should not be included unless it is submitted via written or paper submissions following detailed instructions provided by the FDA. By following these guidelines and utilizing case studies as references, you can ensure a timely and comprehensive 510(k) submission.
Preparing the Submission: Key Sections and Components
A comprehensive understanding of the medical device in question is paramount when preparing a 510(k) submission. This encompasses a thorough knowledge of the device's users, which may range from clinicians and physicians to dentists and patients, as well as detailed instructions for use, including critical warnings and cautions. In collaboration with Marketing teams, it's essential to investigate the competitive environment, paying special attention to devices that are similar in intended use and technological characteristics.
The process involves extensive review of materials such as research literature, clinical studies, and competitor labeling to construct a comparative table that outlines potential predicate devices.
Once potential predicate devices have been identified, the next step is to delve into the Summaries of Safety and Effectiveness Data (Seeds) available in the FDA's 510(k) database. This examination helps to discern the similarities and differences that will be crucial in drafting the submission. It is equally important to ensure the confidentiality of sensitive information throughout the submission process.
Submitters must be vigilant not to include personal medical information, Social Security numbers, or proprietary business details in publicly accessible comments and should follow specific instructions if submitting confidential information.
Cover Sheet Forms and Public Information
When preparing the 510(k) submission, the cover sheet forms and public information section are crucial elements requiring meticulous attention. It is essential to provide a comprehensive overview of the medical device, including its intended use, users (such as clinicians, physicians, dentists, and patients), and detailed instructions, with emphasis on warnings and precautions. Collaborate with marketing departments to understand the competitive landscape, identifying predicate devices and creating comparative analyses.
This involves scrutinizing research literature, clinical studies, and product labeling to ensure your device aligns with one that has previously been cleared by the FDA, noting both similarities and differences via a comparative table.
Moreover, it's important to navigate the regulatory environment with care. When submitting comments or additional information electronically, remember that all submissions become public records. Ensure that no confidential information is inadvertently disclosed.
If necessary, confidential information should be submitted in written form, following the specific instructions for written/paper submissions to maintain confidentiality.
Stay informed about the latest FDA guidelines and adopt clear, consumer-friendly language in all sections of the submission, including the presentation of data and safety information. This not only facilitates FDA review but also aligns with the agency's standards for transparency and clarity, as seen in their recent final rule regarding direct-to-consumer prescription drug advertisements.
Lastly, leverage resources such as the FDA’s 510(k) database, which contains Summaries of Safety and Effectiveness Data (SSEDs) for approved devices, providing valuable insights into the agency's decision-making process. This comprehensive approach to completing the 510(k) submission's cover sheet forms and public information section will facilitate a smoother FDA review and contribute to a more efficient regulatory process.
Comparing Your Device to the Predicate Device
Demonstrating substantial equivalence to a predicate device is an integral part of a 510(k) submission. Achieving a comprehensive understanding of the device in question, including its intended user base—ranging from clinicians and physicians to patients—is essential. Detailed knowledge of the device's instructions for use, alongside awareness of any potential warnings and cautions, forms the foundation for a successful comparison to a predicate device.
A proactive approach involves collaborating with Marketing teams to explore the competitive environment and home in on devices that may be suitable predicates. The creation of a comparative table, informed by an array of sources like research literature, clinical studies, and product labeling, is a critical step. Such a table should juxtapose the new device with identified predicate devices, highlighting their intended uses and technological characteristics.
Once potential predicates are pinpointed, a thorough review of their Summaries of Safety and Effectiveness Data (SSEDs) accessible through the FDA's 510(k) database is imperative. This evaluation must meticulously assess the similarities and differences between the devices in question.
It's also important to navigate the sharing of feedback with the FDA with caution. Public comments, including attachments submitted electronically, will be made fully available in the docket, so it's the submitter's responsibility to omit confidential details such as personal medical information or sensitive business data. For those preferring to keep certain information private, the FDA provides clear instructions for written/paper submissions that ensure confidentiality.
This meticulous preparatory work, combined with a strategic understanding of FDA expectations and submission pathways, lays down a solid groundwork for arguing substantial equivalence in a 510(k) submission. The end goal is not only to fulfill FDA requirements but also to expedite the journey towards a decision of substantial equivalence, ultimately advancing the device's trajectory to market.
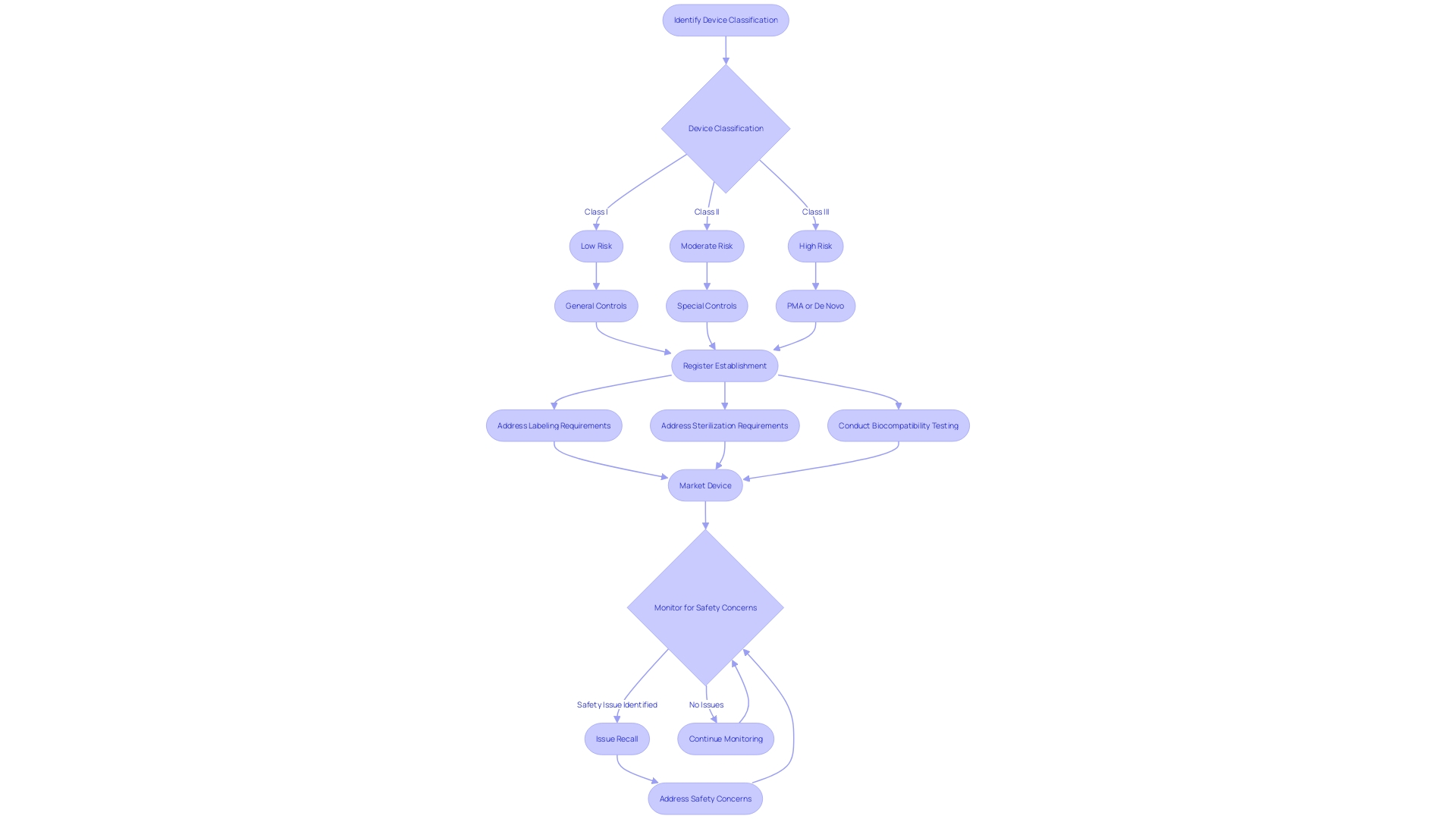
Ensuring Patient Safety: Labeling, Sterilization, and Biocompatibility
Ensuring patient safety is a paramount concern in the realm of medical device approvals. A crucial aspect of this process is addressing the stringent requirements for labeling, sterilization, and biocompatibility testing. Labeling must provide clear, accurate information to facilitate safe usage.
Sterilization protocols are critical to prevent contamination and infection, while biocompatibility testing assesses the potential for adverse reactions with human tissue.
Take, for instance, the HeartMate 3 Left Ventricular Assist System, a device designed to aid the heart's blood-pumping ability. Despite its life-sustaining role, a recall was issued not for product removal but as a corrective action, underscoring the importance of continuous monitoring and responsiveness to potential safety concerns.
Moreover, the case of Profemur artificial hips illustrates the dire consequences of device failure. The device featured a "dual modular neck" for customization, which, despite initial innovation acclaim, became a liability as over 750 instances of fractures were reported, necessitating emergency surgeries for affected patients.
In response to the evolving landscape of medical device safety, UL Solutions has launched testing services in Michigan to address quality, safety, and cybersecurity risks. Their Rochester Hills laboratory exemplifies the industry's drive to enhance medical device reliability, emphasizing the reduction of contaminants like volatile organic compounds.
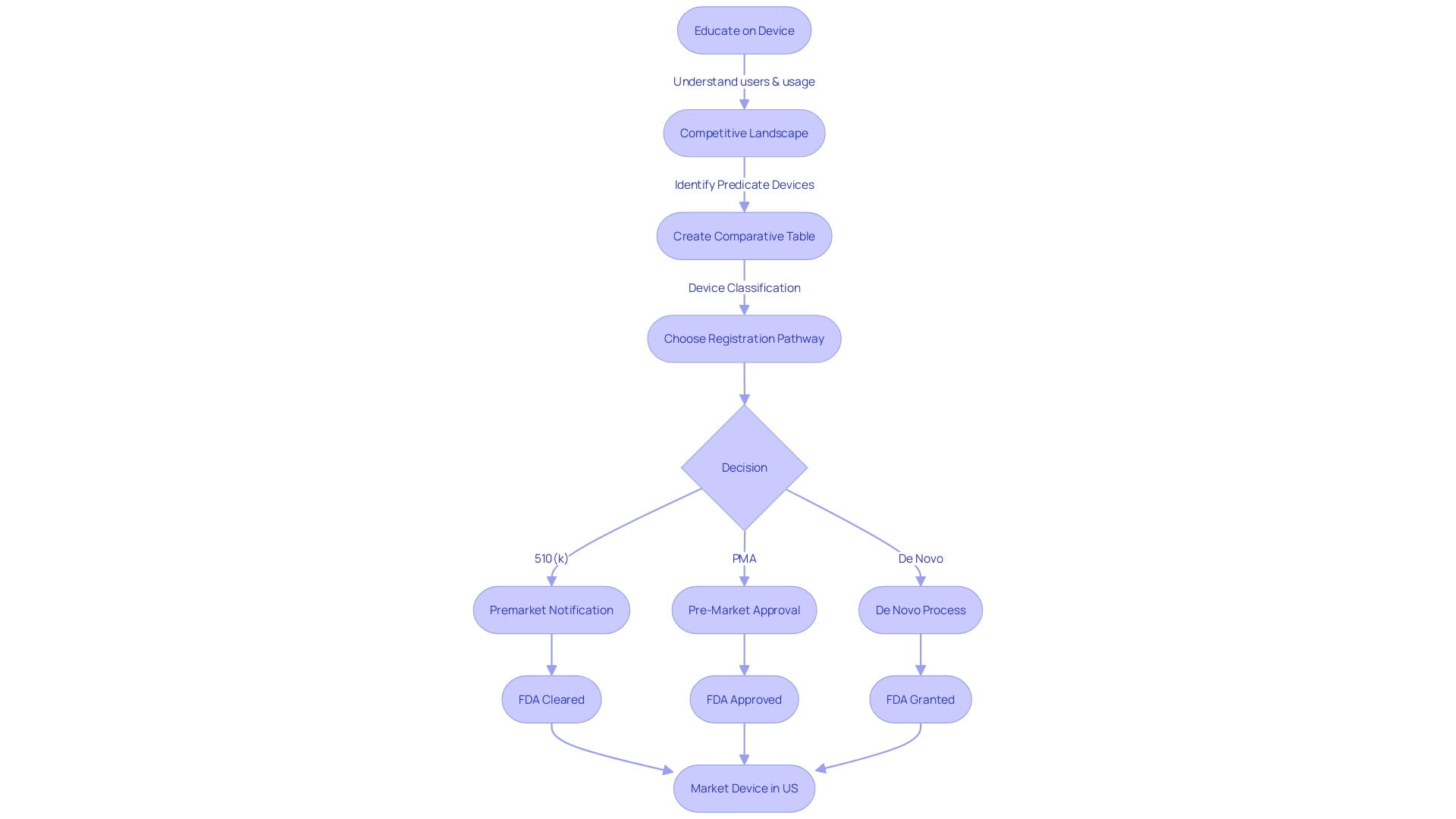
To navigate the regulatory pathway, it is essential to understand the FDA's three-tier classification system, which correlates to the patient risk associated with each device. The chosen registration pathway, be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process, determines the extent to which a device must be scrutinized before it can legally enter the U.S. market.
The distinctions between FDA terms such as Registered, Cleared, Approved, and Granted are significant. Each term represents a different stage and rigor of review, reflecting the device's compliance with the required standards for patient safety and efficacy. As the industry continues to evolve, professionals must remain vigilant in understanding these terms and applying them correctly to uphold the highest safety standards.
Truthful and Accurate Statements and Certifications
A successful 510(k) submission necessitates clear, truthful, and accurate documentation regarding the medical device in question. Each submission should include a signed original and the requisite number of copies, complete with all exhibits as outlined in the regulatory guidelines. It is important to note that initial filings and any amendments that change previously submitted information must be carefully prepared to maintain transparency and comply with the legal requirements.
To illustrate the gravity of this, consider the FDA's role in safeguarding public health by ensuring the safety and efficacy of medical devices. The agency's mandate extends beyond just medical devices to a broad range of health-related products, highlighting the importance of strict adherence to regulatory standards.
In the context of enforcement and regulatory compliance, the Securities and Exchange Commission (SEC) provides a pertinent example of an organization that emphasizes the underlying economic realities over mere labels. As per the Supreme Court's Reves decision, the essence of a product's nature takes precedence over its nomenclature. Applying a similar principle, it's crucial for those involved in 510(k) submissions to focus on the substance over form, ensuring that the information provided accurately reflects the device's intended use and complies with regulatory frameworks.
Recent discussions by the SEC's Division of Enforcement resonate with the medical device industry's need for candid dialogue and cooperation. Emphasizing transparent communication, the SEC encourages industry participants to maintain integrity and prioritize the importance of their work, mirroring the ethos necessary for effective 510(k) submissions.
Moreover, the credibility of submissions is paramount, as evidenced by investor interviews that highlight the need for high-quality, verifiable data. Just as in financial markets, where independent assurance plays a vital role in bridging credibility gaps, in the medical device sector, meticulous documentation and verification are essential for successful 510(k) submissions.
In conclusion, the process demands not only compliance with the procedural aspects of submissions but also a commitment to the integrity of the information provided, mirroring the broader regulatory landscape's emphasis on truthfulness and accuracy.
Submission Process: Electronic Submissions and User Fees
To streamline the FDA 510(k) submission process for medical devices, it's crucial to adhere to the agency's explicit guidelines. Submissions must be electronic and include certain forms and fees. It's essential to follow the FDA's instructions meticulously when submitting comments, as they become part of the public docket.
Comments, along with their attachments, will be posted unchanged; therefore, it's your responsibility to omit any confidential details, such as personal medical data, Social Security numbers, or proprietary business information.
When sending electronic submissions, be mindful that including personal identifiers in your comments means this information will be publicly accessible. If your submission requires confidentiality, opt for a written or paper submission, following the detailed instructions for 'Written/Paper Submissions'. For these, you may send your documents to the Dockets Management Staff at the FDA headquarters in Rockville, MD.
The FDA has a broad mandate, ensuring the public health by regulating a range of products from drugs and medical devices to food and tobacco. This comprehensive oversight stresses the importance of a robust and transparent submission process, especially for medical devices which directly affect patient care and safety.
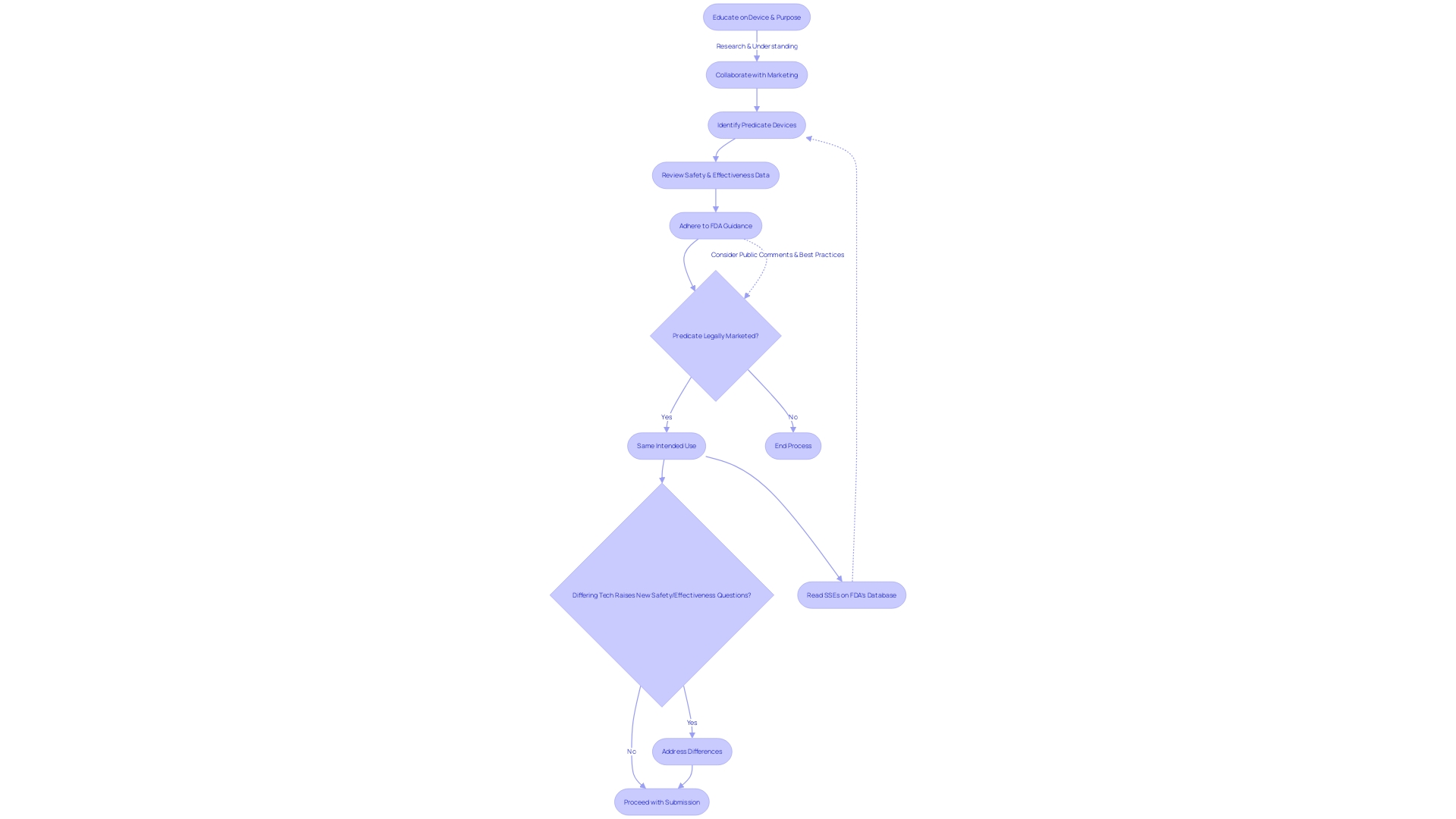
FDA Review Process and Timeline
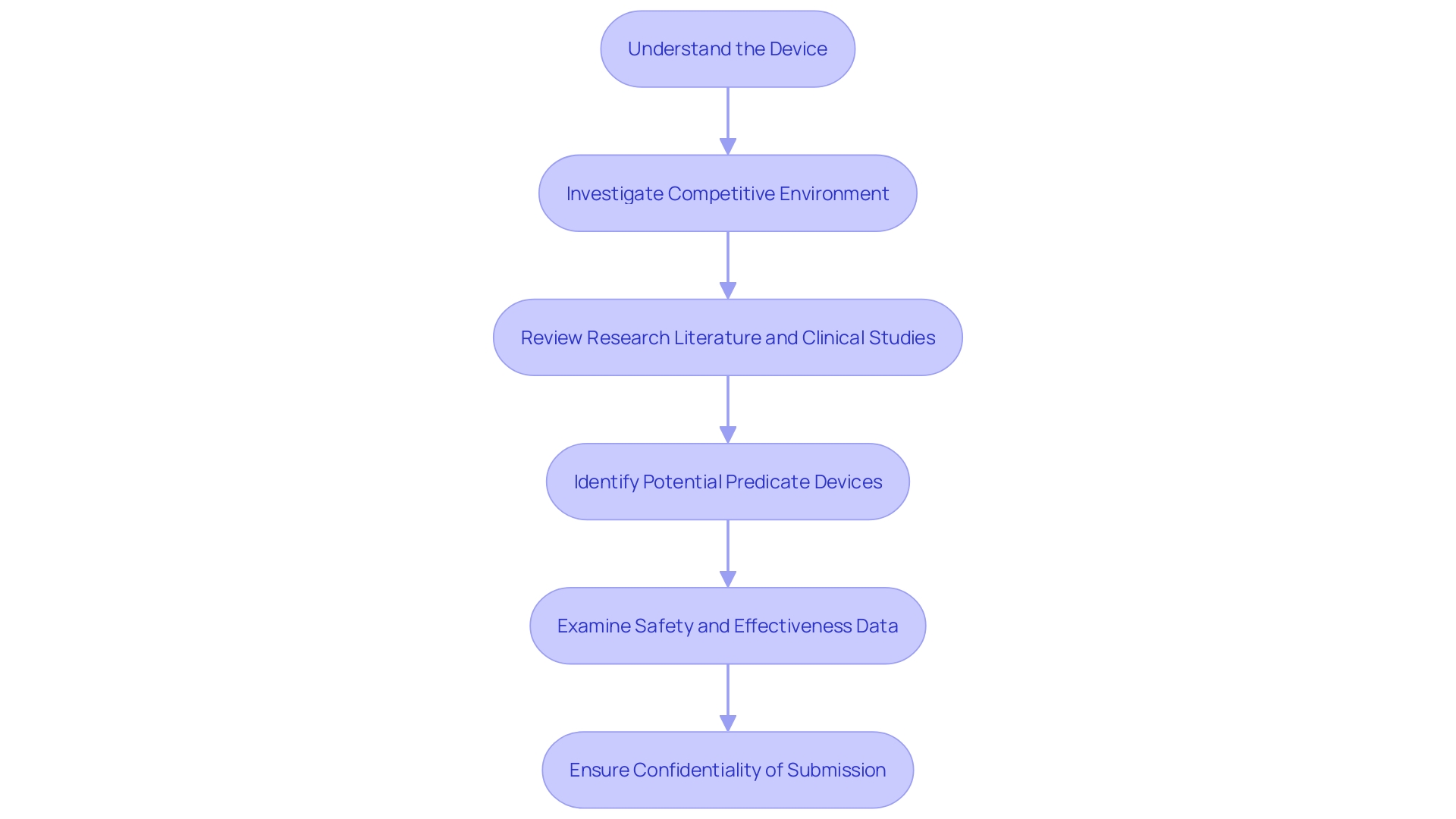
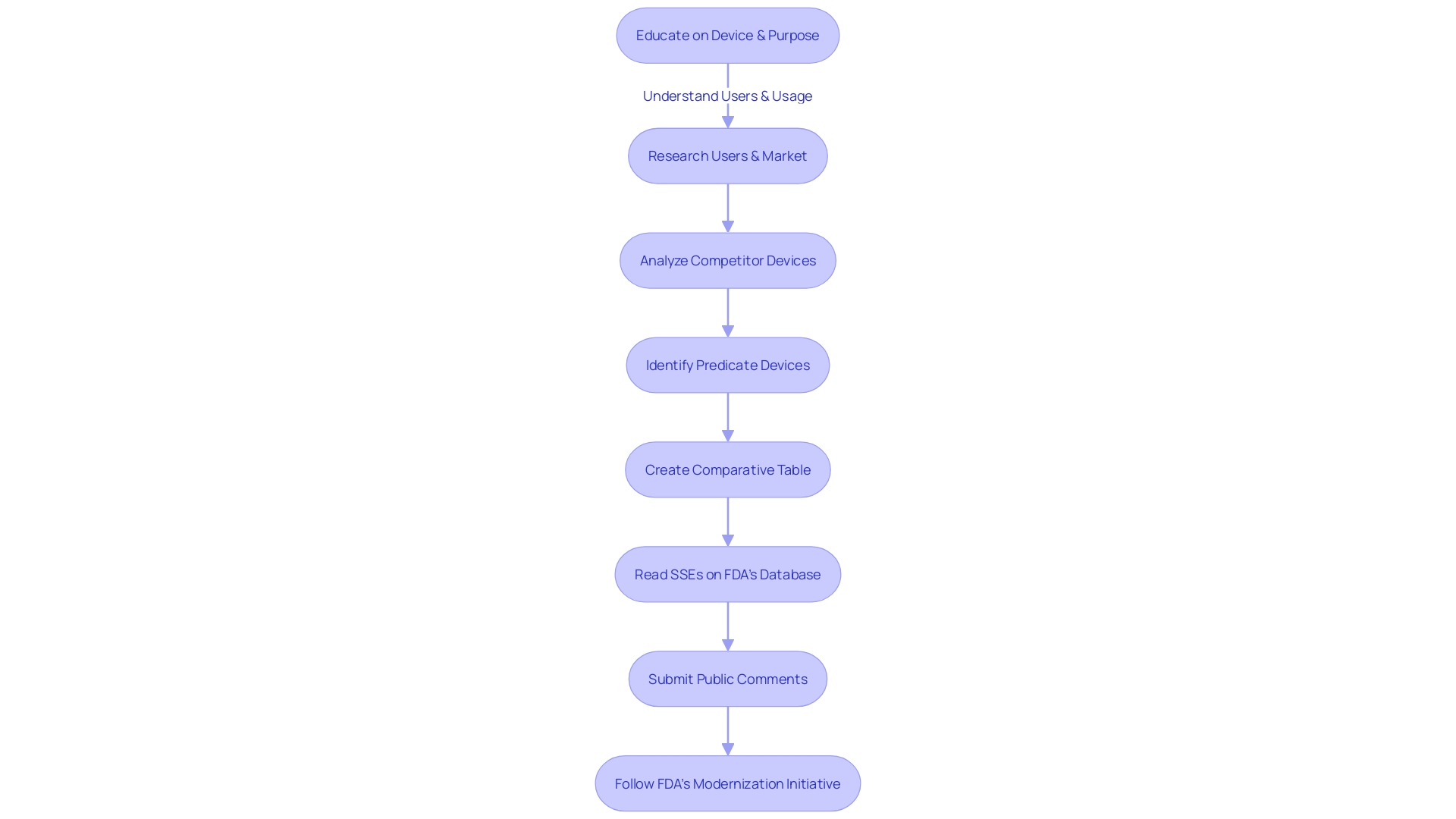
Gaining a thorough comprehension of the medical device in question, encompassing its purpose, the users it serves, and the instructions for utilization, is pivotal. This includes a clear understanding of any warnings or precautions that may be associated with the device. Collaboratively working with marketing teams can illuminate the competitive field, identifying and comparing similar devices on the market.
Such an analysis often involves reviewing available literature, clinical studies, and marketing materials to discern potential predicate devices that share the intended use and technological characteristics of your product.
Creating a comparative table becomes an essential step in organizing this data, leading to an informed selection of an appropriate predicate device. This selection is critical as it forms the basis for the 510(k) submission, which argues that the new device is as safe and effective as the existing predicate. Once potential predicate devices are identified, thorough examination of the summaries of their safety and effectiveness data available in the FDA's 510(k) database is crucial for evaluating both the similarities and differences to your device.
Navigating the FDA's regulatory landscape demands an appreciation of the agency's role in safeguarding public health through the rigorous evaluation of drugs and medical devices. The process of device approval, while complex, is designed to ensure that every product meets stringent safety and efficacy standards. For medical devices, the 510(k) clearance process allows for a more expedited review if a device can be shown to be substantially equivalent to another legally marketed device.
However, it's important to acknowledge that not all devices require clinical trials for approval, as highlighted by the documentary 'The Bleeding Edge,' which casts a critical eye on the FDA's 510(k) process. This underscores the necessity for manufacturers to thoroughly understand and adhere to submission requirements to avoid delays and ensure compliance. As such, the meticulous preparation of a 510(k) submission is not only a regulatory requirement but a fundamental responsibility to future patients who depend on the safety and effectiveness of medical innovations.
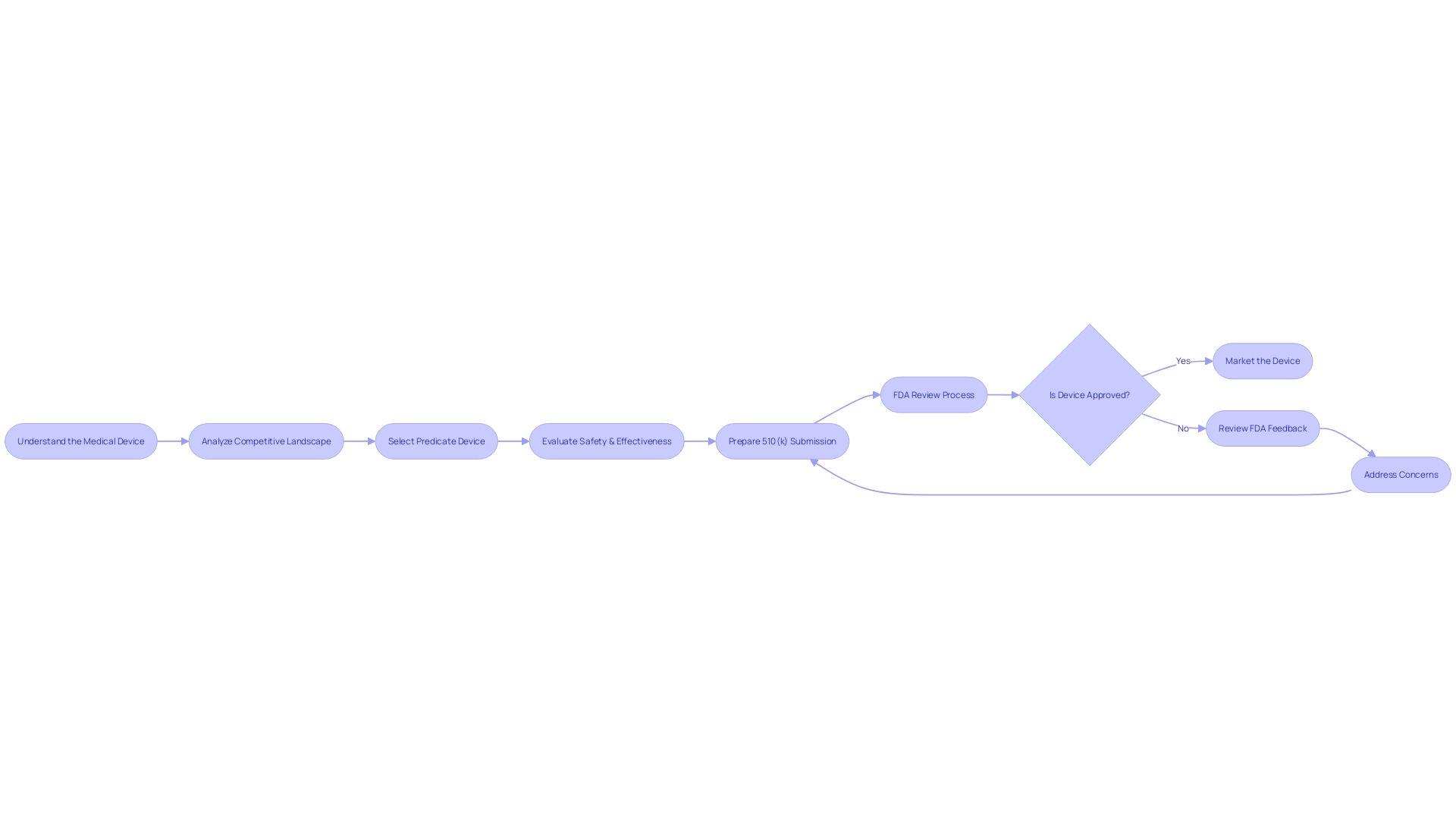
Common Challenges and Tips for Success
Navigating the FDA 510(k) submission process for medical device manufacturers involves a thorough understanding of the device itself, its users, and the broader market landscape. Manufacturers must delve into comprehensive research, learning about users such as clinicians, physicians, dentists, and patients, and meticulously reviewing instructions for use, which include crucial warnings and cautions. Collaborating with marketing teams, manufacturers should analyze competitor devices and create a detailed comparative table based on information from research literature, clinical studies, and other marketing materials.
Once potential predicate devices are identified, manufacturers should consult the FDA's 510(k) database to scrutinize the Summaries of Safety and Effectiveness, comparing similarities and differences. This step is critical in the selection of an appropriate predicate device, which must be legally marketed and registered with the FDA, with the same intended use and technological characteristics that do not raise new safety or effectiveness concerns.
Public comments are an integral part of the FDA's regulatory process. When submitting comments electronically, it is essential to avoid including confidential information, as these will be made public upon posting. If confidentiality is required, comments should be submitted in writing, following detailed instructions provided by the FDA.
The FDA's ongoing modernization initiative includes developing best practices for predicate selection, as highlighted in a draft guidance document released on September 7, 2023. This initiative aims to bring efficiency to the review process, with a focus on leveraging older predicates to capitalize on long-term safety data.
Moreover, industry leaders emphasize the importance of understanding the competitive landscape. This includes not just product-specific knowledge but also insights into successful business strategies for medical device companies. Acquisition, IPOs, licensing, partnerships, and strategic alliances are all viable pathways for a successful exit, as they can provide significant returns and growth opportunities.
To provide context, the FDA categorizes medical devices into three classes based on risk, with class one and two devices typically undergoing the 510(k) clearance process. This process allows a new device to be compared to an existing predicate device, facilitating a more expedited market entry. However, the complexity of the device and the nature of the medical condition it addresses can impact regulatory approval times, which vary across regions.
In summary, manufacturers must adopt a meticulous and strategic approach to the 510(k) submission process, ensuring a deep understanding of their device, the competitive market, and the regulatory landscape. By doing so, they can navigate the challenges of the FDA 510(k) process and achieve successful outcomes for their medical devices.
Conclusion
In conclusion, navigating the FDA's 510(k) submission process for medical devices requires a comprehensive understanding of the different pathways available. Thoroughly analyzing the device's intended user base, understanding its usage instructions, and comprehending all warnings and precautions are crucial steps. Collaborating with the marketing department can provide valuable insights into the competitive landscape and potential predicate devices.
Consulting the FDA's 510(k) database for Summaries of Safety and Effectiveness Data (SSEDs) is essential for discerning the nuances among devices.
By meticulously preparing the submission and aligning it with the specific requirements of the chosen pathway, medical device manufacturers can navigate the FDA approval process more effectively, bringing their products to market with confidence and compliance.
Identifying an appropriate predicate device is pivotal, involving a thorough investigation into existing devices that are similar in intended use and technological characteristics. Collaborating with the marketing department can provide valuable insights into the competitive landscape, which is beneficial when reviewing research literature, clinical studies, and other informational materials.
Navigating FDA guidance documents is critical for compliance with regulatory standards. Understanding that these documents offer clarity and recommendations on regulatory issues is important. Locating these documents through the FDA's website and following proper procedures when submitting comments or feedback is essential.
Embracing new methods and tools is necessary to maintain compliance while expediting product development.
Preparing a traditional 510(k) submission involves a thorough understanding of the device and its intended use. Researching the competitive landscape and creating a comparative table to juxtapose potential predicate devices is crucial. Analyzing the Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA's 510(k) database is indispensable.
Ensuring patient safety is paramount in the medical device approval process. Addressing requirements for labeling, sterilization, and biocompatibility testing is crucial. Compliance with regulatory standards and the commitment to truthfulness and accuracy in documentation are essential.
To streamline the FDA 510(k) submission process, adhering to electronic submission guidelines is crucial. Gaining a thorough comprehension of the medical device, collaborating with marketing teams, and reviewing Summaries of Safety and Effectiveness Data (SSEDs) are pivotal in the review process.
In summary, manufacturers must adopt a meticulous and strategic approach to the 510(k) submission process, ensuring a deep understanding of their device, the competitive market, and the regulatory landscape. By doing so, they can navigate the challenges of the FDA 510(k) process and achieve successful outcomes for their medical devices.




