Introduction
The 510(k) submission process is a crucial pathway for medical device manufacturers to demonstrate the safety and effectiveness of their products to the U.S. Food and Drug Administration (FDA). This premarket submission requires thorough research, including understanding the device, analyzing the competitive landscape, and establishing a predicate device comparison. The FDA's review process, including the 510(k) pathway, is constantly scrutinized to ensure patient safety.
Different types of 510(k) submissions exist, each with its own specific criteria and assessment protocols. The traditional pathway involves a comprehensive review, while the special pathway is for modifications to devices with existing clearance. The abbreviated pathway leverages guidance documents and recognized standards for faster clearance.
A successful 510(k) submission requires meticulous preparation, understanding the device's intended use, user needs, and adhering to the FDA's stringent standards. The submission includes essential components such as cover sheet forms, public information about the device, and templated sections. Submissions must be made electronically, following the FDA's guidelines for comment submissions and safeguarding sensitive information.
The review process involves meticulous evaluation of safety and efficacy, consideration of public comments, and adherence to regulatory frameworks. Navigating the 510(k) submission process requires a strategic approach, understanding the device, conducting a comparative analysis, and following the FDA's guidance documents to ensure successful clearance.
What is a 510(k) Submission?
The 510(k) process is a crucial pathway for medical manufacturers to show the U.S. Food and Drug Administration (FDA) that their product is comparable to a legally marketed item and is safe and effective for use. This premarket submission is required for products that do not belong to the exempt category or are not subject to an alternative regulatory framework. Thoroughly researching the object in question, including understanding its users, proper usage, potential warnings, and cautions, is essential for manufacturers. It's also important to analyze the competitive landscape by reviewing research literature, clinical studies, and marketing materials of similar products to establish a predicate product comparison. This comparison helps in creating a comprehensive table, which is a fundamental step in the 510(k) preparation process. Manufacturers should further analyze the Summaries of Safety and Effectiveness Data (Seeds) on the FDA's 510(k) database to distinguish the similarities and differences with potential predicate products.
Furthermore, it is crucial to highlight that the FDA's evaluation process for medical instruments, which includes the 510(k) pathway, is continuously examined to guarantee patient safety. It is widely acknowledged that not all gadgets necessitate clinical trials, which was a point of scrutiny in the 2018 documentary 'The Bleeding Edge.' The documentary highlighted how devices could be fast-tracked through the 510(k) process if they are substantially equivalent to existing devices, sometimes leading to patient harm. This emphasizes the significance of a thorough and complete assessment by manufacturers when preparing their 510(k) filings.
Finally, in the broader context of FDA regulations, the agency has established clear guidelines for public commentary. These guidelines include instructions for electronic sending to ensure the confidentiality of sensitive information. As part of its mandate, the FDA is committed to the safety, effectiveness, and security of various healthcare-related products, not limited to medical devices, but also including drugs, biological products, and more. Manufacturers must comprehend and comply with these regulations when making their contributions, as part of their duty to safeguard public health.
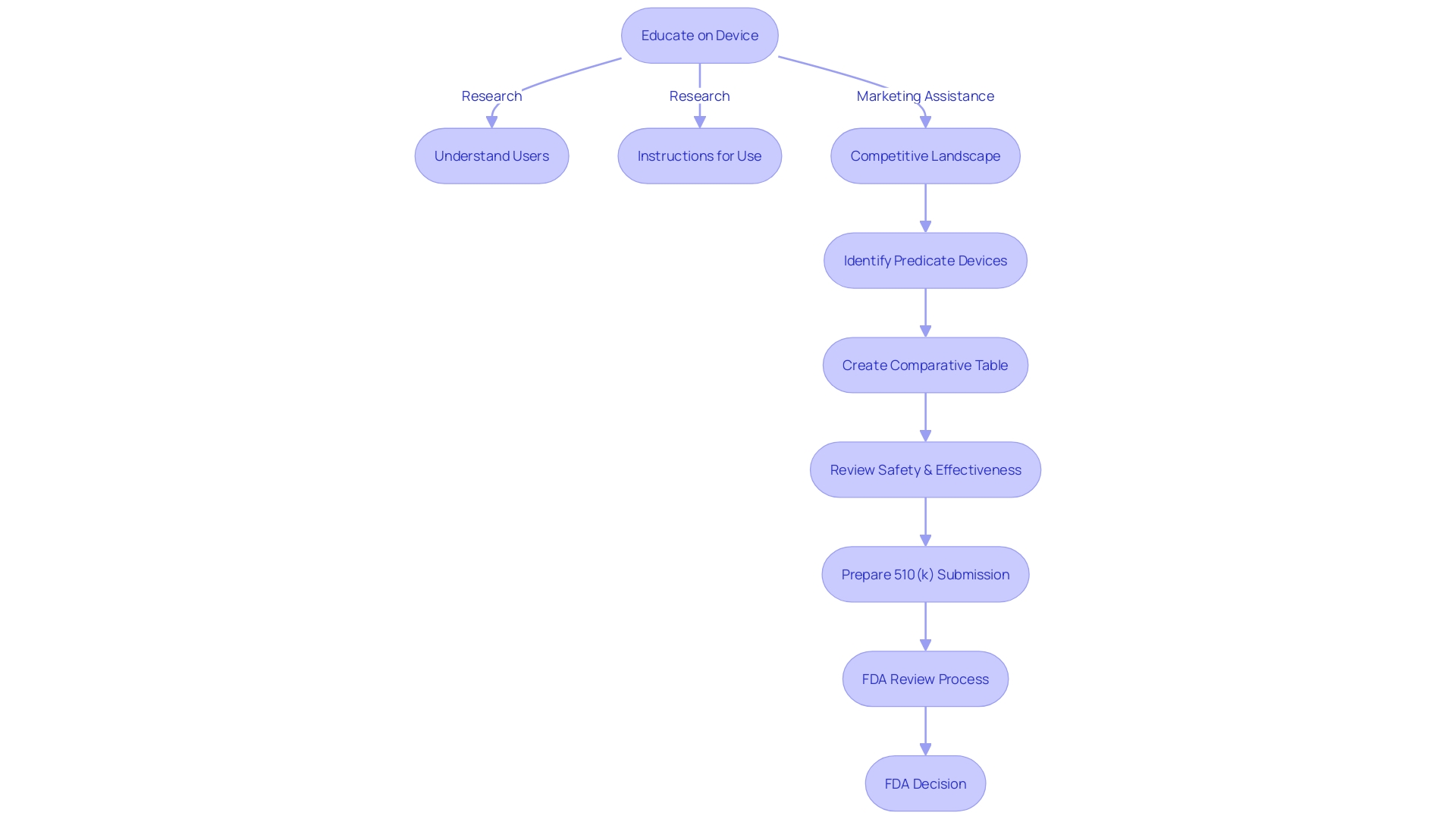
Types of 510(k) Submissions
The FDA classifies 510(k) applications into three different types, each with its own specific criteria and assessment protocols. Traditional 510(k) applications are the most common pathway, offering a thorough evaluation of a medical instrument in comparison to a legally marketed precedent equipment. This pathway requires a detailed dossier of evidence demonstrating that the new product is as safe and effective as the predicate. Special 510(k) filings are meant for alterations to a product that already has 510(k) approval, where the modifications do not impact the safety or efficacy of the product. It allows for a more streamlined review process given that the original equipment's performance characteristics are well understood. The Abbreviated 510(k) use guidance documents, special controls, and recognized standards to ease the review process, potentially expediting the clearance of a medical instrument that adheres to existing guidelines. Each pathway demands a strategic approach to compiling and presenting data, where understanding the product's intended use, user needs, and the competitive landscape becomes crucial. This includes making comparative tables and aligning with the FDA’s strict standards for safety and efficacy, where even the presentation of data in applications follows specific requirements, such as those recently specified for direct-to-consumer drug advertisements. Industry professionals must navigate this terrain by enhancing efficiency in regulatory documentation and keeping up-to-date with the changing regulatory landscape to ensure timely and successful clearances.
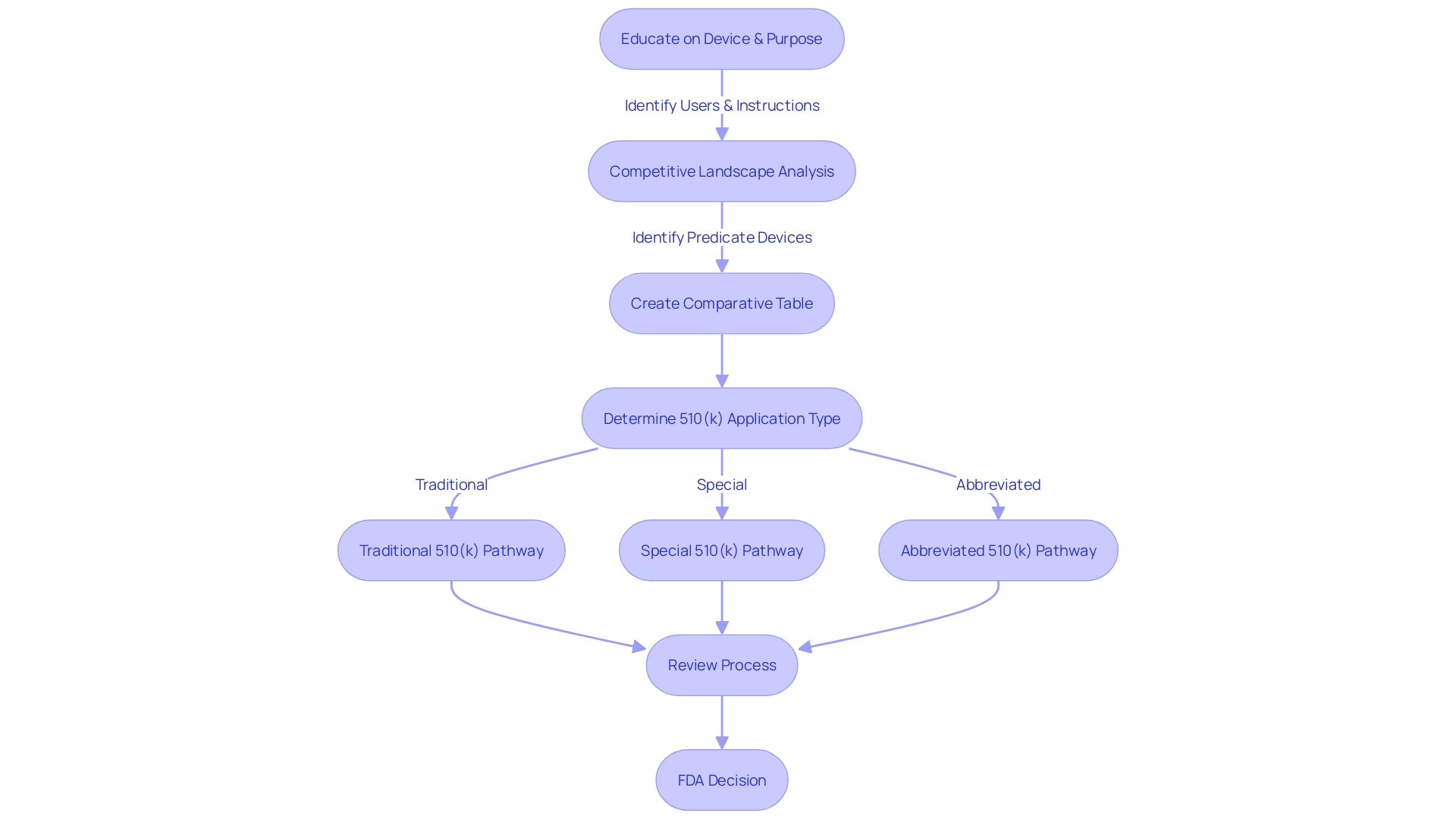
Traditional 510(k) Submissions
Navigating the traditional 510(k) submission pathway is a critical step in the medical equipment approval process. This pathway requires a thorough comparison of the new product to one that is already legally marketed, ensuring that it is both safe and effective for its intended use. An essential part of this procedure is the development of a comparative table that outlines the resemblances and distinctions between the new apparatus and its precursor, which entails a thorough examination of the subject tool, its users, and the current competitive environment.
To thoroughly evaluate the safety and efficacy of a product, it is vital to scrutinize the instructional materials, including any warnings and precautions. Furthermore, exploring research literature, clinical studies, and existing marketing materials helps in identifying a suitable predicate product. Reading the Summaries of Safety and Effectiveness Data (SSEs) available in the FDA's 510(k) database is an integral part of this analysis, as it provides insights into the predicate's performance characteristics.
The evaluation procedure for conventional 510(k) applications frequently necessitates a substantial quantity of clinical data, which is thoroughly scrutinized for its scientific merit. The scientific background and preliminary data are assessed to determine whether they justify the proposed study. Also considered is the level of innovation and its impact on the field, as well as the feasibility and scientific quality of the proposed work.
In the realm of regulatory compliance, it is imperative to follow the instructions for submitting comments meticulously, as public comments are a part of the regulatory review process. Submitters must be cautious not to include any confidential information in their comments, as these will be made public unless appropriately submitted as confidential.
In general, the conventional 510(k) pathway is intended to promote innovation while guaranteeing that new products fulfill the rigorous safety and effectiveness criteria necessary to safeguard public health, reflecting the commitment of the medical tool industry in developing solutions that are both pioneering and dependable.
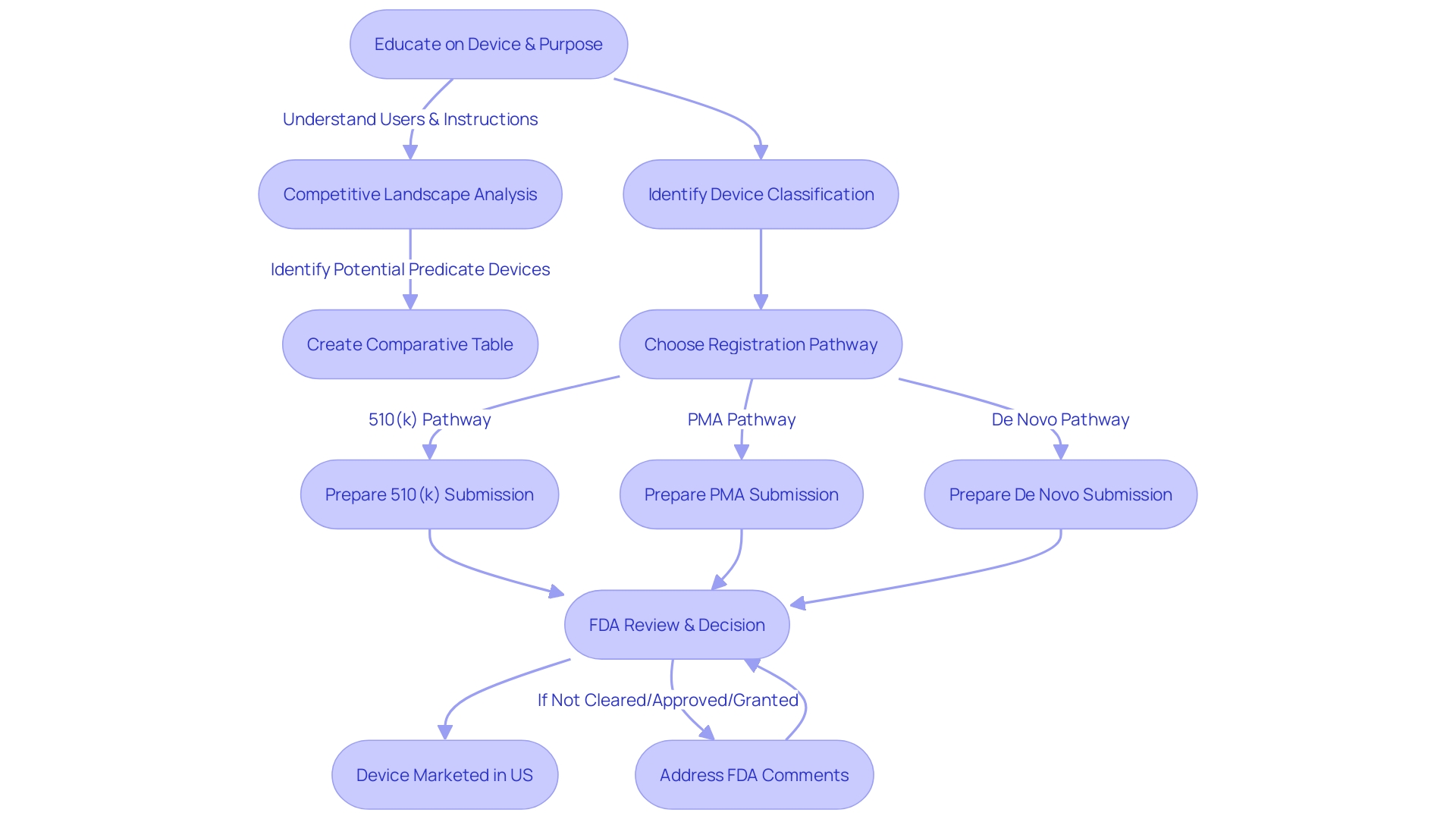
Special 510(k) Submissions
Special 510(k) cater to modifications where the existing product already holds FDA clearance, and the changes do not pose new safety and efficacy questions. The accelerated assessment for these submissions depends on a thorough understanding of the instrument, including its function, users, and instructions for use, which must be free of any unclear warnings and cautions. A comparative analysis with similar existing instruments is vital, leveraging resources such as research literature, clinical studies, and competitive data to substantiate the instrument's equivalence. In addition, by interacting with the FDA's publicly accessible 510(k) database to examine Summaries of Safety and Effectiveness Data (SSED), it guarantees a thorough assessment of the resemblances and distinctions with precedent items. Industry professionals must navigate this process with acute awareness of the regulatory landscape, following protocols for public commentary and safeguarding confidential information. The FDA's commitment to transparency is exemplified by initiatives like the EDGAR system and public meetings, which offer insights into the FDA's methodologies and invite stakeholder participation. This streamlined approach to Special 510(k) reviews underscores the agency's efforts to facilitate innovation while maintaining rigorous standards for public health.
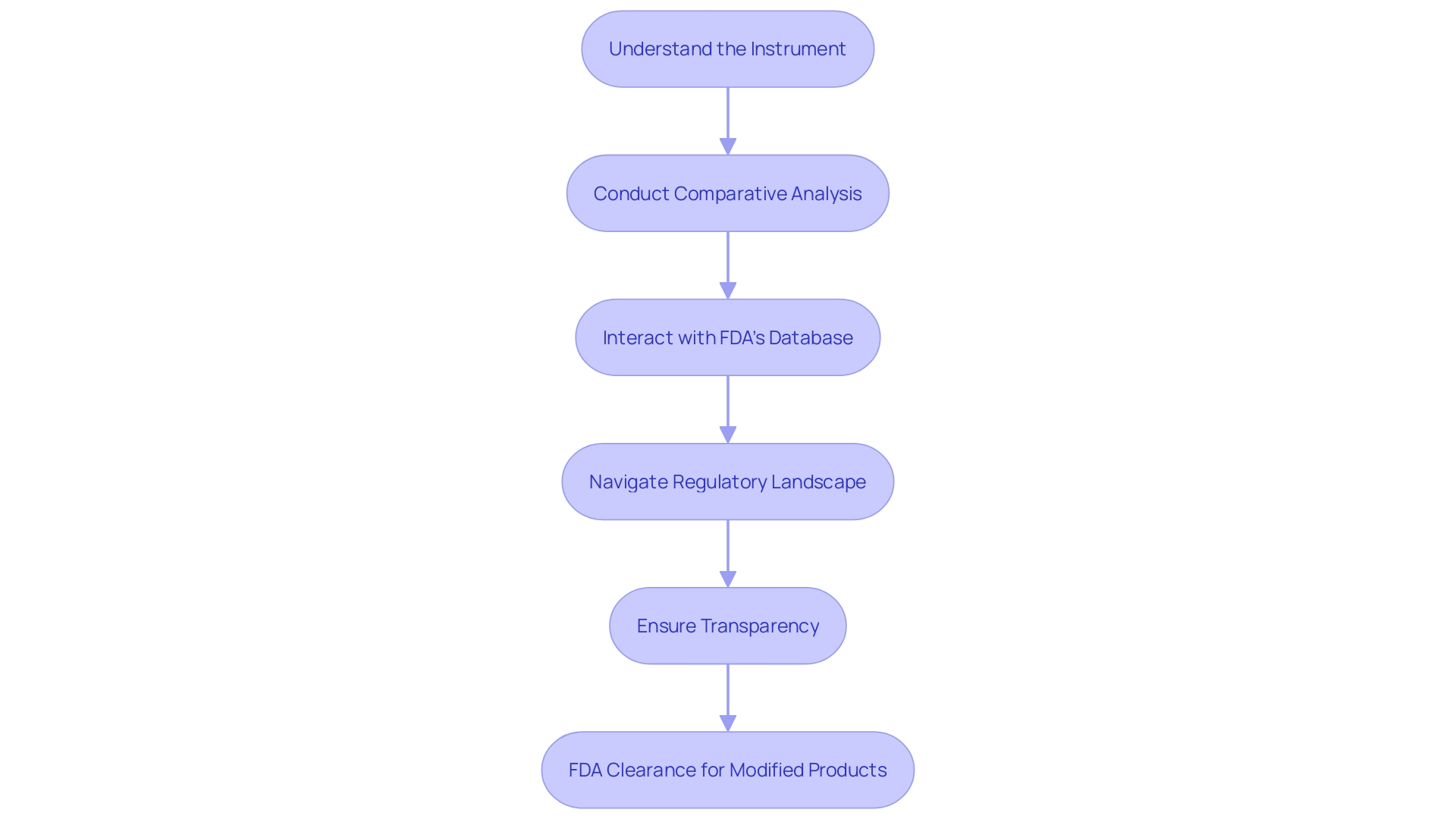
Abbreviated 510(k) Submissions
The streamlined Abbreviated 510(k) pathway offers a faster review process for medical products that have previously received clearance. This type of application utilizes established FDA guidance documents, special controls, or recognized consensus standards to demonstrate that the new equipment is essentially the same as a previously authorized reference equipment. By showcasing this significant similarity, manufacturers can bypass additional documentation, as long as they carefully adhere to the FDA's guidance and ensure the product fulfills all identified requirements.
Thorough preparation is crucial for a successful process, beginning with a thorough comprehension of the users of the apparatus, such as clinicians and patients, and the instructions for use of the apparatus. In addition to this, manufacturers need to analyze the competitive environment, reviewing research literature, clinical studies, and existing marketing materials to identify appropriate reference products. A comparative analysis is essential, drawing on resources such as the FDA’s 510(k) database, where Summaries of Safety and Effectiveness provide valuable insights into potential predicates.
The FDA's role as a protector of public health underscores the importance of compliance with its regulations. Medical manufacturers must make sure that their documents are transparent, well-documented, and free of sensitive information that shouldn't be disclosed. It's crucial to adhere to the FDA's instructions for commenting to prevent unintentional disclosure of sensitive data.
The FDA’s recognition of standards plays a vital role in the Abbreviated 510(k) process. The historical record of FDA's standard recognitions, available on the Federal Register, along with resources such as CDRH Learn, guide manufacturers through the conformity assessment necessary for demonstrating a product’s compliance with regulatory requirements. The rigorous application of these voluntary consensus standards, developed with a commitment to transparency and balance, contributes to regulatory quality and public health safety.
Components of a 510(k) Submission
A 510(k) application, a crucial stage in acquiring FDA clearance for medical devices, is a comprehensive document comprising vital particulars that back the agency's assessment of the device's safety and effectiveness. The submission is composed of several crucial sections:
-
Cover Sheet Forms provide the FDA with an initial overview of the submission, which includes the submitter's identification and the device's classification.
-
Public Information About Your Gadget section must be crafted with meticulous attention to detail, providing a comprehensive account of the gadget's intended use, technological attributes, and user information. This data is often dispersed throughout an organization and may encompass experiment reports, meeting notes, and more, underscoring the importance of a strategic approach to compiling this multifaceted information.
-
Templated Sections are structured to streamline the compilation process, guiding submitters through the various components that need to be addressed, such as comparisons to predicate instruments and demonstrations of safety and effectiveness.
The importance of a well-prepared 510(k) for the FDA's role in protecting public health through thorough device evaluation cannot be overstated. Furthermore, the recent FDA publication on direct-to-consumer prescription drug advertisements emphasizes the significance of clear and understandable information presentation, a principle that is equally relevant to 510(k) applications. Submitters are strongly encouraged to adhere to the FDA’s guidance documents and fully comprehend the process of submitting, which can be intricate and time-consuming. The agency anticipates a thorough comprehension of the subject, competitive landscape, and a comparative analysis of potential predicate options, as indicated by the Summaries of Safety and Effectiveness Data (SSEs) available in the FDA’s 510(k) database. In the end, the process should assist the FDA in determining the substantial equivalence of the product to an already legally sold item.
Cover Sheet Forms
Starting a 510(k) necessitates a thorough comprehension of the medical equipment in question, from its intended usage to the intricacies of its operation. The process begins with the submission of essential cover sheet forms that serve as the submission's foundation. The Medical Device User Fee Cover Sheet (Form FDA 3601) and the CDRH Premarket Review Submission Cover Sheet are crucial in this initial phase, providing essential information about the apparatus and its manufacturing entity. These forms are more than just bureaucratic necessities; they are the stepping stones to a comprehensive comparative analysis that will eventually be used to demonstrate the apparatus's similarity to existing, approved devices, known as predicates.
This comparative analysis is not to be taken lightly. One must delve into extensive research, encompassing a thorough review of clinical studies, user instructions, and warnings, coupled with an understanding of the competitive landscape, as advised by industry experts. Such diligence is evidenced by the insights gained from the documentary The Bleeding Edge, which underscores the critical nature of the 510(k) clearance process in ensuring patient safety, despite the controversy regarding the necessity of clinical trials for equipment approvals.
The importance of these initial forms is further emphasized by the evolving digital landscape in healthcare. As the industry shifts rapidly, with a growing number of private practices being acquired, the ability to navigate the regulatory environment with agility and precision becomes paramount. This is where the 510(k) database comes into play, offering access to Summaries of Safety and Effectiveness that are instrumental in evaluating your instruments against their predecessors.
By initiating the process with well-prepared cover sheet forms, manufacturers set the stage for a successful review process. This focus on particulars demonstrates a dedication to fulfilling the FDA's requirements and adjusting to the ever-changing regulatory requirements of the industry, ultimately resulting in improved efficiency in preparing regulatory documents and a streamlined route to market entry for crucial medical products.
Public Information About Your Device
The central documentation within a 510(k) submission contains crucial information about the medical instrument, including its intended use and performance characteristics. Key elements of this documentation include the 510(k) Cover Letter, Indications for Use Statement, and the 510(k) Summary, which together communicate the purpose, application, and compliance with relevant standards. A comprehensive understanding of the device necessitates examining its use in clinical settings, potential warnings, and how it compares to existing market offerings. This requires extensive research into clinical literature, studies, and regulatory filings to ensure a comprehensive comparison with predicate devices—those with identical intended uses and technological characteristics.
To illustrate, Medtronic plc, the global healthcare technology leader, exemplifies the importance of insight-driven care and innovative solutions, showcasing a wide array of technologies that address numerous health conditions. Their method of open communication and comprehension of competitive environments is in line with the recommended methods for 510(k) filings. Furthermore, the FDA's recent rule on direct-to-consumer prescription drug advertisements emphasizes the need for clear, consumer-friendly language in medical communications—a principle that also applies to 510(k) documentation.
In accordance with openness, experts creating 510(k) applications are urged to foster comprehensibility and transparency regarding the apparatus's reasoning and results. This ensures that critical information impacting patient care and device risks is effectively communicated. The process of public comment as described by the Health and Human Services Department further emphasizes the value of transparency and public engagement in the regulatory process.
Having real-time access to filings and documents through platforms such as the Sec's EDGAR system can be a valuable example for those trying to navigate the complexities of 510(k) filings. With the ability to sort through large quantities of data and retrieve particular information relevant to medical equipment, researchers and professionals can more effectively match their products with regulatory requirements and industry norms.
Templated Sections
An application for clearance to the FDA is an important stage in introducing a medical instrument to the market, and it necessitates careful record-keeping to prove that the instrument is secure and efficient. Among the vital elements of this statement are the Truthful and Accuracy Statement, which serves as a legal attestation to the veracity of the information provided; the Class III Summary and Certification, which provides an overview of the product and asserts that it meets the necessary criteria for a medical instrument in Class III; the Financial Certification or Disclosure Statement, detailing any financial ties that may influence the product's evaluation; and the Declarations of Conformity and Summary Reports, which confirm that the product conforms to recognized standards and summarizes the supporting data.
These sections constitute the main component of the document, presenting fundamental information that the FDA utilizes to evaluate the device's qualification for clearance. Moreover, they must be prepared with the understanding that any inaccuracies or omissions could have significant ramifications not only for the approval process but also for the trustworthiness of the submitting entity. Software developers, such as those in digital-asset companies who are accustomed to a rapidly evolving industry, can appreciate the complexity of establishing and adhering to such regulatory frameworks. The need for meticulous focus on every aspect in these regulatory filings is similar to the deliberate development of compliance programs they encounter, especially when constructing such systems from scratch.
The importance of public commentary in shaping these regulations cannot be overstated, with a 60-day comment period providing an opportunity for stakeholders to contribute valuable insights. All comments are publicly posted, reflecting the transparency and responsiveness of the Health and Human Services Department to industry and consumer feedback.
In the context of healthcare, where the stakes are high, and the well-being of patients is on the line, the rigor of the 510(k) submission process—underscored by the use of prior authorization practices in Medicare Advantage—highlights the systemic commitment to ensuring that only products that are thoroughly vetted for safety and efficacy make it to the market. This gatekeeping role, performed by entities like the FDA, parallels the function of prior authorization in managing healthcare service utilization, both aiming to balance access to necessary services with the imperative of maintaining high standards of care.
Electronic Submission Requirements
Submissions for 510(k) clearance, a crucial step in bringing medical products to the U.S. market, must be submitted electronically to the FDA. Utilizing the FDA's Electronic Submission Gateway (ESG) or the CDRH eSubmitter, this process is designed for the secure and efficient handling of documents. It's crucial for applicants to fully comprehend the object under consideration, including its users and usage instructions, along with any associated warnings. A comprehensive analysis should include a deep dive into the competitive landscape, which can be achieved with the help of Marketing teams. Examining research literature, clinical studies, and competitor materials is crucial for identifying potential predicate objects that have the same intended purpose and technological features. Creating a comparative table is a strategic step in this process.
Additionally, public feedback is an integral part of the FDA's approach to regulatory processes. Applicants must follow specific instructions when submitting comments, ensuring the exclusion of confidential information to avoid unwanted public disclosure. Comments, which are made publicly available after review, can also be attached as files or supported with documents. As an agency dedicated to safeguarding public health, the FDA ensures the safety and effectiveness of products within its jurisdiction, which encompasses medical equipment. The agency's responsibilities extend to the security of the nation's food supply and regulation of other consumer products.
For those getting ready to submit a 510(k), it is recommended to go through the Summaries of Safety and Effectiveness (SSEs) accessible in the FDA's 510(k) database. This allows for a detailed comparison of the proposed mechanism against existing ones, highlighting similarities and differences. It is also crucial to emphasize that any personal information provided will be disclosed publicly in accordance with FDA policies, highlighting the importance of thoughtful deliberation regarding the content shared electronically.
Finally, the FDA's commitment to transparency and public involvement is further emphasized through open comment periods for documents under review. Stakeholders are encouraged to contribute their insights within specified timelines, reinforcing the collaborative nature of medical equipment regulation.
Submission Process
Ensuring a successful FDA 510(k) submission for medical instruments is a meticulous process that requires careful preparation of detailed documentation. The documentation must include a comprehensive description of the apparatus, such as its intended use for diagnosing, treating, preventing, curing, or mitigating any disease or condition. In addition, it should specify the patient population for which the equipment is intended. Submissions must be thorough, including pictorial representations, specifications, and if applicable, engineering drawings. It is essential to identify each component or ingredient if the apparatus is composed of multiple elements and describe the properties of the instrument that are relevant to its intended medical purpose.
Additionally, all FDA-assigned reference numbers for legally marketed medical instruments that are intended to be utilized in combination with the filing must be incorporated. As per recent guidance, feedback can be solicited through the Q-Submission Program, which outlines mechanisms for submitters to request interactions with the FDA regarding medical applications. This guidance underscores the importance of differentiating between informal communication and formal Pre-Submission feedback.
The FDA encourages public commentary on draft guidances, highlighting the transparency and value of stakeholder input in shaping regulatory policies. All comments are made public post-review, emphasizing the responsibility of the submitter to exclude confidential information. This participatory approach not only informs policy but also ensures that submitters are aware of the significance and impact of their contributions on regulatory practices.
The process of providing the necessary documentation is not only about compliance but also about contributing to the regulatory framework that governs medical device safety and efficacy. By following the FDA's procedural and documentation requirements, applicants can navigate the landscape of applications more effectively, ultimately contributing to the advancement of medical technology and patient care.
Review Process
The FDA's meticulous 510(k) review process commences upon receipt of a submission. The initial administrative review ensures all necessary documentation is present. Afterward, a thorough review is conducted, examining the medical equipment's safety and effectiveness, based on comprehensive data provided. The FDA's objective, as a component of the U.S. Department of Health and Human Services, is to safeguard public health by ensuring that medical equipment is secure and efficient for its intended purpose. The review process also involves considering public comments, as all are deemed public and can be submitted online for consideration by the Health and Human Services Department. Alongside the examination, it is recommended for stakeholders to fully familiarize themselves with the apparatus, including user demographics, usage instructions, and potential risks. A comparative analysis with existing products in the market, focusing on products with similar intended uses and technological characteristics, can be instrumental in understanding the competitive landscape. This context is critical for the FDA, which recently updated its list of AI/ML-enabled medical products that have been legally marketed in the U.S., reflecting the agency's commitment to transparency in this rapidly evolving field. The review process is not just about regulatory compliance but also about understanding the scientific and market dynamics that support the medical industry.
Tips for Successful 510(k) Submissions
Navigating the FDA 510(k) submission process requires a strategic and well-informed approach. Begin by acquiring a strong comprehension of the medical apparatus you are working with, including its purpose, users—whether they be clinicians, physicians, dentists, or patients—and its instructions for use, paying special attention to any warnings and precautions. Collaborate with your marketing team to conduct a comprehensive review of the competitive landscape. This entails examining research literature, clinical studies, websites, brochures, sell-sheets, instructions for use, and labeling to identify potential precedent objects that have the same intended purpose and technological characteristics. This knowledge is essential in creating a comparative table that will serve as a vital component of your document.
The next step involves a deep dive into the Summaries of Safety and Effectiveness (SSEs) available on the FDA's 510(k) database. Here, you will assess the similarities and differences between your device and identified predicate devices, which is a critical component in demonstrating substantial equivalence.
The complexities of the process also require you to familiarize yourself with the FDA's guidance documents and understand the agency's expectations. Make use of the resources available on the FDA website to understand the intricacies of what needs to be included in your application and the steps to follow, ensuring that your application is not only accepted but also meets the FDA's criteria for a determination of substantial equivalence.
As you get ready to present your remarks and paperwork, make sure that they are free of sensitive data that should not be disclosed unless you choose a written/paper entry, as indicated by the FDA's guidelines. By adhering to these guidelines, you can significantly enhance the probability of a successful 510(k) submission, thus contributing to the advancement of medical device technology and patient care.
Conclusion
In conclusion, the 510(k) submission process is crucial for medical device manufacturers to demonstrate the safety and effectiveness of their products to the FDA. It involves thorough research, understanding of the device, and a comprehensive analysis of the competitive landscape. The FDA's review process prioritizes patient safety.
There are three types of 510(k) submissions: traditional, special, and abbreviated, each with specific criteria and assessment protocols. Successful submissions require meticulous preparation, adherence to FDA standards, and understanding of the device's intended use and user needs.
The submission process includes essential components such as cover sheet forms, public information about the device, and templated sections. Submissions must be made electronically, following FDA guidelines for comment submissions and safeguarding sensitive information. The review process involves evaluating safety and efficacy, considering public comments, and adhering to regulatory frameworks.
Navigating the 510(k) submission process requires a strategic approach, understanding the device, conducting a comparative analysis, and following FDA guidance. Manufacturers must be well-prepared, adhere to requirements, and stay informed of the evolving regulatory landscape. By doing so, they can contribute to the advancement of medical device technology and patient care while meeting the FDA's expectations for safety and effectiveness.




