Introduction
Good Manufacturing Practices (GMP) are indispensable in the medical device industry, ensuring that products are consistently produced and controlled according to stringent quality standards. Authorities such as the FDA and ISO provide detailed regulations that manufacturers must adhere to, ensuring product safety and efficacy. For instance, the FDA, part of the U.S. Department of Health and Human Services, plays a crucial role in safeguarding public health by regulating medical devices among other products.
Compliance with these regulations not only mitigates risks but also enhances the credibility of medical devices in the market.
The qualification of medical devices is a complex process faced with numerous challenges, including regulatory constraints and confidentiality concerns. GMP offers a structured framework for this qualification process, helping to maintain consistent quality and adherence to international standards like ISO 13485. This is crucial because gaining market approval and ensuring compliance remains top priorities for medical device leaders.
Moreover, the evolving landscape of regulatory demands continually reshapes the development and market entry of medical devices. Industry experts emphasize the importance of agility and adaptability in building effective quality management systems, which are essential for navigating these regulatory changes efficiently. This ensures that every device not only meets the required standards but also maintains its integrity and efficacy throughout its lifecycle.
Key Regulations for GMP Compliance
'Good Manufacturing Practices (GMP) are essential in the healthcare product industry, ensuring that items are consistently produced and controlled according to rigorous quality standards.'. Authorities such as the FDA and ISO provide detailed regulations that manufacturers must adhere to, ensuring product safety and efficacy. For example, the FDA, a component of the U.S. Department of Health and Human Services, has an essential role in protecting public health by overseeing healthcare tools among other products. Adhering to these regulations not only reduces risks but also boosts the trustworthiness of health products in the market.
The certification of healthcare instruments is a complicated procedure confronted with many obstacles, such as regulatory limitations and privacy issues. GMP offers a structured framework for this qualification process, helping to maintain consistent quality and adherence to international standards like ISO 13485. 'This is essential because obtaining market approval and ensuring compliance remain top priorities for health technology leaders, as identified in industry surveys.'.
Moreover, the evolving landscape of regulatory demands continually reshapes the development and market entry of medical instruments. Industry experts emphasize the importance of agility and adaptability in building effective quality management systems, which are essential for navigating these regulatory changes efficiently. This ensures that every piece of equipment not only meets the required standards but also maintains its integrity and efficacy throughout its lifecycle.
Understanding 21 CFR Part 820
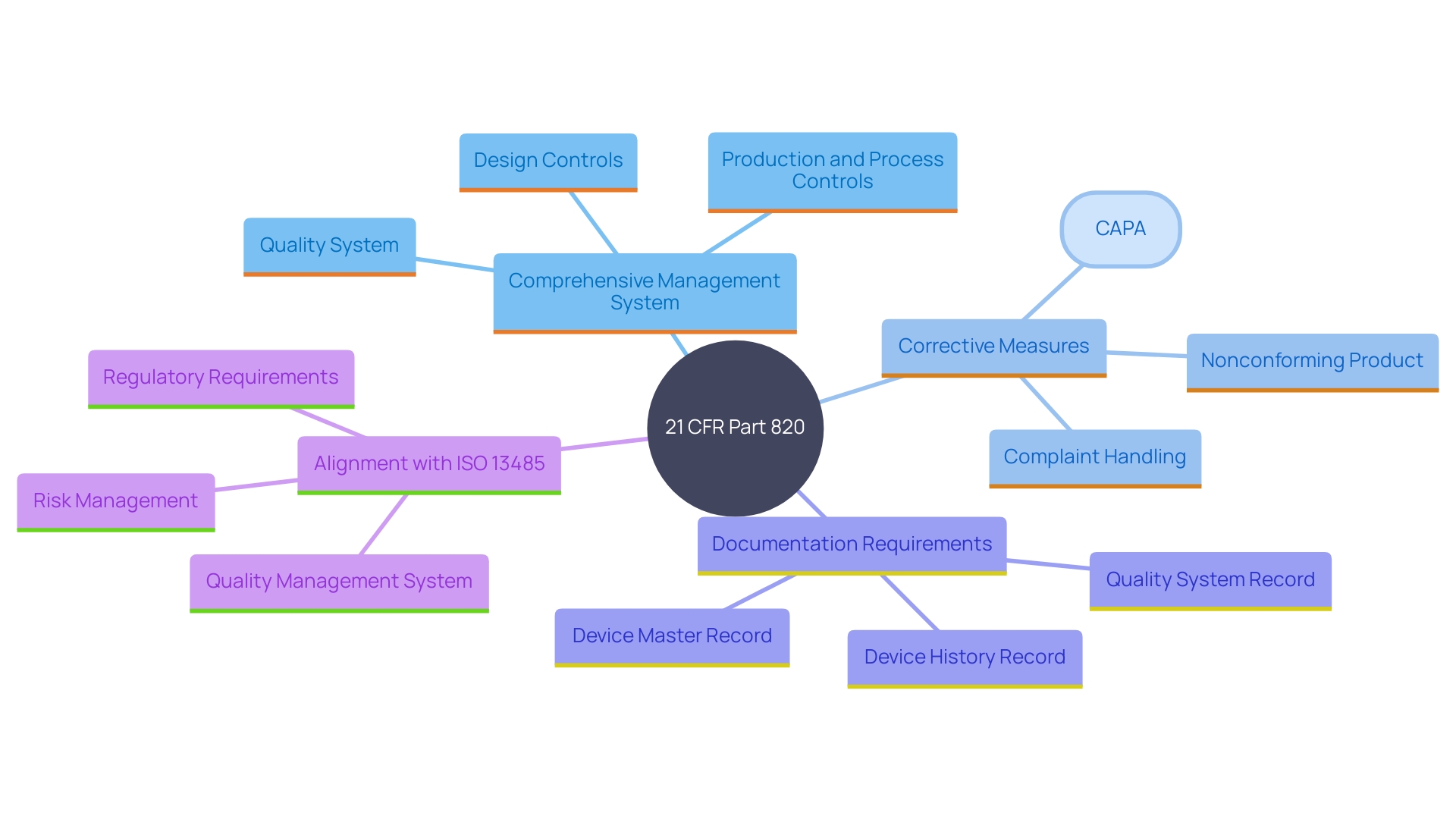
21 CFR Part 820, established by the FDA, sets forth the Quality System (QS) requirements for medical device manufacturers. This regulation mandates a comprehensive management system encompassing all facets of production, from design and manufacturing to distribution. Ensuring adherence to these regulations is critical for meeting safety and effectiveness standards.
The FDA’s emphasis on promoting and consistently enhancing the standard, safety, and effectiveness of medical devices aligns with 21 CFR Part 820's stringent requirements. The regulation highlights systematic corrective measures, such as those observed in recent company responses tackling matters like complaint coding and data analysis. For instance, firms are required to document adverse event information, evaluate reportability, and maintain deliberation records as per 21 CFR 803.18(b)(1)(i).
Moreover, the FDA's efforts to harmonize its Quality System regulation with ISO 13485 reflect a commitment to global regulatory consistency. ISO 13485 requires documented procedures for managing management system documents, ensuring accessibility, and preventing the use of outdated documents. Top management must demonstrate their commitment to maintaining and improving the quality management system, ensuring resources are adequate for compliance.
The FDA's regulatory structure, backed by voluntary consensus norms, promotes innovation and uniformity. 'These criteria are created based on concepts of openness, involvement of interested parties, and equitable representation, aiding in a strong regulatory framework that guarantees the safety and effectiveness of healthcare tools.'. By following 21 CFR Part 820, manufacturers can guarantee their products meet the highest criteria, thereby boosting trust and credibility with customers.
Elements of a Quality System Regulation
A robust Quality System Regulation (QSR) encompasses critical elements such as Design Controls, Document Controls, Production and Process Controls, and Corrective and Preventive Actions (CAPAs). These components collectively ensure that medical devices are developed and manufactured to meet rigorous criteria throughout their lifecycle.
Design Controls, similar to Application Lifecycle Management (ALM) in software development, ensure that the final product is safe, effective, and of the utmost standard. They include rigorous processes for updates and changes, which can even be managed using Agile methodologies. Document Controls ensure that all documentation, from clinical evaluation plans to risk management files, is meticulously analyzed and consistently presented. This prevents disconnection between conclusions and actual data, a common challenge in clinical data presentation.
Production and Process Controls concentrate on preserving the integrity and excellence of the manufacturing process, following Good Manufacturing Practice (GMP) guidelines. This framework is essential for guaranteeing adherence to international guidelines such as ISO 13485 and FDA regulations. Lastly, CAPAs are essential for identifying and addressing potential issues proactively, leveraging post-market surveillance data with the same rigor as primary data to enhance product excellence and safety.
By incorporating these measures, healthcare equipment producers can methodically tackle compliance demands and uphold rigorous levels of excellence, ultimately guaranteeing patient safety and product effectiveness.
Harmonization with International Standards (ISO 13485:2016)
'ISO 13485:2016 is a globally acknowledged guideline that sets forth criteria for a thorough quality management system (QMS) specifically for the creation and production of healthcare products.'. 'This guideline is pivotal for ensuring that organizations can consistently meet both customer and regulatory requirements.'. By aligning with ISO 13485, medical product manufacturers can enhance their market access and consumer trust, as it demonstrates a commitment to producing high-quality, safe medical products.
The importance of this standard is underscored by the way it mandates documented procedures for controlling all aspects of the QMS. This includes the approval, review, updating, and retrieval of documents to guarantee their accessibility and prevent the unintended use of obsolete documents. Furthermore, ISO 13485 emphasizes the need for top management to demonstrate their commitment to the QMS by ensuring customer requirements are met and maintaining the integrity of the system when changes are implemented. Adequate resources must be allocated to sustain the QMS, impacting human resources, infrastructure, and work environments.
Risk management is a critical aspect of ISO 13485, emphasizing the need to proactively identify and mitigate potential risks throughout the design, manufacturing, and post-market surveillance processes. This approach not only guarantees adherence to regulatory standards but also results in the creation of high-quality products that deliver optimal patient outcomes.
Adhering to ISO 13485 also facilitates compliance with global regulations, which is crucial given the varying regulatory landscapes across different regions. By being globally compliant, manufacturers can streamline their operations and reduce the complexity associated with meeting multiple regulatory requirements. This worldwide strategy for adherence is progressively significant as healthcare instruments become more advanced and their regulatory landscapes more rigorous.
In summary, ISO 13485:2016 is a cornerstone for manufacturers of healthcare instruments, ensuring that they can consistently produce safe and effective products while meeting both regulatory and customer expectations. Its emphasis on documented procedures, risk management, and resource allocation makes it a crucial standard for maintaining the highest levels of quality and safety in the medical device industry.
Conclusion
The article provides a comprehensive overview of the critical role that Good Manufacturing Practices (GMP) play in the medical device industry. It highlights the necessity for compliance with regulations set forth by authorities such as the FDA and ISO, which are essential for ensuring product safety and efficacy. The structured approach of GMP facilitates the qualification process of medical devices, allowing manufacturers to navigate the complexities of regulatory demands while maintaining high quality standards.
Additionally, the significance of 21 CFR Part 820 is emphasized, outlining its requirements for a robust Quality System that encompasses all aspects of production. This regulation not only promotes safety and effectiveness but also aligns with international standards like ISO 13485. The integration of these regulations fosters a culture of continuous improvement and innovation within the industry, ensuring that medical devices meet the highest quality benchmarks.
Key components such as Design Controls, Document Controls, and Corrective and Preventive Actions (CAPAs) are essential for maintaining compliance and enhancing product quality. These elements collectively support manufacturers in addressing regulatory requirements and safeguarding patient safety throughout the lifecycle of medical devices.
In conclusion, adherence to GMP and associated regulations is vital for medical device manufacturers aiming to establish credibility and trust in the market. By prioritizing quality management systems and aligning with international standards, organizations can not only ensure compliance but also contribute to the overall safety and effectiveness of medical devices. This commitment to quality not only meets regulatory expectations but also serves to protect public health and enhance consumer confidence in medical technology.




