Introduction
In clinical trials and healthcare, the Informed Consent Form (ICF) stands as a cornerstone document, pivotal for ensuring that participants are fully informed about the studies they consider joining. This document encapsulates critical information such as the research's purpose, the procedures involved, potential risks and benefits, and the study's duration. Additionally, it underscores the rights of participants, including their ability to withdraw from the study at any time.
The primary function of the ICF is to protect the autonomy of individuals, allowing them to make well-informed decisions regarding their participation.
Regulatory bodies advocate for presenting key information upfront in the ICF, enhancing participants' understanding and supporting informed decision-making. Empirical research continues to refine these forms, aiming to improve participant comprehension and satisfaction. This ensures that the consent process is accessible and tailored to participants' unique needs, thereby fostering an ethical and transparent environment in clinical research.
This article delves into the essential elements, ethical and legal requirements, and the overarching importance of ICFs in protecting participant rights, ultimately highlighting their role in the informed consent process.
What is an Informed Consent Form (ICF)?
An Informed Consent Form (ICF) is a pivotal document in clinical trials and healthcare settings designed to ensure individuals are thoroughly informed about the study they are considering. It succinctly presents essential information such as the purpose of the research, the procedures involved, potential risks and benefits, and the study's duration. This essential document also emphasizes the rights of individuals, including their right to withdraw from the study at any time.
The ICF's primary function is to safeguard the autonomy of individuals, ensuring they make well-informed decisions about their participation. According to the National Organization for Rare Disorders (NORD), it is vital that consent be presented in ways that are accessible and tailored to a participant's unique needs, whether through simplified language, videos, or illustrations. This approach helps overcome barriers such as language differences, sensory impairments, and varying levels of health literacy.
Furthermore, draft guidance from regulatory bodies recommends that key information be presented at the beginning of the informed consent document. This includes the purpose of the study, potential risks and benefits, and the duration and procedures of the investigation. Such clarity not only aids potential contributors in understanding the study but also supports ongoing consent discussions between investigators and contributors.
Empirical research, such as that conducted by Janssen R&D, is critical to refining the ICF. Their research aims to assess understanding and satisfaction with various types of consent documents, striving to improve usability and guarantee knowledgeable decision-making. This data-driven method is essential for creating consent forms that effectively convey necessary details, thereby facilitating informed participation or refusal in clinical trials.
Key Elements of an ICF
A successful Informed Consent Form (ICF) should offer a clear overview of the study's aim, thorough descriptions of the procedures individuals will experience, and details about possible risks and advantages. It must also assure individuals of their confidentiality and emphasize the voluntary nature of their involvement. Additionally, the ICF should specify the study's duration and outline any compensation or costs associated with participation. Based on preliminary recommendations, presenting crucial details at the start of the consent document can greatly improve participants' comprehension of why they may or may not wish to take part in the study. This approach ensures that essential details such as the research purpose, potential risks and benefits, study length, and procedures are communicated effectively, thus supporting knowledgeable decision-making. The National Organization for Rare Disorders (NORD) has praised the draft guidance for allowing innovative approaches, such as videos, to make the informed consent process more accessible. These methods assist in tackling challenges such as language barriers and health literacy skills, guaranteeing that everyone involved can completely grasp the consequences of their involvement.
Ethical and Legal Requirements for ICF
The development and use of Informed Consent Forms (ICFs) are governed by stringent ethical guidelines and legal regulations. These encompass principles detailed in the Declaration of Helsinki and local regulations concerning human subject studies. Researchers must ensure that the ICF is written in clear and comprehensible language, reflecting the ethical duty to respect participant autonomy and safeguard their welfare.
Effective informed consent starts with delivering essential details regarding the study in a clear and concise manner. The draft guidance from regulatory bodies highlights that essential details should encompass important subjects such as the aim of the study, the possible risks and advantages, and the duration and methods of the investigation. This approach aids individuals in grasping the reasons why they might choose to engage in the research.
Furthermore, the draft guidance promotes the use of various methods to present this key data, including written, oral, media, and electronic formats. This flexibility ensures that the informed consent process is accessible to all individuals, considering factors like language barriers, hearing or vision impairments, and health literacy competencies. The National Organization for Rare Disorders (NORD) applauded these recommendations for their inclusivity and innovative approaches.
Moreover, it is crucial for researchers to use key information as a guide during the consent discussion with potential subjects. This practice not only aids understanding but also aligns with the revised Common Rule, harmonizing human subject protection regulations with the U.S. Department of Health and Human Services (HHS) standards. By following these guidelines, clinical studies can be carried out more effectively while safeguarding the rights of individuals and promoting medical progress.
Importance of ICF in Protecting Participant Rights
The ICF is instrumental in protecting rights of individuals by ensuring informed decision-making. It starts with delivering essential details regarding the study in a clear and succinct way. This includes the purpose of the study, possible risks and benefits, and the study’s length and procedures. By supplying this crucial data in advance, individuals can make knowledgeable choices regarding their engagement. This process also includes the right to decline involvement without any repercussions, reinforcing ethical research practices and fostering trust between researchers and those involved. The draft guidance motivates researchers to utilize essential details as a reference to aid conversations with prospective subjects, making the consent process more reachable and comprehensible.
The Informed Consent Process
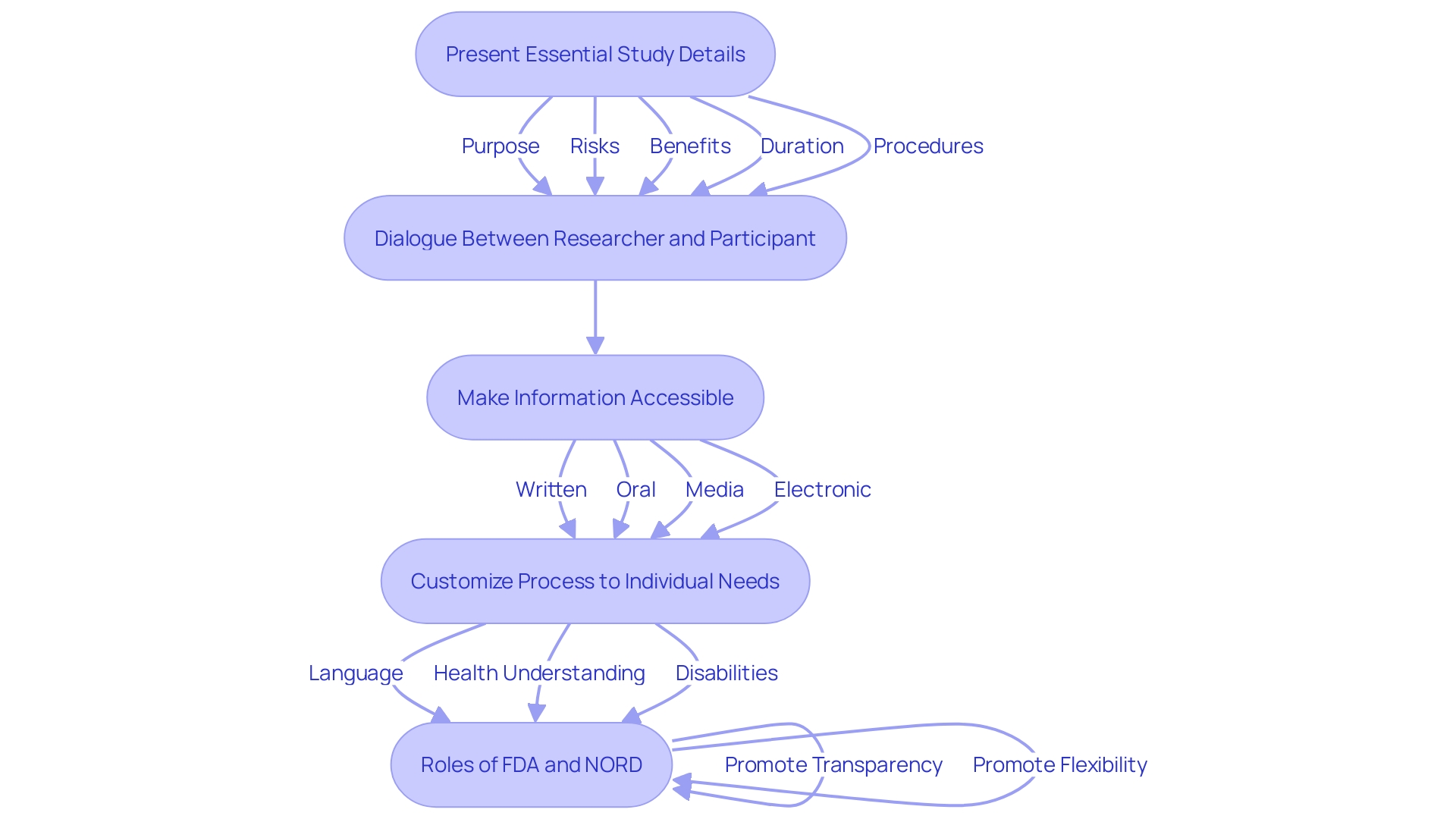
The consent procedure involves more than merely a signature; it is a crucial conversation between the researcher and the individual involved. This process begins with presenting essential details about the study in a clear and concise manner, such as the purpose of the research, possible risks and benefits, and the study’s duration and procedures. The draft guidance promotes the incorporation of this essential detail at the start of the informed consent document to enhance comprehension and aid the consent dialogue between the investigator and prospective individuals.
Ensuring that participants comprehend their rights and the details provided is crucial for fostering an environment of trust and respect. It is recommended that researchers use various methods to make this information accessible, including written, oral, media (such as videos), and electronic consent. Dr. Robert M. Califf, Commissioner of Food and Drugs, emphasizes the importance of clinical trial transparency and the FDA’s oversight in harmonizing clinical research regulations to facilitate the development of medical products that benefit public health.
Additionally, the National Organization for Rare Disorders (NORD) endorses creative methods for obtaining consent, acknowledging the necessity to customize the process to individuals' distinct requirements, including language obstacles, health understanding, and disabilities. This flexibility in implementing the key data requirement ensures that the informed consent process is accessible and understandable for all participants.
Differences Between ICF for Research and Treatment
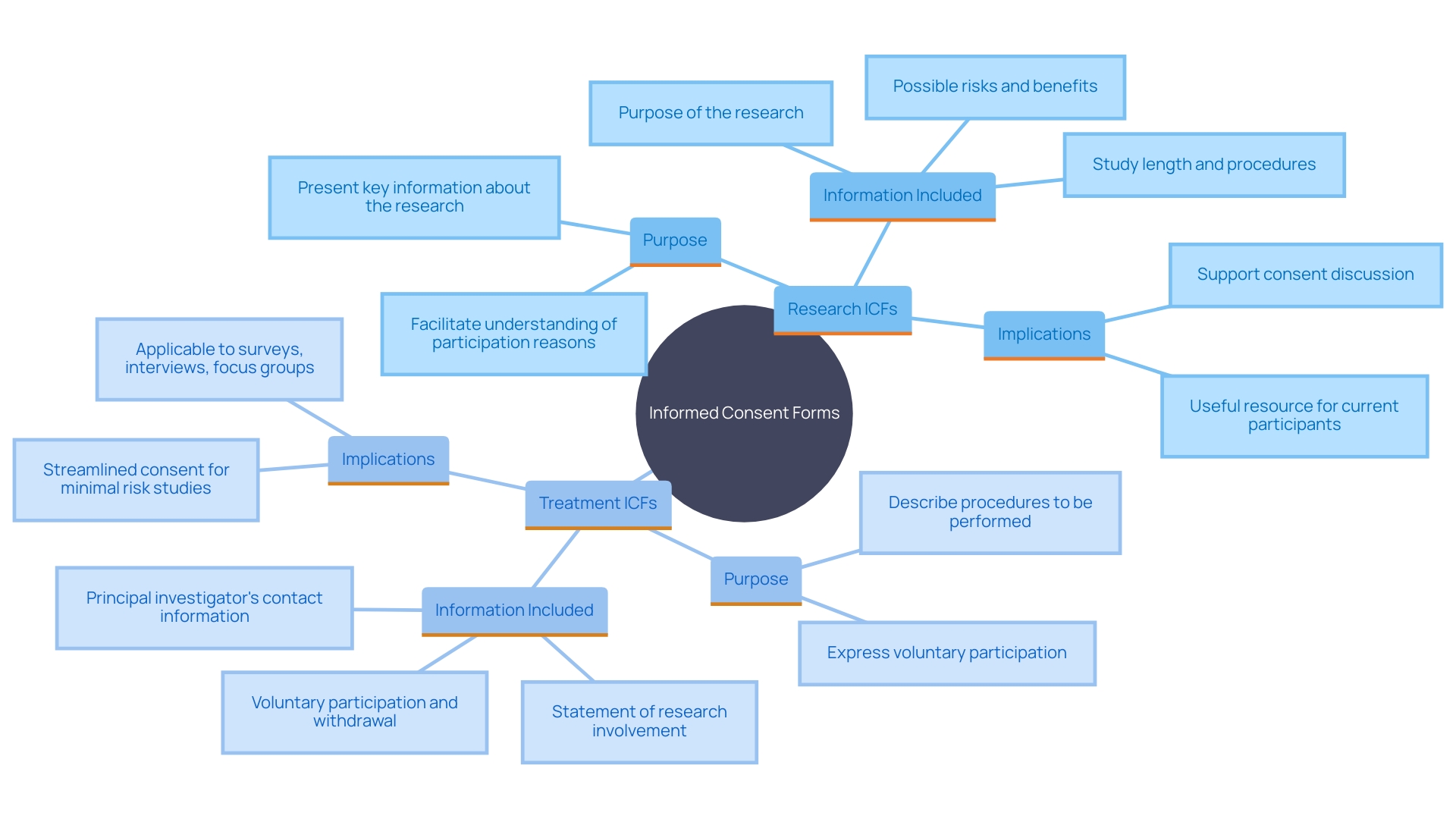
Informed Consent Forms (ICFs) for research and treatment, while similar in structure, diverge significantly in their purpose and content. Research ICFs are designed to provide comprehensive details about experimental procedures, potential risks, benefits, and uncertainties related to the outcomes. This transparency assists participants in making knowledgeable choices about their involvement. For example, the FDA's draft guidance emphasizes presenting key information at the beginning of the informed consent document. This includes details such as the purpose of the research, possible risks and benefits, and the study's duration and procedures.
On the other hand, treatment ICFs focus on established medical interventions, detailing proven treatments and standard care protocols. This distinction is crucial for those involved as it impacts their understanding and expectations of what they are consenting to. The addition of essential details in consent documents, as suggested by the FDA, acts as a beneficial resource for current and potential study contributors, ensuring they understand the reasons for and consequences of their involvement. This approach aligns with the broader goal of making clinical research more efficient while safeguarding the rights of individuals involved.
Confidentiality and Data Protection in ICF
Confidentiality is a cornerstone of the Informed Consent Form (ICF). It guarantees that participants' personal details and data stay secure during the study. The ICF should delineate the measures taken to safeguard data, including anonymization techniques and strict data sharing limits. Participants should be informed that their phone numbers, clinical records, and other personal data will be used responsibly, such as for personalized health information and continuity of care. Furthermore, the ICF must explicitly state that anonymized data will be used for research purposes, such as assessing the quality of care or conducting observational studies, while ensuring that their privacy is not compromised.
Oversight bodies like Research Ethics Committees (RECs) and Institutional Review Boards (IRBs) play an essential role in upholding ethical standards in data management. These bodies face the challenging task of balancing the need for data accessibility with stringent legal and ethical requirements. The sheer number of data access requests can strain their resources, but they are mandated to protect individuals' privacy rigorously.
A systematic review highlighted the importance of consent quality across various socio-economic contexts, noting factors such as comprehension of study information and understanding the right to withdraw. Ensuring that individuals are fully aware of the nature of their involvement, the risks, and the benefits is vital. 'This process must be conveyed in a manner that participants can easily understand, allowing them to make knowledgeable decisions without feeling pressured.'.
The FDA's 2018 proposed rule to revise informed consent regulations aligns with these principles, emphasizing that informed consent should be clear and concise, presenting key information upfront. This encompasses the aim of the study, possible risks and advantages, as well as the procedures and time frame involved. These guidelines assist in guaranteeing that individuals' rights and privacy are honored, preserving the integrity and credibility of the study process.
The Covid-19 pandemic emphasized the significance of data sharing for advancing studies while highlighting the need for strong data privacy measures. Using state-of-the-art methods in Data Protection by Design and by Default ensures data privacy without compromising data quality and utility. This method shows that it is possible to safeguard individuals' privacy while enabling important studies, as evidenced in the investigation carried out in a Portuguese hospital during the pandemic.
Regulatory Bodies and ICF Approval
Compliance with regulations set forth by governing bodies such as the Food and Drug Administration (FDA) and Institutional Review Boards (IRBs) is crucial for the integrity and ethical conduct of clinical trials. These organizations provide comprehensive guidelines to ensure that Informed Consent Forms (ICFs) adhere to both ethical and legal standards.
The FDA, for instance, has made significant strides in harmonizing human subject protection regulations with the U.S. Department of Health and Human Services (HHS) Common Rule. This alignment aims to make clinical studies more efficient while safeguarding participants. The FDA's recent guidance mandates that consent starts with essential details regarding the study, presented clearly to aid comprehension. This approach is based on research regarding patient comprehension of information found in prescription drug labeling.
Additionally, the FDA issued a final rule allowing an exception to obtain consent under specific conditions when clinical investigations pose no more than minimal risk. This rule includes appropriate safeguards to protect the rights, safety, and welfare of participants, thus advancing medical product development without compromising participant protection.
IRBs play a crucial role in this process by making sure that all studies involving pharmaceutical products include consent without exceptions. Steven Kritz, MD, emphasizes that the insistence on informed consent by IRBs is fundamental in maintaining ethical standards in clinical research.
Moreover, the FDA and OHRP have provided recommendations to develop a key information section in clinical trials, using plain language and formatting tools to enhance understanding. This effort is part of a broader initiative to advance the generation of evidence needed to demonstrate the safety and effectiveness of medical products, ultimately facilitating medical advances while protecting participant rights.
Conclusion
The Informed Consent Form (ICF) is a critical component of clinical trials and healthcare, ensuring that participants are fully informed about the studies they consider. It outlines essential information, including the study's purpose, procedures, potential risks and benefits, and participants' rights. This transparency protects individual autonomy and fosters trust between researchers and participants, emphasizing the right to withdraw at any time.
ICFs are developed under stringent ethical and legal guidelines, with regulatory bodies like the FDA and IRBs requiring clear communication of key information. Innovative approaches, such as multimedia and simplified language, are encouraged to accommodate diverse participant needs, enhancing understanding across varying levels of health literacy.
Confidentiality and data protection are also crucial in the ICF framework. Safeguarding personal information and ensuring participants' privacy are paramount throughout the research process. Recent developments in informed consent regulations highlight the need to balance data accessibility with strong privacy measures.
In summary, the ICF is essential for ethical clinical research. It ensures that participants are adequately informed and their rights protected, thereby supporting informed decision-making and enhancing the integrity of the research process. Continuous improvements in the clarity and accessibility of ICFs will further promote ethical conduct in clinical trials, benefiting participants and advancing medical science.




