Introduction
The Food and Drug Administration (FDA) plays a critical role in ensuring the safety and effectiveness of medical devices in the United States. Manufacturers must navigate a complex regulatory landscape, including device classification and regulatory pathways, to bring their devices to market.
One important aspect of this process is obtaining an Investigational Device Exemption (IDE) for conducting clinical investigations. This article will delve into the requirements and process for securing an IDE, the different types of IDEs, determining significant risk, IDE application contents and submission, the role of Institutional Review Boards (IRBs), labeling and advertising requirements, monitoring and reporting requirements, and common FAQs and additional resources. By understanding these key aspects of the IDE process, manufacturers can successfully navigate the FDA approval process and ensure their devices are legally marketed in the U.S.
What is an Investigational Device Exemption (IDE)?
The Food and Drug Administration (FDA) plays a pivotal role in public health by ensuring the safety and efficacy of a wide array of products, including medical devices. To bring a medical device to the U.S. market, manufacturers must navigate a complex regulatory landscape that begins with device classification. The FDA categorizes devices into three classes based on the level of risk they pose to patients, with Class I representing the lowest risk and Class III the highest.
High-risk Class III devices, which are critical to sustaining life such as pacemakers, are subject to the most stringent regulatory scrutiny, often requiring a Pre-Market Approval (PMA) due to their significant role in patient care. Conversely, Class I and II devices may be eligible for the less rigorous 510(k) clearance process, which involves demonstrating that the new device is substantially equivalent to an already legally marketed device. Understanding these classifications and regulatory pathways, including the Investigational Device Exemption (IDE) for conducting clinical investigations, is essential for manufacturers to successfully navigate the FDA approval process and ensure their devices are legally marketed in the U.S.
IDE Requirements and Process
Securing Investigational Device Exemption (IDE) from the FDA is a critical step for medical device manufacturers aiming to conduct clinical studies in the United States. The FDA, integral to the U.S. Department of Health and Human Services, mandates a thorough application process.
Manufacturers must demonstrate that their device is properly classified according to the FDA's three-tier system, reflecting the device's associated patient risk. For high-risk class three devices, such as life-sustaining implantables, the scrutiny is more intense due to their significant role in patient care.
The application must detail the device's specifications, the objectives of the proposed investigation, and a meticulously designed study protocol. This protocol is crucial as it must be capable of generating reliable scientific data while concurrently safeguarding participant rights and well-being. The FDA's review process is rigorous, ensuring only devices that meet stringent safety and effectiveness standards are permitted to enter the clinical trial phase. This regulatory vigilance is emblematic of the FDA's overarching mission to protect public health by ensuring the security and efficacy of medical interventions.
Types of IDEs: Abbreviated and Full IDEs
Integrated Development Environments (IDEs) are essential tools in the realm of software development, offering a centralized suite of functionalities that cater to code creation, modification, and testing. Distinct types of IDEs exist, each tailored to specific needs within the development cycle.
Abbreviated IDEs cater to devices that echo the design and features of previously approved devices, facilitating minor tweaks or adaptations. These Ideas are particularly beneficial when the modifications are not substantial enough to warrant an exhaustive review.
Conversely, Full IDEs are indispensable when introducing devices that represent a significant departure from existing models or carry an inherent high risk to participants in clinical studies. The choice of IDE is crucial, as it aligns with the complexity and novelty of the device under development, ensuring that the software tools available are commensurate with the demands of the project at hand. The evolution of Ideas reflects the dynamic nature of software development, with advancements ranging from the early use of punch cards to the sophisticated, feature-rich platforms of today such as PyCharm, which boasts robust refactoring capabilities and support for Computer-Aided Software Engineering (CASE) tools. These developments underscore the importance of selecting an IDE that not only streamlines the development process but also aligns with the regulatory compliance and market dynamics critical to the success of medical device development.
Significant Risk and Non-Significant Risk Determination
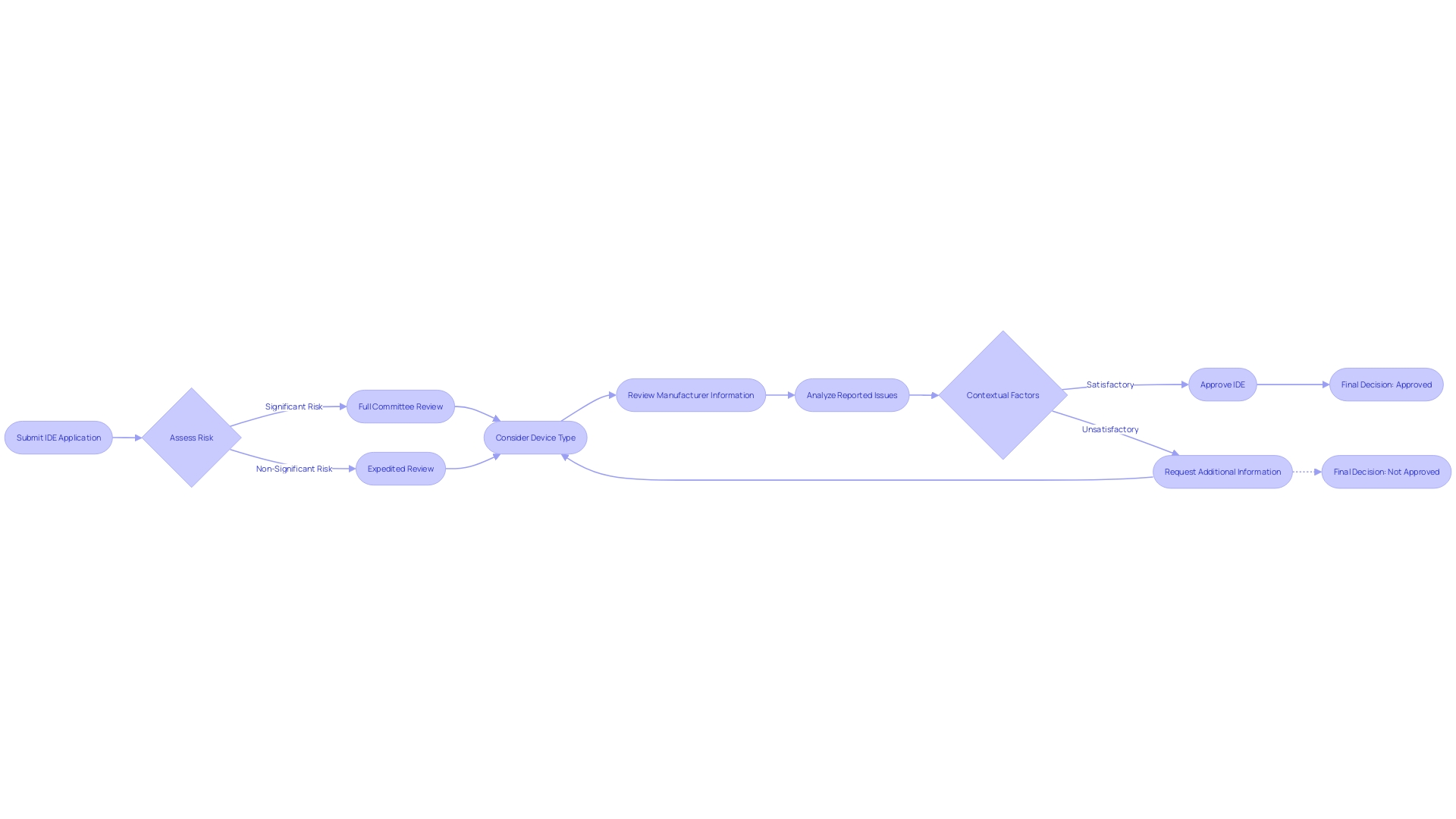
When submitting an Investigational Device Exemption (IDE) application, the FDA meticulously evaluates the level of risk associated with the medical device in question. This assessment categorizes devices as either significant risk (SR) or non-significant risk (NSR).
SR devices are subjected to a more thorough review process due to their potential to pose a greater danger to participants. This evaluation includes considering the device type, the manufacturer, brand name, and the specific lot number if applicable.
The FDA also examines whether any reported issues with the device are due to defects, malfunctions, or breakages, and whether the device was being operated at the time of the event. Additionally, the context of the device's use is scrutinized, such as whether other therapies administered to the patient could have influenced the event. This rigorous process is part of the FDA's overarching mission to ensure public health by verifying the safety, effectiveness, and security of medical devices, along with a broad spectrum of other consumer products and therapies.
IDE Application Contents and Submission
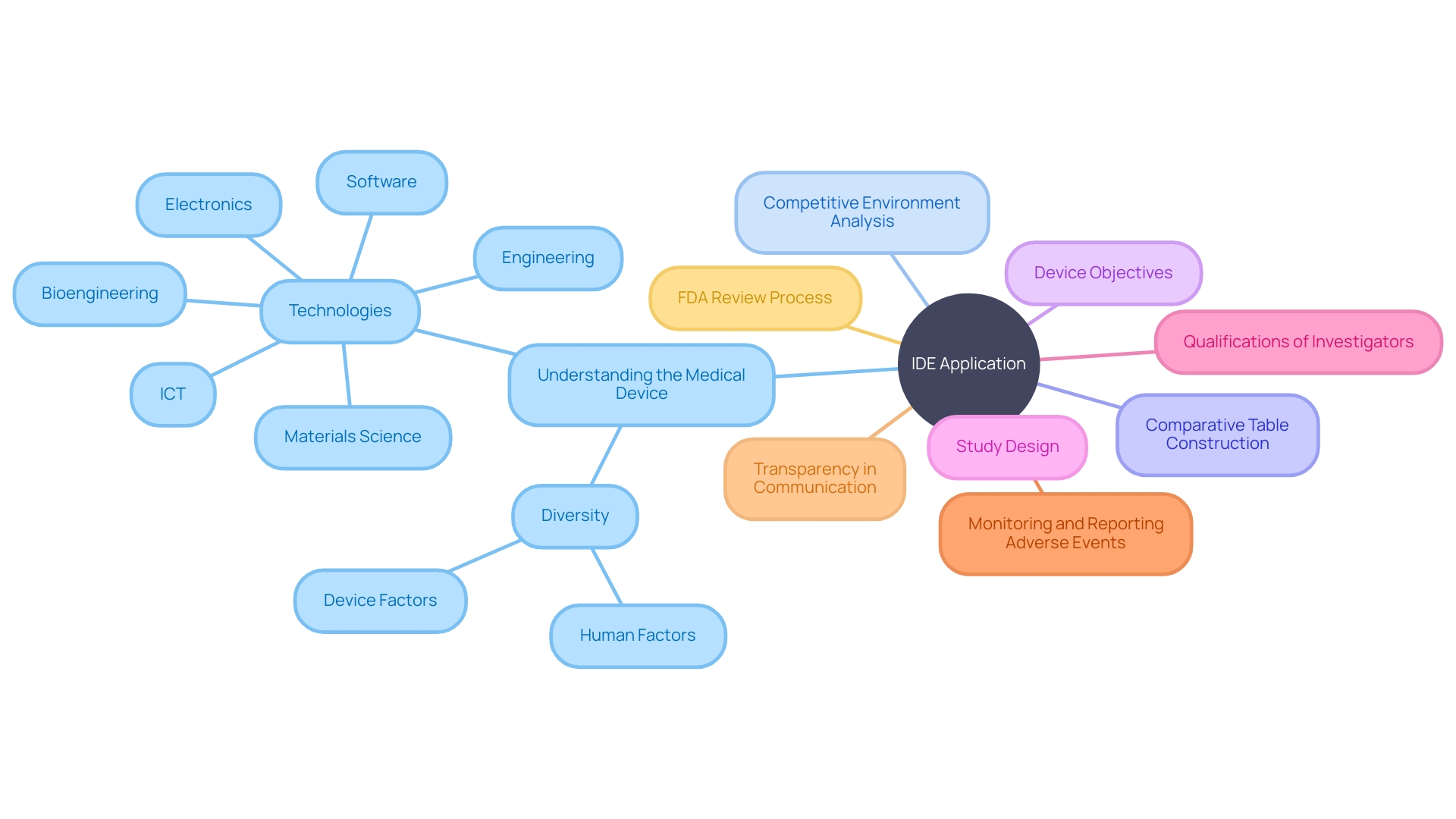
In preparing an Investigational Device Exemption (IDE) application, it is vital to thoroughly understand the medical device in question. This includes grasping its intended use and the nuances of its operation, which extends to being well-versed in the user manual, inclusive of any warnings and precautions. It's also essential to be aware of the competitive environment, identifying analogous devices with similar intended uses and technological attributes.
This knowledge aids in the construction of a comparative table, which is a critical component of the IDE submission process. The application must encapsulate comprehensive details about the device, including its objectives and study design. It should also detail the qualifications of the investigators involved, alongside a robust plan for monitoring and reporting any adverse events that may occur.
A quote that encapsulates the essence of what the application should achieve is: “In this document, 'transparency' describes the degree to which appropriate information about a Machine Learning Medical Device (MLMD) is clearly communicated to relevant audiences.” The application should ensure that any information which could impact risk assessments and patient outcomes is effectively conveyed, taking into consideration what the intended user or audience requires and the context in which the information is used. This approach emphasizes the utilization of optimal media, timing, and strategies to facilitate successful communication. Once the IDE application meticulously addresses these components, it is ready to be submitted to the FDA for a rigorous review and subsequent approval, paving the way for clinical trials and, ultimately, advancements in medical technology.
Institutional Review Board (IRB) and Its Role
The pathway to securing FDA clearance or approval for a medical device is multi-faceted, beginning with the critical step of classifying the device according to the FDA's three-tier system based on patient risk. Once the device is appropriately classified, the manufacturer must navigate the appropriate registration pathway, be it the 510(k) Premarket Notification, Pre-Market Approval (PMA), or De Novo process. Each term - Registered, Cleared, Approved, and Granted - holds distinct regulatory significance, often misunderstood even among industry professionals.
Prior to marketing a device in the United States, it must be FDA Cleared, Approved, or in the case of the De Novo process, Granted. Part of this rigorous process involves obtaining approval from an Institutional Review Board (IRB), which serves as an independent ethics committee. The IRB plays a pivotal role in safeguarding the rights and welfare of study participants by meticulously reviewing the study protocol, informed consent documents, and any other materials pertinent to the ethical conduct of the study, ensuring compliance with regulatory standards.
Labeling and Advertising Requirements
For medical device manufacturers, navigating the regulatory landscape is critical, especially when conducting Investigational Device Exemption (IDE) studies. Prior to marketing the device, the manufacturer must determine the appropriate FDA classification for the device, which is categorized into three levels based on patient risk.
Following classification, the device must follow the correct registration pathway, which could be the Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. The FDA's terminology for market authorization varies: a device may be 'Registered,' 'Cleared,' 'Approved,' or 'Granted' depending on the pathway pursued.
It is imperative for the labeling of the investigational device to state 'for investigational use only' and to refrain from commercial distribution. Furthermore, any advertising must strictly align with the approved investigational use, avoiding promotion for other purposes. This ensures compliance with FDA regulations and protects patient safety during the investigational phase.
Monitoring and Reporting Requirements
In the investigational device exemption (IDE) phase, the onus is on the medical device manufacturer to meticulously oversee the study's progression. This includes a critical watch over participant safety, coupled with the diligent reporting of any adverse events.
These reports must be meticulously detailed, capturing the device type, brand, and specific lot number, as well as any device-related issues such as defects or malfunctions. Additionally, it's imperative to record whether the device was in use at the time of an event and if other concurrent therapies might have influenced the outcome.
This comprehensive data collection is not only a regulatory mandate but also a cornerstone in evaluating the device's operational integrity, safety profile, and therapeutic efficacy. Monitoring visits are systematically conducted to gather and assess this data, ensuring robust post market surveillance and adherence to the FDA and IRB's stringent standards. As emphasized by the OECD's Conflict Minerals policy, it's essential for manufacturers to understand the broader implications of their supply chain practices and to maintain compliance with global regulations, which can vary significantly across different markets and regions.
Common FAQs and Additional Resources
Navigating the Investigational Device Exemption (IDE) process for medical device manufacturers is a detailed and intricate affair. To optimize the chances of success, it is not only about adhering to guidelines but also about comprehensively understanding the interplay of various development aspects.
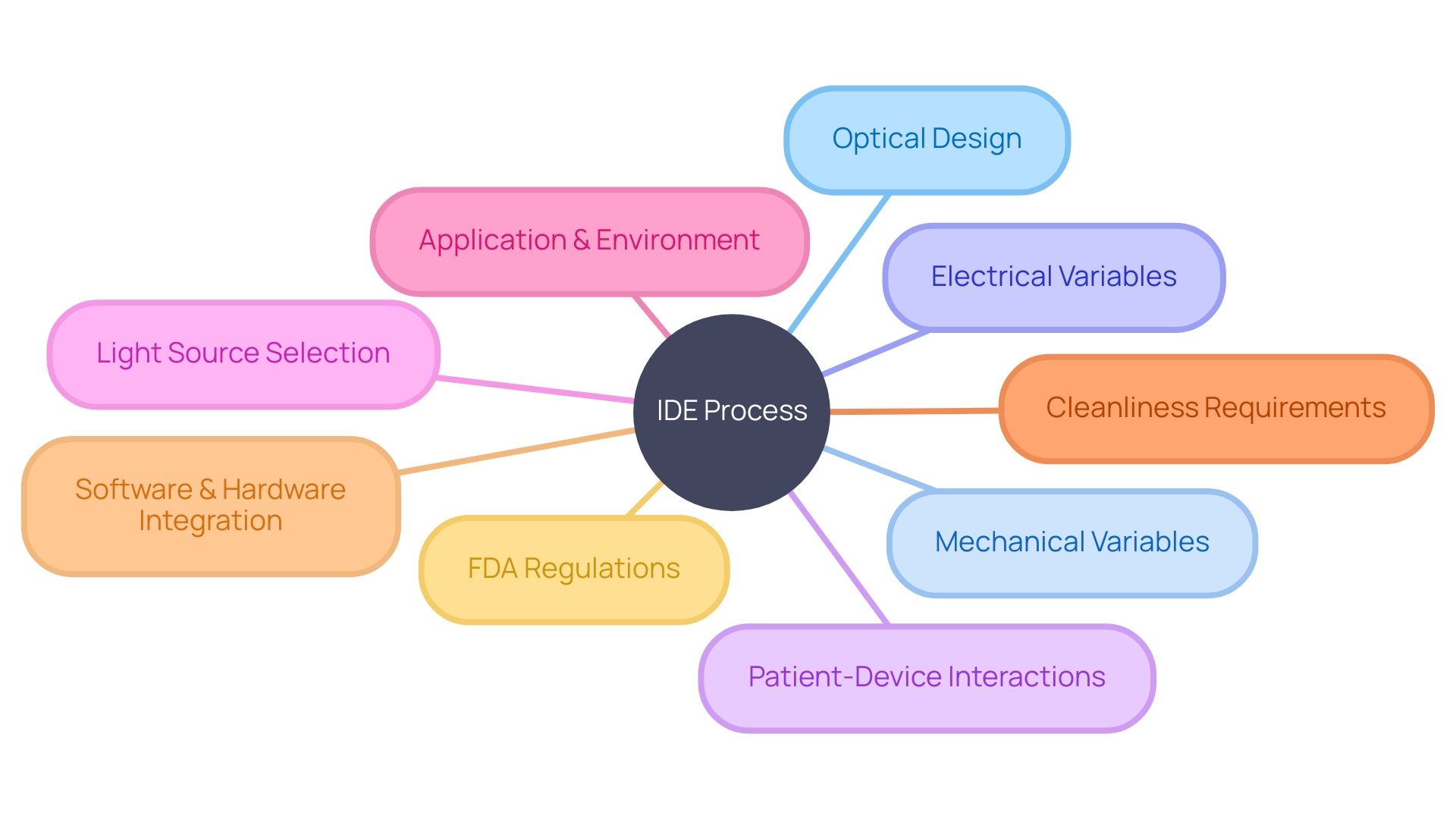
For instance, the integration of optical design requires consideration of mechanical and electrical variables, as well as the potential interactions between patients and the device. This systems approach underscores that an optical problem is not isolated but rather part of a larger, interconnected challenge.
Moreover, the selection of appropriate light sources, with specific attributes like wavelength, power, and type, is crucial for both the functionality and safety of the device. This decision-making process is informed by the intended application and environment in which the device will operate.
As development progresses, one must also bear in mind that initial cleanliness requirements may differ from those of the final product, thereby avoiding unnecessary overhead. Industry leaders in medical device development have demonstrated their ability to successfully launch new products by adeptly navigating this complex process from ideation to market entry.
The integration of software and hardware components, particularly in the burgeoning field of digital health, provides a competitive edge. This is reflected in the industry-leading systems and solutions that are continually emerging in this ultra-competitive market. In line with these considerations, the FDA offers a wealth of resources, including a comprehensive FAQ section, to guide manufacturers through the IDE process. These resources address critical components such as study design, data collection, adverse event reporting, and study termination criteria, all of which are instrumental in assuring the safety, effectiveness, and security of medical devices. With the public's health at the forefront, the FDA's regulatory oversight extends to ensuring that all medical devices meet stringent safety standards before they can be brought to market.
Conclusion
In conclusion, securing an Investigational Device Exemption (IDE) is a crucial step for medical device manufacturers navigating the FDA approval process in the United States. The IDE application requires detailed information about the device's specifications, objectives, and study protocol.
Understanding device classifications and regulatory pathways is essential for successfully navigating the approval process. The FDA's rigorous review ensures that only devices meeting stringent safety and effectiveness standards proceed to clinical trials.
The IDE process involves determining significant risk versus non-significant risk, selecting the appropriate IDE type based on device complexity, obtaining IRB approval, and adhering to labeling and advertising requirements during IDE studies. Monitoring participant safety and reporting adverse events are vital responsibilities during the IDE phase, helping evaluate device integrity and therapeutic efficacy.
Navigating the IDE process requires a comprehensive understanding of various development aspects. Industry leaders emphasize integrating software and hardware components for success in this competitive market. The FDA provides additional resources such as FAQs to guide manufacturers through the IDE process, addressing study design, data collection, adverse event reporting, and study termination criteria. By understanding these key aspects of the IDE process, medical device manufacturers can successfully navigate FDA regulations, ensuring their devices are legally marketed in the U.S. This advancement in medical technology prioritizes public health while meeting stringent safety standards.




