Overview
The article focuses on the key trends shaping the future of Medtech clinical research, including telemedicine integration, wearable technologies, artificial intelligence, and health information interoperability. These trends are supported by evidence of their impact on healthcare delivery and patient outcomes, emphasizing the importance of technology and patient engagement in enhancing clinical research effectiveness and improving health systems overall.
Introduction
The Medtech industry stands at the forefront of innovation, driven by a confluence of emerging trends that are revolutionizing healthcare delivery and enhancing patient outcomes. As the landscape evolves, significant advancements such as:
- Telemedicine integration
- Wearable technologies
- Artificial intelligence
are reshaping the way healthcare is accessed and delivered. These trends not only reflect the industry's response to contemporary challenges but also highlight the critical role of comprehensive clinical trial management services in navigating this dynamic environment. By exploring these transformative developments, the article delves into how Medtech is poised to redefine:
- Patient engagement
- Regulatory navigation
- The utilization of real-world evidence
Ultimately fostering a future where healthcare is more efficient, accessible, and patient-centric.
Emerging Trends Shaping the Future of Medtech
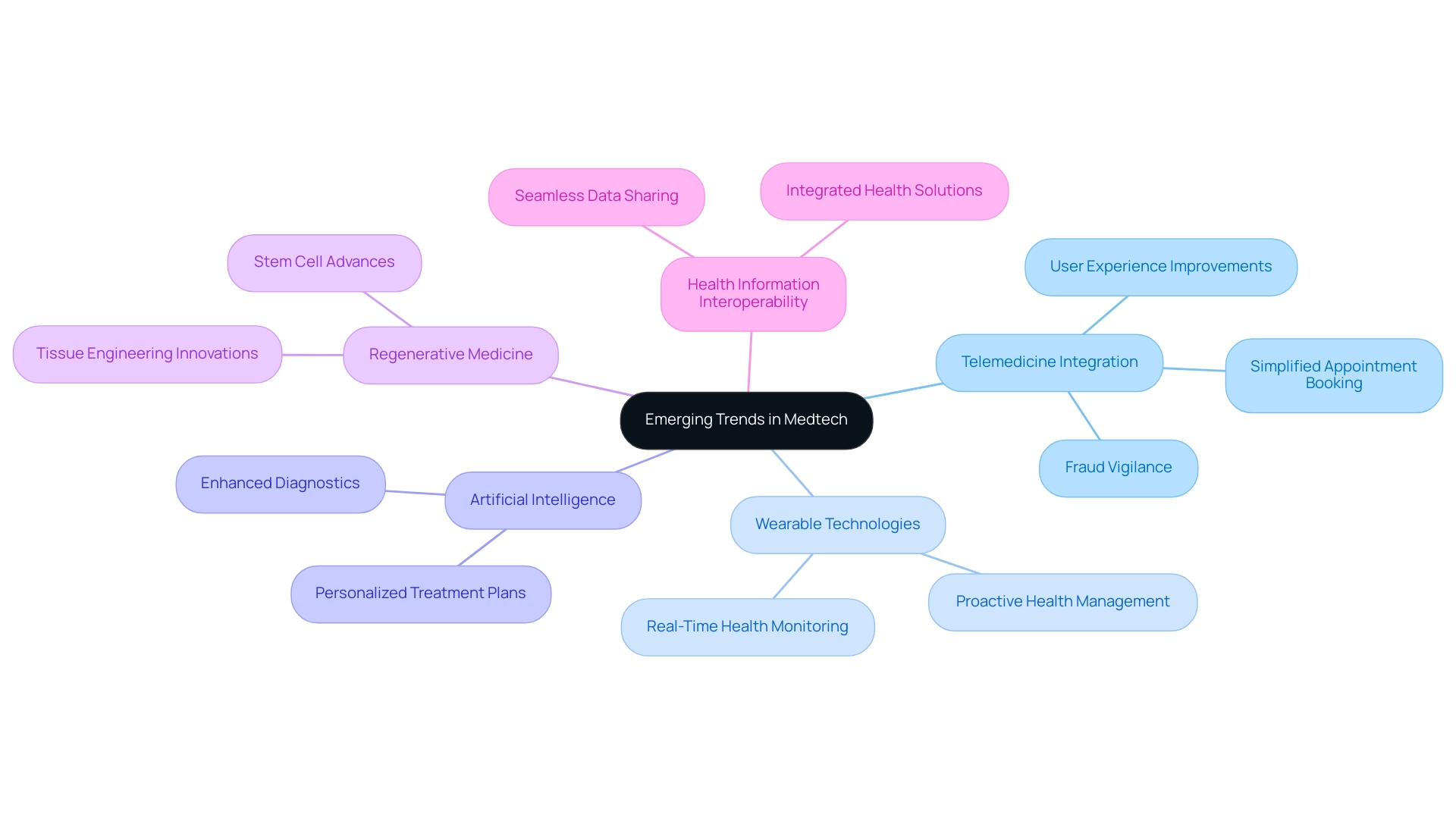
The Medtech industry is currently experiencing several pivotal trends that are reshaping healthcare delivery and individual outcomes, alongside the critical role of comprehensive clinical trial management services:
- Telemedicine Integration: The expansion of virtual consultations has become essential in enhancing access to care, particularly in the wake of the pandemic. Conor Stewart, a research expert in health and pharmaceuticals, notes that almost nine in ten individuals in the U.S. reported that telemedicine has made it easier to access necessary care. However, it's important to note that only 0.2% of all telehealth providers were identified as potentially high-risk for fraud, waste, and abuse, highlighting the need for vigilance in this rapidly growing field. This increase in telemedicine usage is paired with applications intended to simplify appointment scheduling and facilitate consultation payments, greatly enhancing hospital workloads and information management for physicians. The demand for improved user experiences (UX) in these applications has become a major trend, as customers expect clear navigation and quick access to essential functions, which is reflected in recent case studies.
- Wearable Technologies: The proliferation of devices capable of monitoring health metrics in real-time is revolutionizing how individual monitoring and data collection are approached. Recent statistics indicate that the usage of wearable technology in healthcare is anticipated to increase substantially in 2024, reflecting a broader trend towards individual empowerment and proactive health management. As these technologies develop, they are progressively incorporated into everyday medical practices, offering valuable insights for both individuals and healthcare professionals.
- Artificial Intelligence: The incorporation of AI in medical settings is significantly enhancing the accuracy of diagnostics and the development of personalized treatment plans. This trend is essential in enhancing medical study outcomes and reflects the key trends shaping the future of medtech clinical research, enabling more personalized interventions based on individual data and ultimately resulting in better health outcomes.
- Regenerative Medicine: Advances in stem cell studies and tissue engineering are paving the way for innovative treatments that have the potential to revolutionize patient care. These breakthroughs are not only broadening the possibilities for recovery but also introducing new paths for investigation and trials.
- Health Information Interoperability: The critical need for seamless sharing among healthcare providers is becoming increasingly apparent. Enhanced interoperability is crucial for improving patient care and represents one of the key trends shaping the future of medtech clinical research. As the industry moves towards integrated health solutions, the ability to share and access data across platforms will be paramount in driving innovation and improving health outcomes.
In addition to these trends, our service capabilities encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These comprehensive trial management services not only strengthen the local economy through job creation and healthcare improvement but also promote international collaboration in advancing global health. By ensuring adherence to ethical standards and facilitating necessary approvals from ethics committees, we position Medtech at the forefront of improving outcomes and enhancing healthcare delivery.
The Impact of Technology on Clinical Research in Medtech
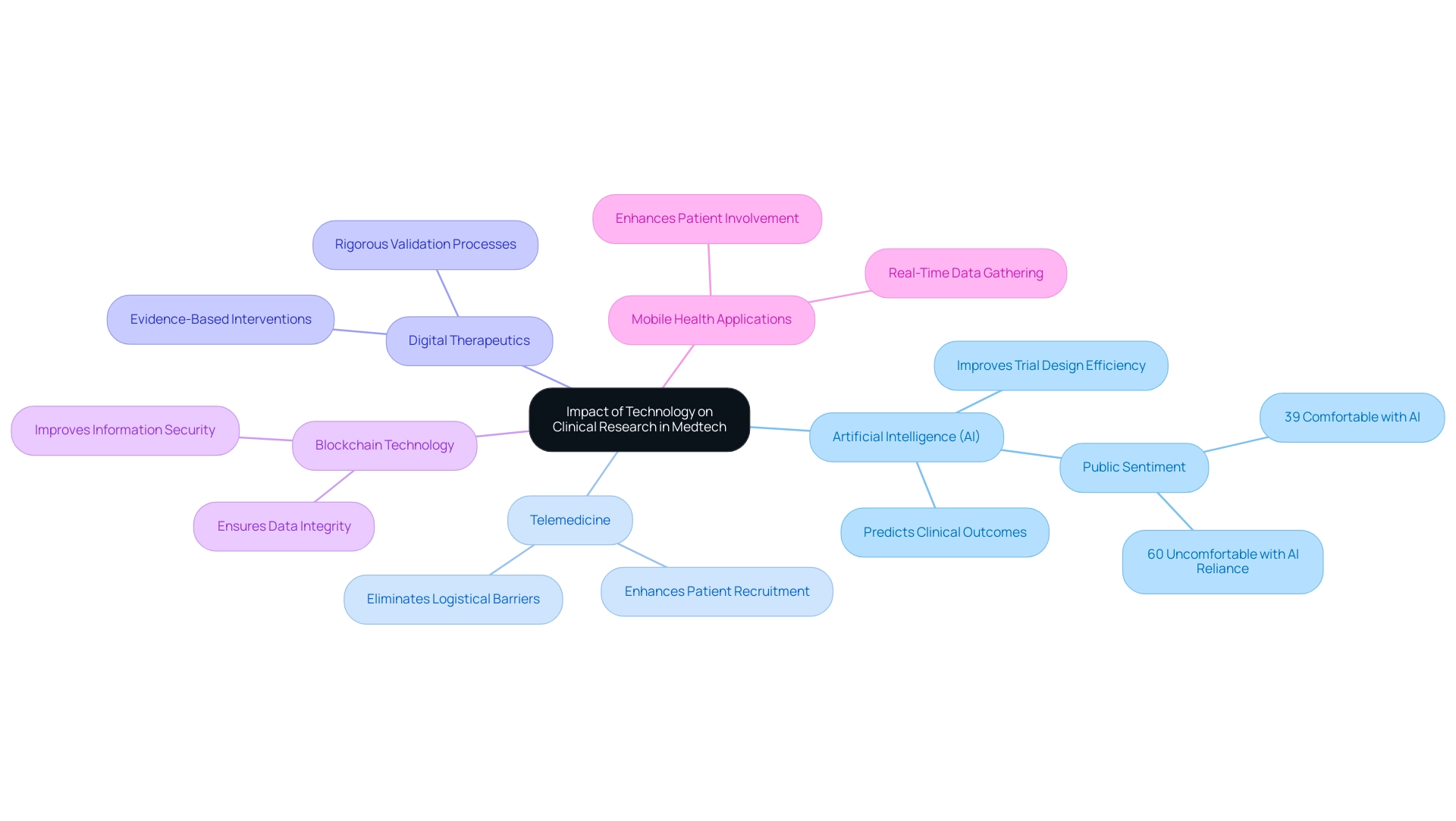
Technology is fundamentally transforming research in the MedTech sector through several key innovations, complemented by comprehensive trial management services that enhance the effectiveness of these technologies:
- Artificial Intelligence (AI): AI algorithms are increasingly employed to analyze extensive volumes of information, predict clinical outcomes, and improve trial design efficiency. Notably, 39% of Americans express comfort with the idea of AI assisting in their medical care, indicating a growing acceptance of AI's role in healthcare. However, data from the Pew Research Center reveals a more complex picture, with 60% of Americans feeling uncomfortable if their healthcare provider relied on AI for medical care. This reflects mixed feelings about AI's role in healthcare and highlights the need for ongoing dialogue about its integration.
- Telemedicine: The integration of remote monitoring and virtual trials is revolutionizing patient recruitment and retention by eliminating logistical barriers. As research trials evolve, telemedicine is becoming indispensable, which reflects the key trends shaping the future of medtech clinical research by enabling better access to trials for diverse populations.
- Digital Therapeutics: These evidence-based interventions deliver therapeutic solutions through software applications, targeting specific medical conditions. Their incorporation into medical research necessitates rigorous validation processes to ensure efficacy and safety.
- Blockchain Technology: By offering an unchangeable record of individual information and consent, blockchain improves information security and transparency in research trials. This technology tackles essential issues regarding information integrity, which is crucial for preserving trust in medical research.
- Mobile Health Applications: These applications enable real-time data gathering and enhance patient involvement, thereby improving the quality of medical data. They play a vital role in capturing patient-reported outcomes and enhancing overall trial efficiency.
In addition to these technological advancements, our comprehensive services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services not only simplify the investigation process but also contribute to job creation, economic growth, and improved healthcare outcomes within local communities. The emphasis on compliance reviews ensures that all studies meet necessary regulations, while robust project management guarantees timely execution and reporting of study status, inventory, and adverse events.
As Noble Shore, VP of Technology Strategy & Product Adoption at Veridix AI, emphasizes, 'AI will be most effective when it augments human capabilities. We need to consider not only the technology but also how it fits into workflows and how to enhance the skills of CROs to get the most out of it.' This perspective highlights the importance of not just adopting new technologies, but also ensuring that clinical research teams are equipped to leverage them effectively. The future of AI's ability to transform healthcare while addressing key trends shaping the future of Medtech clinical research will be crucial in the coming years.
Enhancing Patient Engagement through Co-Design in Medtech
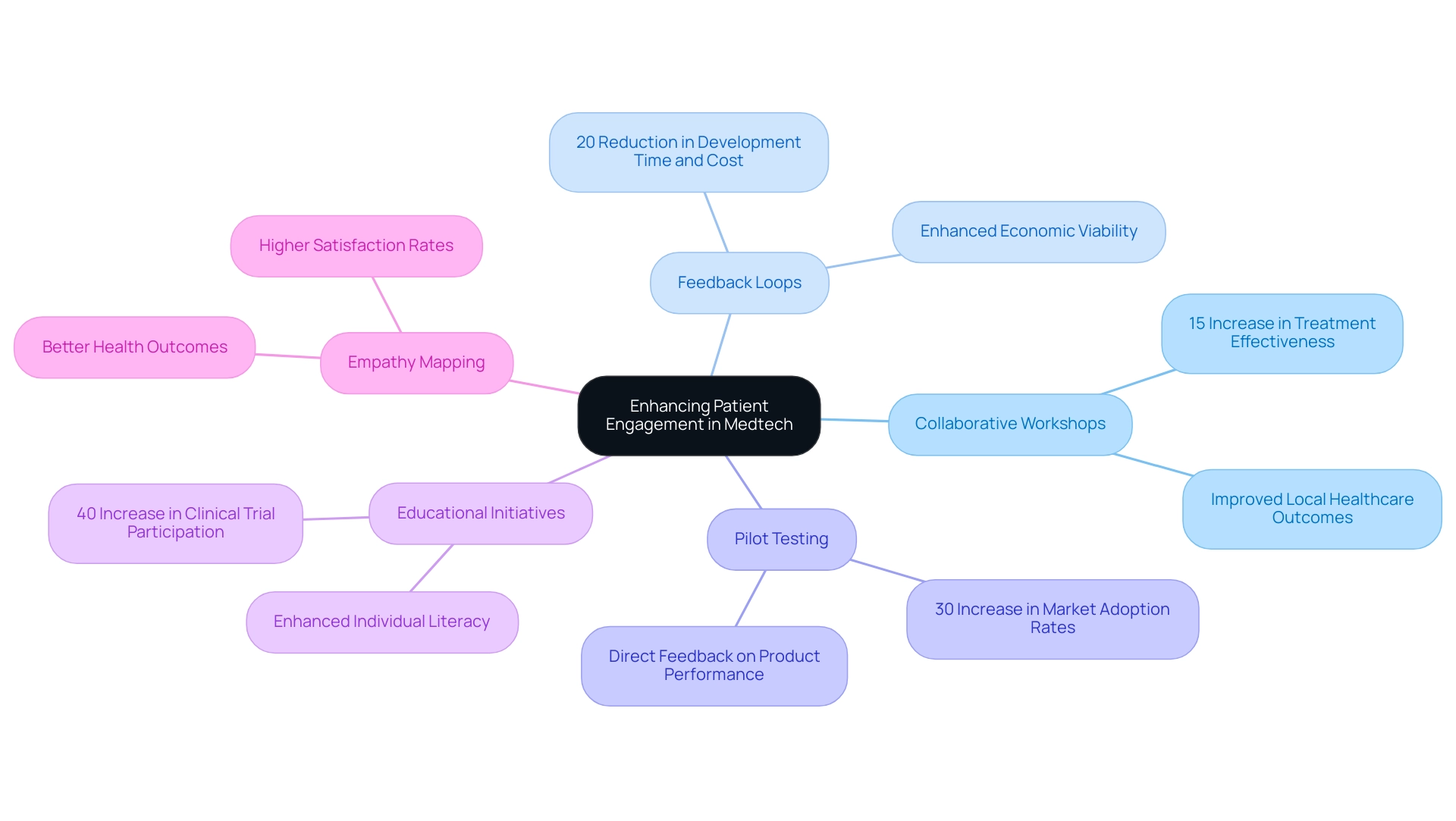
Co-designing Medtech solutions with individuals encompasses several critical strategies that foster collaboration and enhance product development:
- Collaborative Workshops: These sessions are pivotal in engaging individuals to share their experiences and needs, allowing researchers to gather invaluable insights that inform the design process. For instance, a recent workshop led to the development of a new device that significantly enhanced adherence, resulting in a 15% increase in treatment effectiveness, which in turn boosted local healthcare outcomes.
- Feedback Loops: Establishing a continuous feedback mechanism throughout the research and development phases is essential. This iterative process ensures that user input is systematically integrated, refining solutions to better meet expectations. A study found that companies implementing structured feedback loops saw a 20% reduction in development time and cost, enhancing their economic viability.
- Pilot Testing: Trials involving participant volunteers are crucial to evaluate the usability and effectiveness of Medtech solutions prior to full-scale implementation. This real-world testing provides direct feedback on product performance from the end-users. For example, a pilot test for a wearable health monitor not only improved the device's design but also led to a 30% increase in market adoption rates, positively impacting local economies.
- Educational Initiatives: Providing individuals with resources enables them to express their preferences and actively participate in the inquiry process, cultivating a sense of ownership over the solutions being developed. By enhancing individual literacy, these initiatives have been shown to increase participation in clinical trials by 40%, further driving economic growth through increased research activities.
- Empathy Mapping: Utilizing tools such as empathy maps helps visualize and understand the individual's journey, ensuring that the solutions crafted are genuinely aligned with their needs and experiences. This approach has been linked to higher satisfaction rates among individuals, which can translate into better health outcomes and reduced healthcare costs.
These engagement strategies not only enhance product development but also reflect the key trends shaping the future of medtech clinical research and contribute to broader economic impacts. By conducting clinical studies in various countries, Medtech initiatives are part of the key trends shaping the future of Medtech clinical research, which create ripples of positive change, stimulate job creation, promote economic growth, and improve healthcare systems. Recent statistics indicate that individual engagement in Medtech co-design strategies is on the rise; for instance, more than half of healthcare professionals surveyed recognized a significant correlation between feedback loops and improved development outcomes.
Moreover, Practo's remarkable 90% enhancement in EBITDA and 22% increase in revenue highlights the financial advantages of effectively involving individuals in the design process. However, it is essential to recognize that studies on the effect of individual involvement on healthcare quality are still limited and require further exploration, emphasizing existing gaps in the literature. The effectiveness of these strategies is further supported by case studies demonstrating that Medtech content marketing can yield at least a 20% return on investment, showcasing the growing acknowledgment of the importance of user involvement in the design process.
As highlighted by Ensieh Ashrafi, constructive feedback from esteemed reviewers is vital for enhancing the quality of studies, reinforcing the necessity for ongoing dialogue between patients and developers.
Navigating Regulatory Challenges in Medtech Research
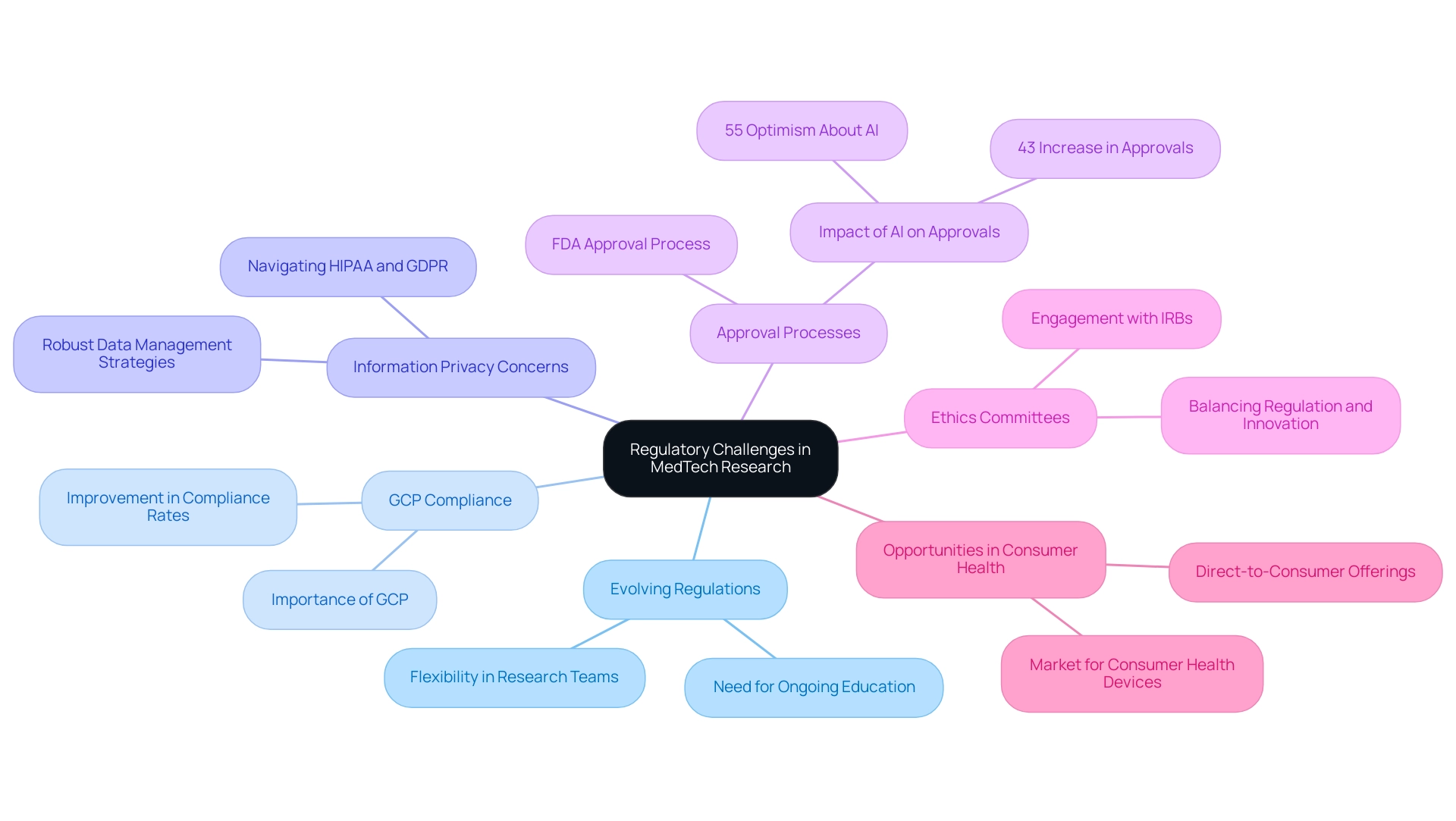
The landscape of MedTech studies is marked by several key regulatory challenges that require strategic navigation:
- Evolving Regulations: Staying abreast of regulatory changes is imperative, demanding ongoing education and flexibility from research teams. As the industry evolves, the need to adapt to new guidelines becomes increasingly vital.
- Compliance with Good Clinical Practice (GCP): Adhering to GCP guidelines is crucial for maintaining the integrity and credibility of trials. Recent statistics indicate that compliance rates with GCP in research trials have shown improvement, underscoring the growing emphasis on reliable outcomes. Regulatory experts, including Katherine Ruiz, an esteemed authority in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, highlight the importance of GCP in fostering trust and ensuring that clinical trials yield valid results.
- Information Privacy Concerns: With increased focus on security, navigating regulations such as HIPAA and GDPR is crucial for safeguarding patient details during study activities. Compliance in this area is increasingly scrutinized, necessitating robust data management strategies.
- Approval Processes: Understanding the intricacies of the FDA approval process is critical for expediting the time-to-market for innovative medical solutions. In 2023, the FDA reported a remarkable 43% year-on-year increase in approvals for MedTech AI algorithms and devices, underscoring the urgency for streamlined pathways. Furthermore, 55% of respondents express optimism about AI's future impact on the MedTech sector, highlighting a positive outlook amidst regulatory challenges.
- Ethics Committees: Engaging effectively with Institutional Review Boards (IRBs) is necessary to uphold ethical standards while simultaneously advocating for innovative investigative methodologies. As highlighted by Jim Welch, EY Global MedTech Leader, the balance between regulation and innovation is pivotal in understanding the key trends shaping the future of MedTech clinical research.
Opportunities in Consumer Health: The growing market for consumer health devices, such as continuous glucose monitors (CGMs), presents MedTech companies with new revenue streams. This opportunity requires navigating the regulatory landscape effectively to capitalize on consumer demand.
In addition to these challenges, our service capabilities support feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and comprehensive reporting. These services are intended to help MedTech firms in navigating regulatory obstacles and ensuring successful trials. Notably, the decrease in innovation capital, which constituted only 44% of financing raised by MedTechs in the past year, down from 67% during the COVID-19 crisis, underscores the pressing need for MedTech companies to leverage our comprehensive services. By effectively navigating existing regulations and adapting to the evolving landscape of medical studies in 2024, companies can leverage the key trends shaping the future of MedTech clinical research to position themselves for success in a challenging environment.
Leveraging Real-World Evidence and Data Analytics in Medtech
Approaches for effectively utilizing real-world evidence (RWE) and sophisticated analytics in medical studies and MedTech are crucial for improving the efficacy and significance of investigations, reflecting the key trends shaping the future of MedTech clinical research and their beneficial influence on local economies. These strategies not only support label extensions and enhance drug safety understanding but also pave the way for gaining international recognition within the industry. Key approaches include:
- Data Mining: By analyzing extensive datasets derived from electronic health records (EHRs), researchers can uncover significant trends and outcomes pertinent to MedTech research. This method is essential as 90% of pharmaceutical firms now possess specialized real-world evidence teams that improve the efficiency of information mining by offering the required expertise to derive actionable insights that guide healthcare decisions, thus aiding economic growth in the areas where studies are carried out.
- Predictive Analytics: Utilizing historical information enables researchers to forecast future outcomes, refining trial design and increasing the likelihood of successful results. This method is particularly valuable in an era where data-driven approaches dictate progress in medical technology, illustrating the key trends shaping the future of MedTech clinical research, which can enhance healthcare delivery and improve local health outcomes.
- Participant Registries: Creating strong participant registries enables the gathering of long-term information on outcomes, improving the significance and usability of medical studies. Such registries can help address critical gaps in understanding treatment impacts over time, further promoting healthcare improvement.
Furthermore, as emphasized by the case study on involvement in clinical trials, addressing obstacles to participation is crucial for ensuring that diverse groups are represented, ultimately enhancing the validity of research findings.
-
Machine Learning: The application of machine learning algorithms facilitates the identification of complex patterns within datasets, which can inform treatment protocols and ultimately enhance care. These advancements are pivotal in adapting to the dynamic healthcare landscape and align with the key trends shaping the future of MedTech clinical research, supporting the development of innovative solutions that benefit local economies.
-
Collaboration with Analytics Scientists: Partnering with analytics experts enhances the ability to interpret intricate datasets effectively. As Dr. James articulated,
Ultimately, the belief is that if we adopt a science- and data-driven approach and apply an ethics- and values-based framework, individuals will benefit. And that’s the ultimate goal.
This ethical framework is particularly relevant when considering patient registries and collaboration with data scientists, as it reinforces the commitment to ethical research practices while maximizing the potential of data analytics in clinical trials.
These strategies emphasize the importance of local economic impacts and international collaboration, ensuring that patient needs remain at the forefront of research efforts. Furthermore, the visual icon representing a globe or network, depicted in a teal color and enclosed within a dashed circular border, symbolizes connectivity and global reach, reinforcing the themes of international collaboration and recognition in the context of healthcare improvement.
Conclusion
The Medtech industry is undergoing a transformative period marked by significant advancements in technology and patient engagement strategies. The integration of telemedicine, wearable technologies, and artificial intelligence is reshaping healthcare delivery, making it more accessible and efficient for patients. As these innovations continue to evolve, they underscore the necessity of robust clinical trial management services that navigate the complexities of regulatory requirements and ethical standards.
Moreover, the emphasis on patient co-design and engagement in product development is proving to be beneficial not only for healthcare outcomes but also for economic growth. By actively involving patients in the design process, Medtech companies can enhance product effectiveness and satisfaction, ultimately leading to improved health results. This collaborative approach fosters a sense of ownership among patients, which is essential for the long-term success of healthcare interventions.
As the Medtech landscape progresses, leveraging real-world evidence and data analytics will be crucial in refining clinical research methodologies. The ability to analyze extensive datasets and apply predictive analytics allows for more informed decision-making, enhancing the efficacy of healthcare solutions. This data-driven approach not only aids in regulatory compliance but also supports the development of innovative treatments that meet the evolving needs of patients.
In conclusion, the future of Medtech is poised for significant advancements that prioritize patient-centric solutions and data-driven strategies. By embracing these emerging trends and fostering collaboration among stakeholders, the industry can navigate the challenges ahead and lead the way toward a more efficient, accessible, and patient-focused healthcare system. The commitment to innovation and ethical practices will be pivotal in shaping the next generation of medical technologies, ultimately improving patient outcomes and contributing to global health advancements.
Frequently Asked Questions
What are the current trends reshaping the Medtech industry?
The current trends include telemedicine integration, wearable technologies, artificial intelligence, regenerative medicine, and health information interoperability.
How has telemedicine impacted healthcare delivery?
Telemedicine has enhanced access to care, with nearly 90% of U.S. individuals reporting easier access to necessary care through virtual consultations. It has also led to the development of applications for better appointment scheduling and payment facilitation.
What role do wearable technologies play in healthcare?
Wearable technologies allow for real-time monitoring of health metrics, empowering individuals to manage their health proactively. Their use in healthcare is expected to increase significantly in 2024.
How is artificial intelligence transforming diagnostics and treatment in Medtech?
AI enhances the accuracy of diagnostics and helps in developing personalized treatment plans, leading to improved medical study outcomes and better health results for patients.
What advancements are being made in regenerative medicine?
Advances in stem cell research and tissue engineering are creating innovative treatments that could revolutionize patient care, expanding recovery possibilities and opening new avenues for clinical trials.
Why is health information interoperability important in Medtech?
Interoperability allows for seamless data sharing among healthcare providers, which is crucial for improving patient care and driving innovation in medtech clinical research.
What comprehensive services are offered in clinical trial management?
Services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all aimed at enhancing healthcare delivery and fostering international collaboration.
How does AI's role in healthcare reflect public sentiment?
While 39% of Americans are comfortable with AI assisting in their medical care, 60% are uncomfortable if their healthcare provider relies solely on AI, indicating mixed feelings about its integration.
What are digital therapeutics and their significance in research?
Digital therapeutics are software-based interventions targeting specific medical conditions, and their incorporation into medical research requires rigorous validation to ensure efficacy and safety.
How does blockchain technology enhance clinical research?
Blockchain provides an unchangeable record of individual information and consent, improving information security and transparency, which is essential for maintaining trust in medical research.
What benefits do mobile health applications offer?
Mobile health applications facilitate real-time data gathering and enhance patient involvement, improving the quality of medical data and overall trial efficiency.

