Introduction
The regulatory landscape for medical device clinical trials in Latin America is complex and diverse, with varying requirements from country to country. Understanding these regulatory frameworks is crucial for companies looking to expand their innovative product offerings in this region. From navigating local patient population data requirements to identifying target markets based on disease incidence and prevalence, there are several factors to consider when entering Latin American markets.
Additionally, innovation pathways in countries like Canada, Australia, the US, and South Korea can expedite product development and regulatory approval. This article explores the regulatory frameworks, key regulations and guidelines, clinical trial design and approval process, ethical considerations and informed consent, data management and reporting requirements, labeling and packaging regulations, and post-market surveillance and vigilance in Latin America. By staying informed and adapting to local regulations, companies can navigate the regulatory environment and ensure the safety and efficacy of their medical devices.
Objective and Scope
Latin America presents a unique landscape for medical device clinical trials, especially for first-in-human (FIH) and early-feasibility studies (EFS). Understanding the regulatory frameworks in this region is crucial, as the requirements can vary significantly from country to country. For instance, while some nations accept clinical trial data from international studies for regulatory approval, others like Japan, China, and Hong Kong mandate local patient population data, potentially increasing development costs.
With the global medical device market expanding, companies like Inspira Technologies are extending their innovative product offerings to Central American countries, including Mexico. This expansion is often contingent on navigating the regulatory environment effectively, exemplified by Inspira's usability study conducted in the US for its INSPIRA ART100 blood circulation device, highlighting the importance of understanding the context of intended use.
Moreover, devices range broadly in complexity, from simple tools like tongue depressors to advanced implants and diagnostic software. The World Health Organization defines medical devices as those aiding in diagnosis, treatment, and quality of life improvements for patients. This definition underscores the diversity of devices and the necessity for tailored regulatory approaches.
When entering Latin American markets, identifying target markets based on disease incidence and prevalence is essential. Companies must select representative locations for Voice of Customer (VoC) research, despite variations between countries. Finding the right local partners is also critical for grounding research and understanding regional specifics.
Furthermore, innovation pathways in countries such as Canada, Australia, the US, and South Korea can facilitate faster product development and regulatory approval.
Regulatory pathways like the De Novo process in the US classify novel medical devices and create new regulatory categories, setting a precedent for future submissions. These pathways emphasize the importance of robust and transparent clinical trial data to ensure the safety and effectiveness of medical devices. As the industry evolves, comprehensive legal and health policy research becomes increasingly vital, as does public access to clinical data, which is supported by initiatives like the Laura and John Arnold Foundation and the Global Health Justice Partnership.
Regulatory Frameworks for Medical Device Clinical Trials
Latin America presents a dynamic regulatory landscape for medical device clinical trials, with national regulatory bodies playing pivotal roles in supervising the development of medical technologies. As emerging markets in Latin America become more significant in the pharmaceutical sector, understanding the governance of these technologies is crucial, particularly in the context of ethical, legal, and social issues.
Case studies in health and medicine suggest that market incentives and intellectual property rights are among the factors influencing the evolution of medical technologies. This is evident in the governance frameworks developed within the region, which may create challenges or promote success in the management of first-in-human (FIH) and early-feasibility studies (EFS).
For instance, the International Medical Device Regulators Forum (IMDRF) works to foster global convergence and cooperation with key regional organizations. Understanding the role of such entities is central to navigating the Latin American regulatory environment. The IMDRF's engagement with groups like the APEC LSIF Regulatory Harmonization Steering Committee and the Pharmacy and Poisons Board can provide insights into harmonization efforts.
Innovation pathways have emerged as a means to expedite the approval of novel medical devices, similar to the De Novo process. This pathway enables the classification of new devices based on risk and establishes a regulatory category ensuring safety and effectiveness. Learning from the De Novo model, which was underused for years due to procedural requirements, Latin American regulatory bodies are adapting to streamline their processes for the benefit of innovation.
Recent developments, such as the UK's Medicines and Healthcare products Regulatory Agency (MHRA) introducing a new system for faster approval of low-risk clinical trials in October 2023, underscore the global trend towards more efficient regulatory practices, which Latin America could emulate.
Furthermore, the unique challenges and opportunities in Latin America are highlighted by the fact that while some countries allow the use of clinical trial data from other nations for regulatory approval, others, such as Japan, China, and Hong Kong, mandate local clinical trial data, affecting the cost and development strategy of medical products.
The discourse on Decentralized Clinical Trials (DCTs) and their global growth, with a compound annual growth rate of 30.1% expected between 2021 and 2026, also touches upon the region. The expansion of DCTs raises questions about standardization, regulatory and ethical uncertainties, and the need for best practices to ensure quality results, as noted by experts in the field.
With increased investment by governments, universities, and businesses in research and development (R&D), middle-income countries, including those in Latin America, are becoming more influential in medical innovation. This shift is accompanied by the rising prevalence of chronic conditions and emerging infectious diseases, calling for a profound understanding of biology and chemistry to develop effective treatments.
In summary, regulatory bodies in Latin America are integral to the safety and efficacy of medical devices, and their functions are shaped by various factors, including international cooperation, innovation pathways, and the growth of DCTs. Navigating this environment requires a strategic approach that considers the complex interplay of market dynamics, intellectual property, and ethical considerations.
Key Regulations and Guidelines
Successful execution of first-in-human (FIH) and early-feasibility studies (EFS) in Latin America necessitates a comprehensive understanding of the regional regulatory landscape. Latin America presents unique challenges due to its diverse regulatory environments across different countries. For example, a study examining biometric data protection revealed significant gaps compared to European Union standards.
This highlights the variability in regulatory frameworks that researchers must navigate. In Latin America, the need to adapt European best practices and technologies to local realities is paramount, as evidenced by the urgent call to improve biometric data protection in the face of AI advancements.
The fidelity to regulations is exemplified by the stringent requirements regarding the management of confidential information, where researchers are responsible for ensuring that any public comments or data exclude sensitive details, as outlined by the Food and Drug Administration's submission guidelines. Moreover, the recent instability of Brazil's controlled substances database serves as a stark reminder of the importance of robust data collection systems.
Researchers must also be acutely aware of the ethical considerations involved in participant selection and consent. Definitions and requirements for vulnerable populations, such as minors and those mentally impaired, demand careful attention. Informed consent forms must encompass essential elements, such as participant rights and the conditions for research on special populations like pregnant women, fetuses, and neonates.
It is vital for researchers to recognize the specificities of each country's regulations. For instance, Brazil's ANVISA and Colombia's INVIMA have their own sets of guidelines and procedures that can greatly affect clinical trial design and implementation. The recent confusion surrounding the appointment of a head for INVIMA underscores the complexities and potential delays that can arise from regulatory ambiguities.
To sum up, for those engaging in FIH and EFS in Latin America, it is essential to stay informed on each country's regulatory requirements, maintain vigilance over participant protection and data privacy, and be prepared to address the challenges posed by the region's evolving technological and regulatory environment.
Clinical Trial Design and Approval Process
The clinical trial design and approval process in Latin America requires meticulous attention to regulatory guidelines and ethical considerations. A critical aspect of this process is the protection of vulnerable populations, with specific consent and protection requirements for minors, pregnant women, fetuses, neonates, prisoners, and those who are mentally impaired. Each category demands tailored consent protocols to ensure ethical compliance and safeguard the individuals involved.
During the trial process, clear definitions of investigational products and related terms are crucial, as they form the foundation for regulatory approval. Understanding the distinctions between an Investigational New Drug (IND) application and a New Drug Application (NDA) is essential, as these guide a drug candidate from preclinical studies to clinical trials and ultimately to market approval.
Informed consent is a cornerstone of clinical research, necessitating comprehensive elements in consent forms and other materials that detail participant rights, privacy, appeal, safety, and welfare. This ensures that participants are fully aware of the implications of their involvement and have the necessary information to make an informed decision.
Clinical trials in the region are influenced by a network of health care professionals and stakeholders from across the Americas who collaborate to establish evidence-based frameworks for treatment. Their recommendations are guided by the collective expertise of panel members representing 15 countries, who diligently manage potential conflicts of interest to maintain the integrity of the guidelines.
Moreover, the clinical trial landscape is continually shaped by regulatory developments and safety reviews. For example, the European Medicines Agency's ongoing probe into GLP-1 medicines and their potential association with suicidal thoughts underscores the importance of regulatory vigilance and the need for drug developers to provide comprehensive data for assessments.
Statistical analysis reveals a trend towards lengthier clinical trials, with an increase in the average duration of Phase 2 and Phase 3 trials over recent years. This emphasizes the need for efficiency in trial design and execution, as delays can have substantial implications for patient access to new treatments and a drug's success in the market.
In conclusion, navigating the regulatory environment for first-in-human and early-feasibility studies in Latin America involves a deep understanding of ethical requirements, consent procedures, and regulatory applications, all the while staying current with global safety reviews and market pressures.
Ethical Considerations and Informed Consent
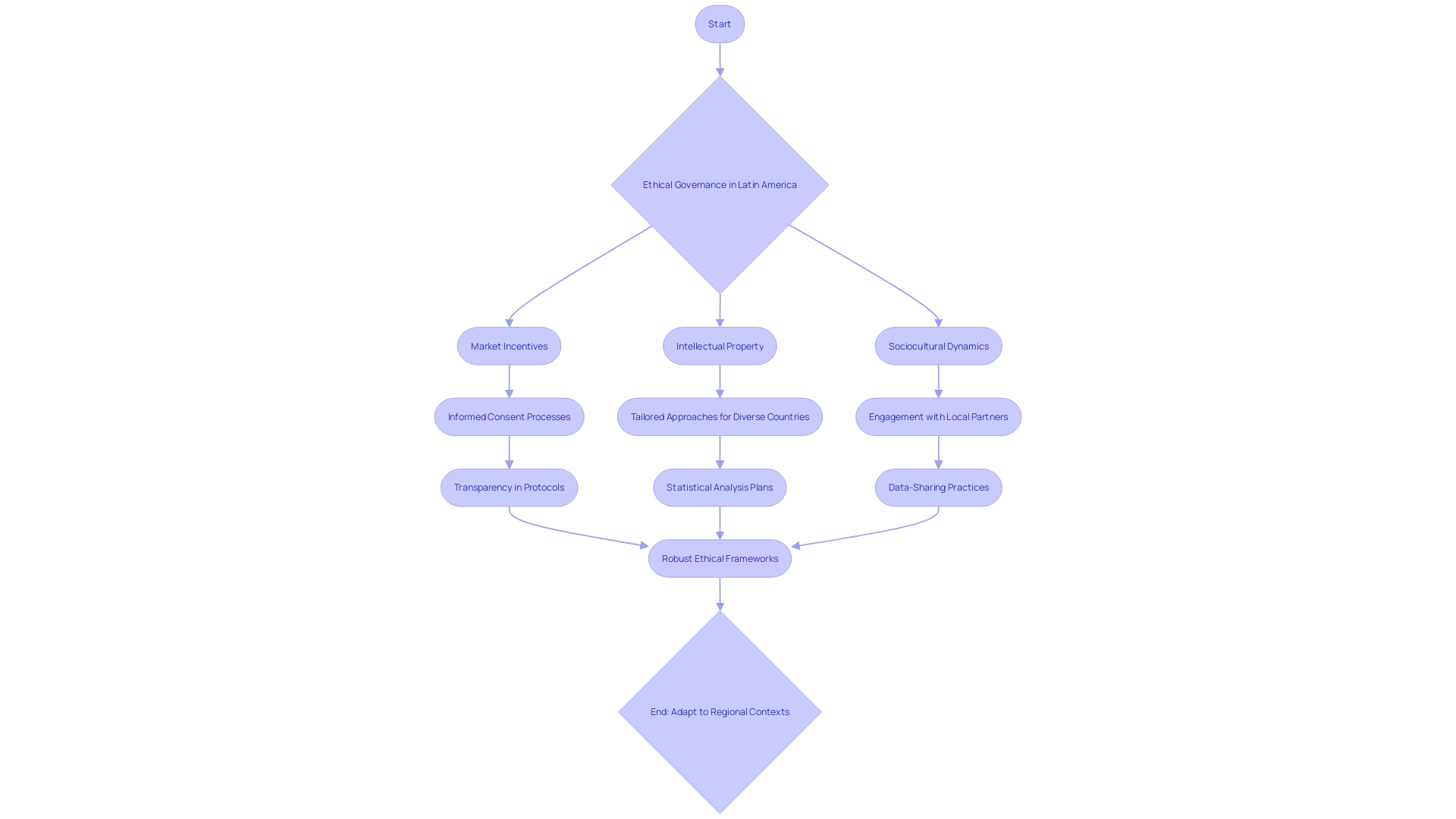
Ethical considerations in medical device clinical trials are paramount, particularly in the diverse and evolving regulatory environments of Latin American countries. The ethical governance and informed consent processes are complex, influenced by market incentives, intellectual property, and sociocultural dynamics. It is essential to navigate these factors to ensure adherence to ethical standards and obtain valid informed consent.
One must consider the heterogeneity of low- and middle-income countries (LMICs), such as the varying disease incidence and prevalence data, to identify target markets for clinical trials. This tailored approach respects local contexts and facilitates ethical study conduct. Additionally, the engagement with local partners is indispensable for grounding research practices in local norms and expectations, thereby upholding ethical integrity.
A noteworthy example of navigating these complexities is Inspira Technologies' expansion into Central America, including Mexico. Their recent agreement anticipates significant revenue contingent upon regulatory approvals, underscoring the criticality of ethical compliance in market access and commercial success. Furthermore, the Usability Study for the INSPIRA ART100, conducted among healthcare professionals, exemplifies the importance of considering the human aspects in clinical trial designs.
Such considerations are integral to ensuring that the studies are ethically sound and that the informed consent process genuinely reflects an understanding of the participant's needs and environments.
As industry involvement in clinical research intensifies, transparency becomes even more crucial. Clinical trials must go beyond mere registry in databases like ClinicalTrials.gov, demanding comprehensive trial protocols and detailed statistical analysis plans. This transparency is vital for reproducibility and trust in clinical research.
However, data-sharing practices remain inconsistent, and access to raw data is often restricted, which can impede the ethical oversight of clinical trials. The dynamic nature of clinical research, with frequent personnel and organizational changes, further complicates the ethical management of trials, highlighting the need for robust and adaptable ethical frameworks in Latin American contexts.
Data Management and Reporting Requirements
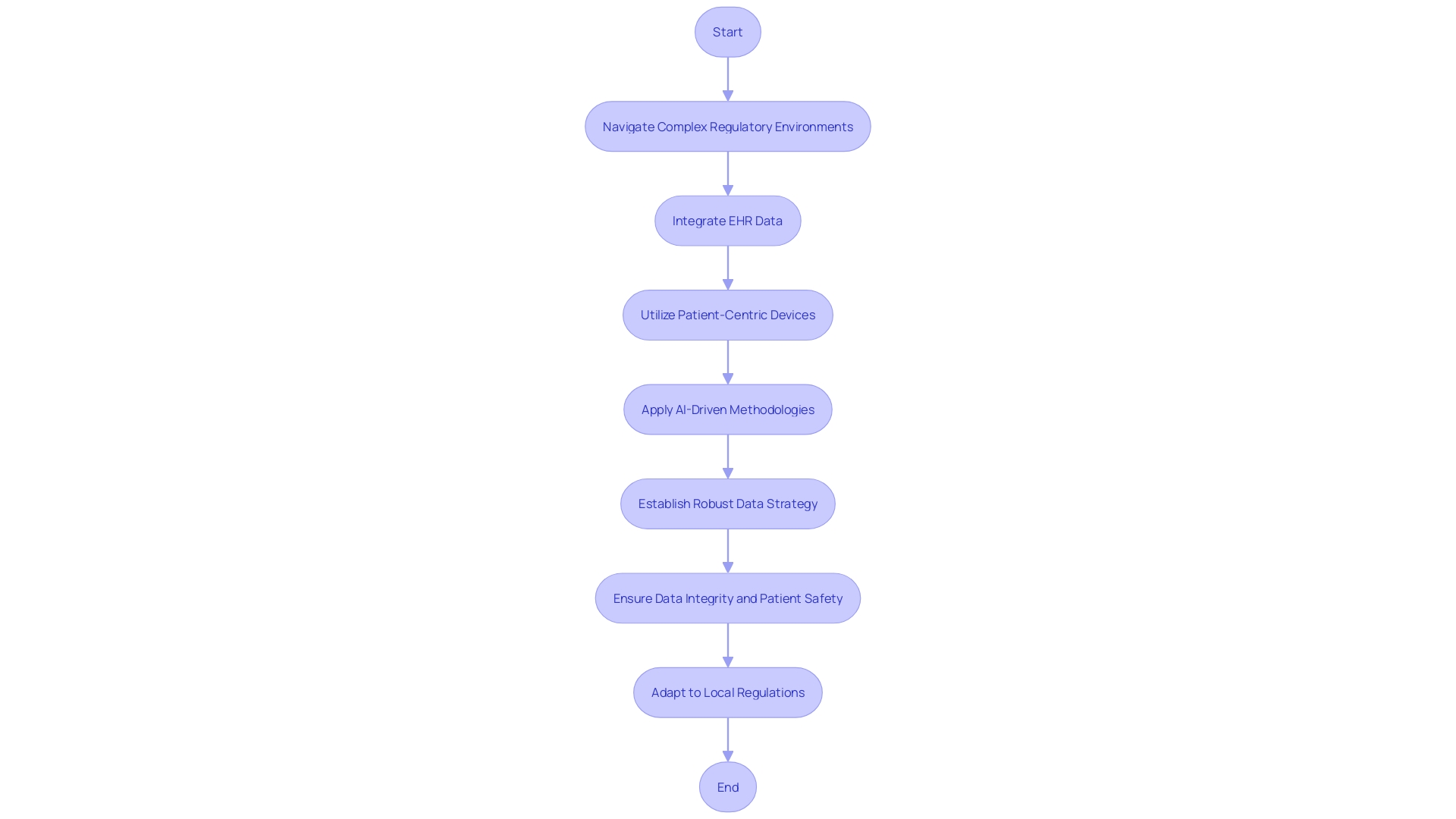
Proper data management and reporting are pivotal for the success and credibility of clinical trials involving medical devices. In the dynamic landscape of Latin America, researchers and sponsors must navigate complex regulatory environments, ensuring their clinical trial data is accurate, secure, and compliant with local standards. Harnessing the power of electronic health records (EHR) can greatly enhance the efficiency and efficacy of these trials.
A recent example includes a multi-center pharmaceutical industry outcomes trial that integrated EHR data to supplement traditional data collection methods, supported by a central coordinating center which addressed the technical, governance, and operational aspects of the trial. Furthermore, the increasing use of patient-centric devices, such as wearables and electronic diaries, combined with artificial intelligence-driven methodologies, has revolutionized data collection, allowing for deeper insights and trends that drive intelligent drug development decisions. However, the sheer volume of data demands a robust data strategy, established prior to trial protocol design, to effectively manage data flow from diverse traditional and digital sources.
According to the World Health Organization, medical devices range from simple tongue depressors and bedpans to complex programmable pacemakers and sophisticated diagnosing systems. This broad spectrum of devices underscores the need for meticulous data handling to ensure patient safety and data integrity. As the medical device landscape evolves with the advent of digital therapeutics for chronic disease management and neurological conditions, the importance of thorough data management becomes even more pronounced, albeit challenging due to the novelty of such therapies in clinical trials.
It is essential to recognize that not all Latin American countries accept clinical trial data from studies conducted elsewhere; for instance, markets such as Japan, China, and Hong Kong necessitate local clinical trial data. This requirement may amplify the cost and complexity of product development. In conclusion, the key to successful data management and reporting in Latin America lies in understanding and adapting to local regulations, leveraging technological advancements, and implementing a well-defined data strategy from the outset.
Labeling and Packaging Regulations
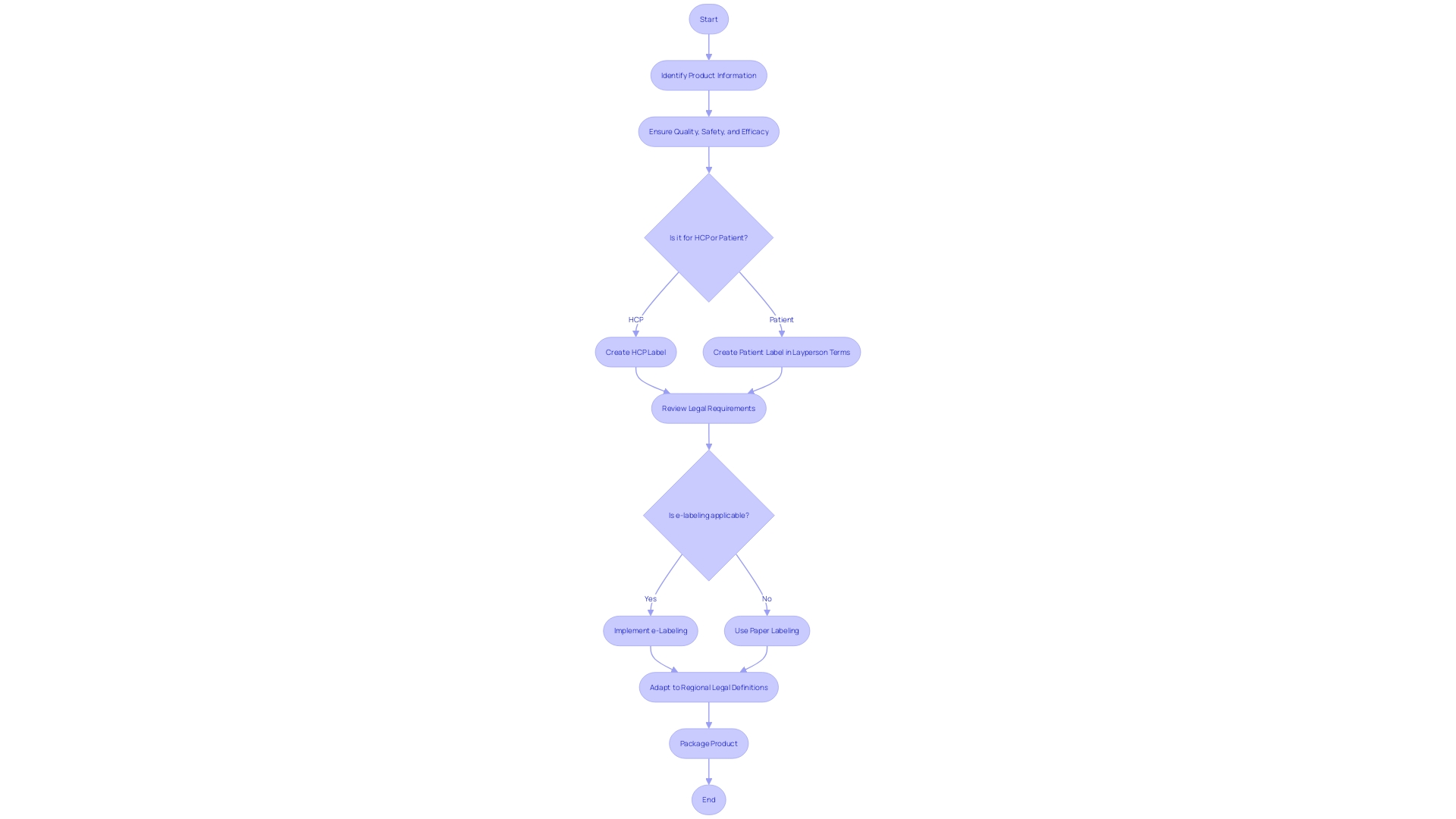
Labeling and packaging of medical devices play a crucial role in ensuring the safety of patients and meeting regulatory standards. In Latin America, the proper presentation of product information is not only a legal requirement but also a key factor in healthcare professionals' (HCPs) ability to choose appropriate treatments and in guiding patients in the safe use of their medicines. The label on both outer and inner packaging should include vital details on the product's quality, safety, and effectiveness.
Moreover, documents provided with the label need to be comprehensible to laypersons, as they are instrumental in patient education regarding medication use.
With the advancement of technology, the digitization of labels, or e-labeling, is becoming increasingly prevalent. This shift to electronic product information offers benefits such as enhanced accessibility and the potential for updated, real-time information. Countries are categorized into Tier 1 or Tier 2 based on their regulatory maturity and operational capabilities, which influence their approach to e-labeling.
Embracing these modern practices can lead to significant improvements in patient care and regulatory operations.
Furthermore, recent developments have highlighted the importance of clear communication in patient-directed materials. For example, the FDA's final rule on direct-to-consumer prescription drug advertisements emphasizes the need for major side effects and contraindications to be presented in a way that is clear and understandable to consumers. Such standards are indicative of the global movement towards patient-centric information, underscoring the need for labels that effectively communicate safety and efficacy.
In the context of Latin America, adapting to these evolving standards means recognizing the diverse legal definitions and terminologies across different regions. Ensuring that labeling practices meet the specific requirements of each country is essential for market authorization holders, sponsors, and applicants to successfully navigate the regulatory landscape and safeguard public health.
Post-Market Surveillance and Vigilance
In the realm of medical technology in Latin America, post-market surveillance (PMS) serves as an essential phase for ensuring medical devices' ongoing safety and effectiveness following market entry. PMS transcends the scope of pre-market assessments by facilitating the constant appraisal and documentation of a device's performance as it encounters real-world usage. This vigilance is key for pinpointing potential safety hazards and advancing device functionality over time.
PMS leverages a spectrum of methodologies to gather pivotal data about the safety and performance of medical devices. These range from passive surveillance systems, where spontaneous reports from healthcare professionals and patients are collected, to active surveillance initiatives that involve registries or studies. Additionally, the integration of electronic health records and administrative databases enriches the continuous monitoring process, shedding light on the enduring safety and efficacy of devices within practical settings.
Latin American regulators have been integrating concepts such as privacy-enhancing technologies (Pets) to mitigate privacy risks and anonymize data, reflecting a broader trend in global policy efforts to adopt such technologies. This includes guidance releases, sandbox initiatives, and amplified investments in Pets research and development. For instance, Brazil's Autoridade Nacional de Proteção de Dados (ANPD) has undertaken technical studies on anonymization and pseudonymization to inform its pending guidance.
Similarly, there is a concerted effort to enhance compliance programs and align them with international best practices across South and Central American competition authorities and courts. This includes considering a company's compliance efforts when determining sanctions for legal infringements. The aim is to foster regional consistency in compliance, promoting legal predictability and business environment certainty, which are pivotal for regions with significant cross-border trade and market integration.
Moreover, the recent FDA final rule on the presentation of major side effects in direct-to-consumer prescription drug ads, emphasizing consumer-friendly language and dual modality in television and radio formats, illustrates the importance of clarity and accessibility in health-related communications. Such regulatory developments underscore the necessity of continuous and transparent monitoring for safeguarding public health.
Through vigilant post-market surveillance, healthcare systems can robustly manage the benefits and risks associated with medical devices, ensuring the trust and safety of patients and users. This ongoing effort is fundamental to maintaining confidence in the health sector and its governing institutions, as evidenced by World Bank case studies on the critical nature of this public health service.
Case Studies and Examples
Understanding regulatory environments for clinical trials in Latin America is crucial for the successful introduction of medical devices. This region presents unique challenges due to its diverse regulatory landscape. As evidenced by the Casea S study led by Dr. Kavita Nanda of FHI 360, innovation is key in addressing healthcare needs, such as providing new contraceptive options.
Similarly, the deployment of Canary Medical's Cardiac Auscultation monitor demonstrates the potential for technology to advance patient care. The WHO defines medical devices broadly, from simple tools to complex machinery, all of which require careful navigation through regulatory processes.
The introduction of new medical devices often necessitates compliance with country-specific regulations, which can complicate the clinical trial process and potentially increase costs. For instance, Japan, China, and Hong Kong mandate local clinical trial data for regulatory approval. This emphasizes the importance of understanding and planning for such requirements in Latin America.
Moreover, the adoption of modern compliance programs, including automation, can facilitate innovation and compliance, despite some resistance within the industry. As the medical device sector evolves, highlighted by significant events from AI regulation to industry changes in 2023, it is evident that staying abreast of regulatory requirements and leveraging technology are key components for success in this dynamic field.
Best Practices for Compliance
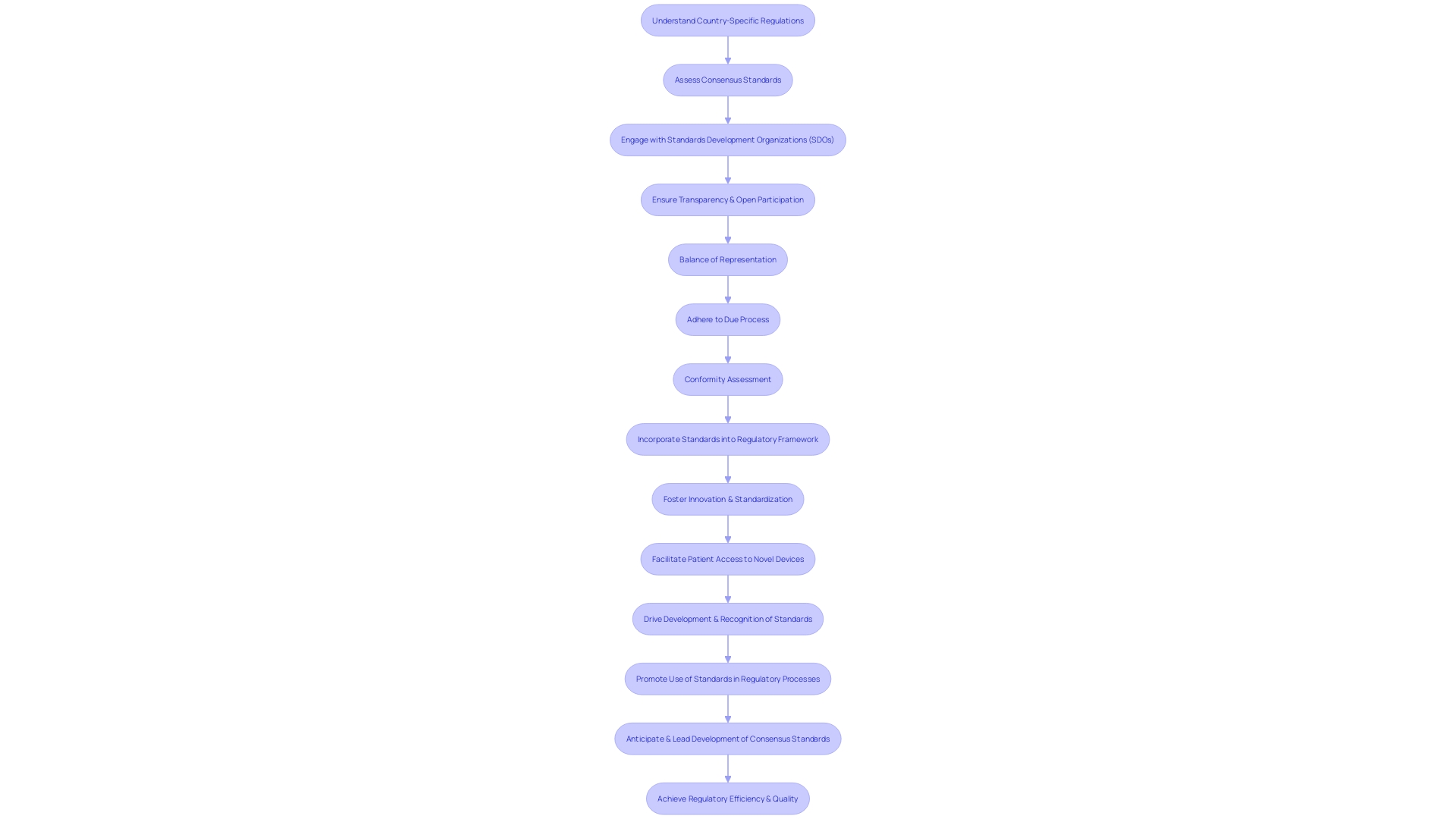
Navigating the regulatory landscape for medical device clinical trials in Latin America involves a multi-faceted approach. To successfully conduct first-in-human (FIH) and early-feasibility studies (EFS), it is essential to recognize the diversity of the regulatory environments across the region. Leveraging insights from recent case studies, such as Inspira Technologies' expansion into Central America, including Mexico, and their usability study for the INSPIRA ART100 blood circulation device, underscores the importance of thorough preparation and local knowledge.
Best practices suggest that researchers and sponsors should focus on a targeted selection of locations for their Voice of Customer (VoC) research by analyzing disease incidence and prevalence data. This focused approach facilitates a more representative understanding of the target markets, despite the inherent differences between countries. Furthermore, the complexities of trials, illustrated by a Phase I study with 52 patients across 105 sites, highlight the need for meticulous resource allocation and protocol familiarization among sites.
The World Health Organization's definition of medical devices and their broad range—from simple tools like tongue depressors to complex machinery—demonstrates the wide array of products that must be considered when navigating regulatory compliance. It's crucial to acknowledge that medical devices significantly enhance patient care, making the regulatory process a critical step in ensuring safety and efficacy.
Finding the right local partners is another critical step. This becomes apparent when considering the rapid changes within medical device companies, such as personnel shifts and contracting, which can impact trial management. Collaborating with local experts who understand the regulatory milieu can streamline the approval process, as exemplified by the verification and validation discussions from the FDA's public meeting on Digital Health Technologies (DHTs) for clinical trials.
Rigorous development and validation of DHTs, with a nod to the CDRH’s approach to software as a medical device (SaMD), are essential for ensuring data quality and usability.
Finally, the importance of adapting clinical trial data to meet the specific requirements of Latin American countries cannot be overstated. Some nations may accept data from international trials, while others, like Japan, China, and Hong Kong, necessitate local patient population data, potentially increasing development costs. This context, along with the insights provided by the Medical Devices: Filings Trends & Signals Q1 2024 report, which serves as a benchmark for evaluating business drivers and corporate actions, should be incorporated into compliance strategies to optimize outcomes in Latin America's diverse regulatory environments.
Conclusion
In conclusion, navigating the complex and diverse regulatory landscape for medical device clinical trials in Latin America requires a deep understanding of the regional frameworks and guidelines. Identifying target markets based on disease incidence and prevalence and leveraging innovation pathways in countries like Canada, Australia, the US, and South Korea can expedite product development and regulatory approval.
Ethical considerations and informed consent are vital in medical device clinical trials. Researchers must navigate participant selection and consent, adhering to each country's specific regulations.
Proper data management and reporting are pivotal for the success and credibility of clinical trials. Researchers and sponsors must ensure accurate, secure, and compliant data collection, adapting to local regulations and implementing a well-defined data strategy.
Labeling and packaging regulations are crucial for ensuring the safety and effectiveness of medical devices. Adapting to evolving standards and meeting specific country requirements are key for successful market authorization and safeguarding public health.
Post-market surveillance and vigilance are essential for ongoing safety and effectiveness assessment. Continuous monitoring, facilitated by various methodologies and electronic health records, ensures the identification of potential safety hazards and advances in device functionality.
In summary, successfully navigating the regulatory environment for medical device clinical trials in Latin America requires a comprehensive understanding of the diverse frameworks, adherence to ethical considerations, meticulous data management, compliance with labeling and packaging regulations, and robust post-market surveillance. By staying informed and adapting to local regulations, companies can ensure the safety and efficacy of their medical devices and effectively expand their innovative product offerings in this region.




