Overview
Medical Device CROs (Contract Research Organizations) in Peru play a vital role in supporting manufacturers by providing essential services such as regulatory approval, trial management, and data analysis, which are crucial for navigating the complex landscape of medical device research and development. The article emphasizes that these organizations not only facilitate compliance with local regulations but also adapt to challenges like resource limitations and economic fluctuations, ensuring that innovative medical technologies can successfully enter the market.
Introduction
In the rapidly evolving landscape of healthcare, Contract Research Organizations (CROs) play a crucial role in the medical device sector, particularly in Peru. As the demand for innovative medical technologies grows, CROs are positioned as essential partners for manufacturers navigating the complexities of clinical trials and regulatory compliance. With a significant portion of the population relying on the services of EsSalud, the urgency for effective medical devices has never been more pronounced.
This article delves into the multifaceted functions of CROs in Peru, highlighting their contributions to:
- Clinical trial management
- Regulatory affairs
- Data analysis
It also addresses the challenges they face in a dynamic regulatory environment and explores future trends that promise to reshape the industry. Understanding the pivotal role of CROs is vital for manufacturers aiming to succeed in this competitive market, as their expertise not only streamlines the development process but also enhances the likelihood of successful market entry for new medical devices.
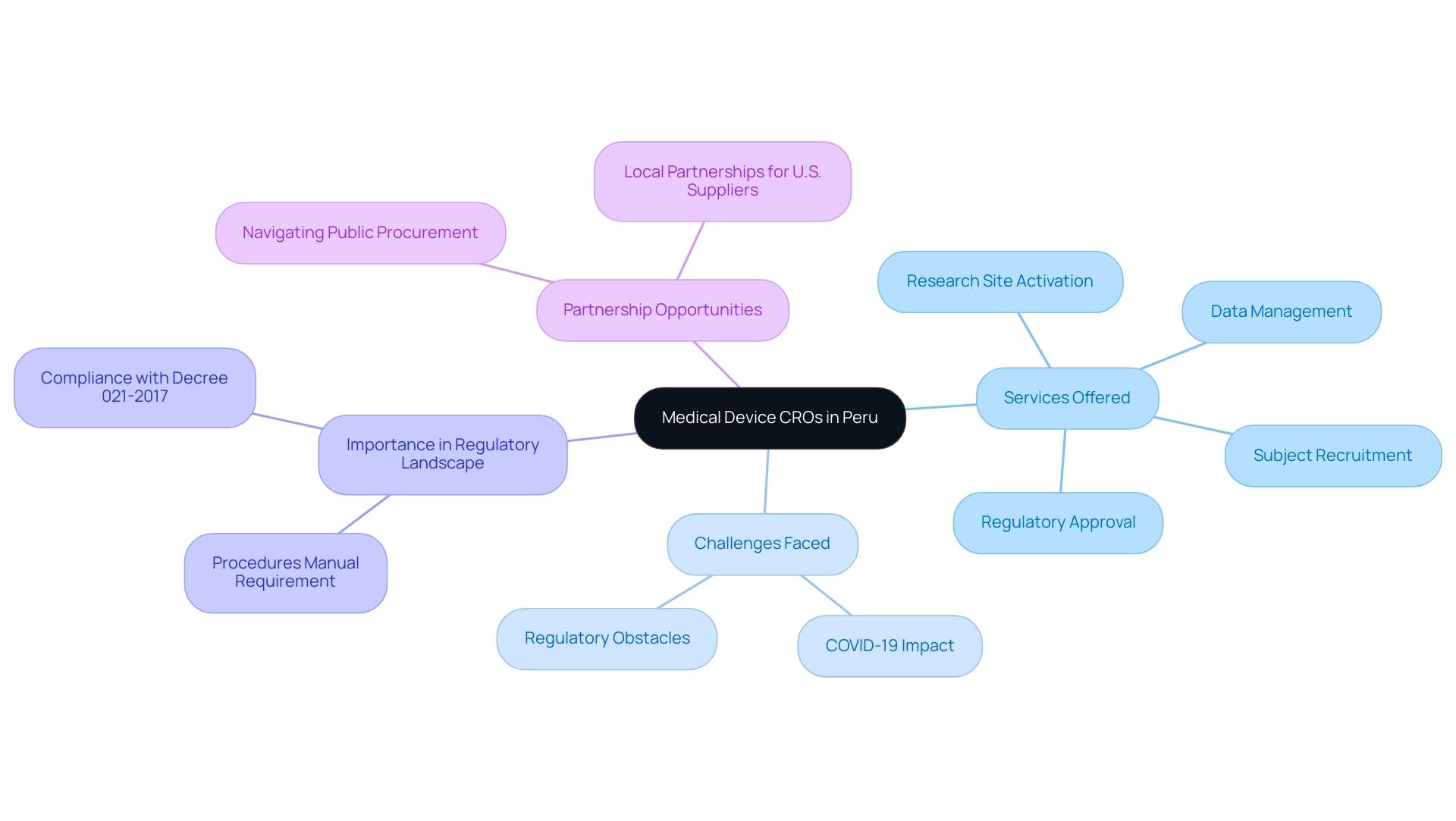
Introduction to Medical Device CROs in Peru
The Medical Device CRO Peru plays a crucial role in the medical equipment sector in Peru by providing specialized services that assist manufacturers throughout the complete research and development process. With EsSalud serving 30 percent of the population, the need for effective medical equipment is paramount. CROss such as bioaccess® are crucial in overseeing research studies, ensuring adherence to both local and international regulations, and aiding the launch of innovative medical devices into the market.
They offer quick, economical, and high-quality medical data, tackling the complex challenges encountered by Medtech startups, including regulatory obstacles and extended subject recruitment timelines. bioaccess® provides a complete range of services, including:
- Regulatory approval processes
- Research site activation
- Subject recruitment strategies
- Data management
These services are vital for navigating the intricate environment of studies. According to the General Office for Research and Technology Transfer of the National Institute of Health, the need for a procedures manual approved by the research institution—outlining operational requirements and monitoring procedures—is critical under Decree 021-2017.
This legal framework emphasizes the significance of clinical research organizations in navigating the complex regulatory landscape. A recent message from the Peruvian Clinical Trials Registry emphasized the halt of administrative processes due to COVID-19, demonstrating the real-world difficulties contract research organizations encounter in sustaining research operations during extraordinary periods. Despite these challenges, bioaccess® has adapted by implementing remote monitoring and flexible trial designs, ensuring continuity in clinical research.
As the demand for advanced medical technologies grows, understanding the role and importance of Medical Device CRO Peru becomes essential for manufacturers seeking to thrive in this competitive environment. Their expertise not only streamlines the development process but also enhances the likelihood of successful market entry for new medical products. Furthermore, the complexities of public procurement in Peru can be arduous, making local partnerships beneficial for U.S. suppliers aiming to penetrate this market.
Key Services Provided by Medical Device CROs in Peru
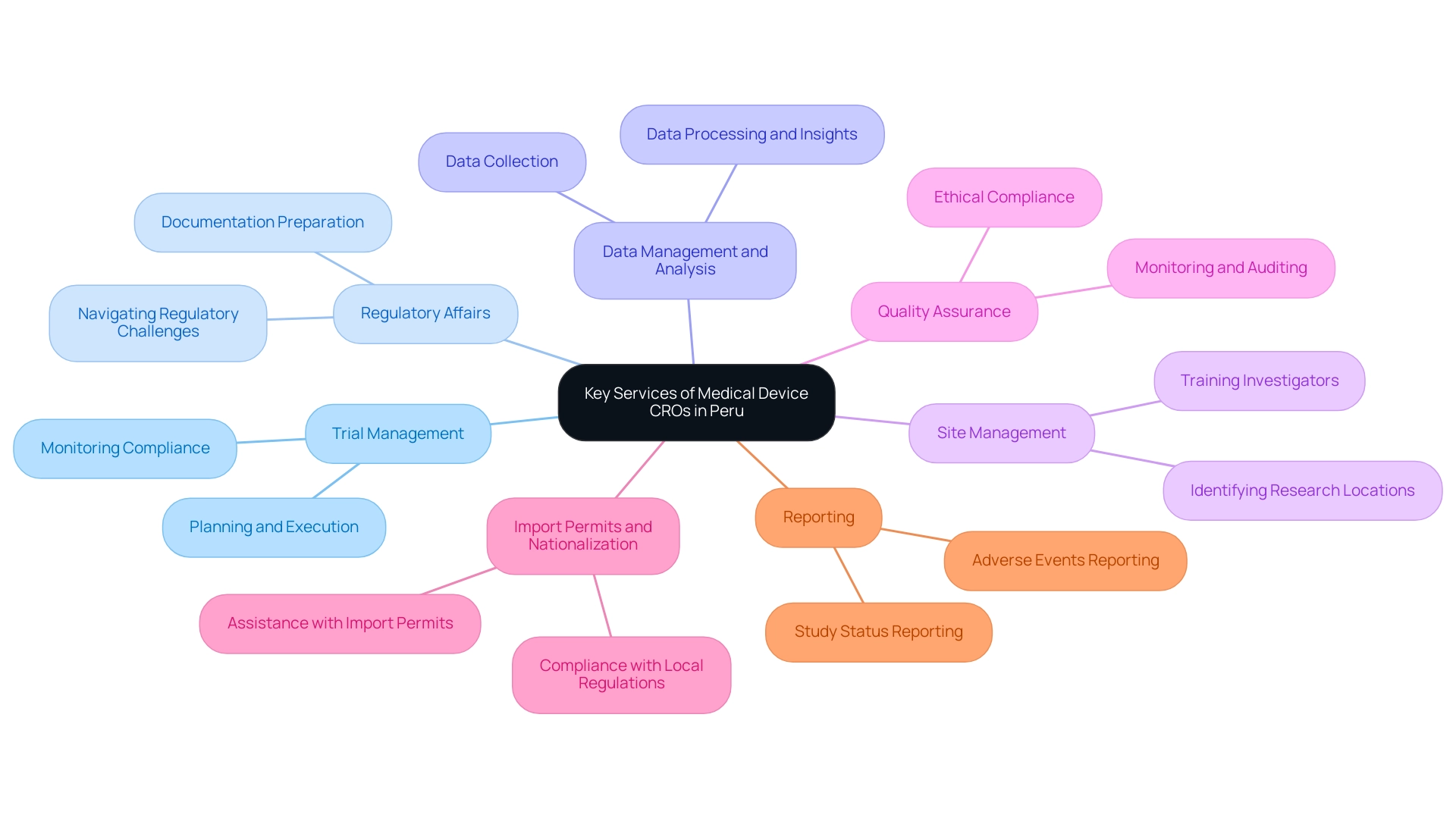
In Peru, the Medical Device CRO Peru provides a comprehensive suite of services aimed at assisting manufacturers during the research process. These services encompass:
-
Trial Management: This involves the meticulous oversight of planning, execution, and monitoring of studies, ensuring that all activities comply with established protocols.
Effective clinical study management is crucial for maintaining the integrity of the research and the safety of participants.
-
Regulatory Affairs: CROs assist in the preparation and submission of necessary documentation to local regulatory bodies, ensuring adherence to regulations stipulated by the National Authority for Pharmaceutical Products, Medical Devices and Medical Products (ANM).
This is essential for obtaining approval for investigational products and ensuring compliance throughout the trial process, especially in navigating the regulatory challenges encountered by medical startups.
-
Data Management and Analysis: Collecting, processing, and analyzing medical data is integral to deriving actionable insights that support regulatory submissions.
This service helps ensure that the data generated is robust and reliable, which is critical for the successful approval of medical devices.
-
Site Management: Identifying, managing, and training clinical research locations and investigators is a key function of contract research organizations.
Effective site management ensures that studies are conducted efficiently and that locations are equipped to meet the specific needs of the research.
-
Quality Assurance: Contract Research Organizations implement rigorous monitoring and auditing practices to ensure that all research activities comply with ethical standards and quality benchmarks.
As mentioned in Decree021-2017, consent may be revoked at any moment without detriment to the participant or legal representative/guardian, highlighting the ethical aspects in research studies.
-
Import Permits and Nationalization: Contract Research Organizations assist in the import permits and nationalization of investigational products, ensuring adherence to local regulations.
-
Reporting: Comprehensive reporting on study status, inventory, and serious and non-serious adverse events is crucial for maintaining transparency and compliance.
Together, these services play a crucial role in the regulatory compliance rates for medical device studies in Peru and are significantly supported by Medical Device CRO Peru, aligning with the latest advancements in research management services and promoting global health enhancement through international cooperation and innovation in Medtech.
Challenges and Considerations for CROs in the Peruvian Market
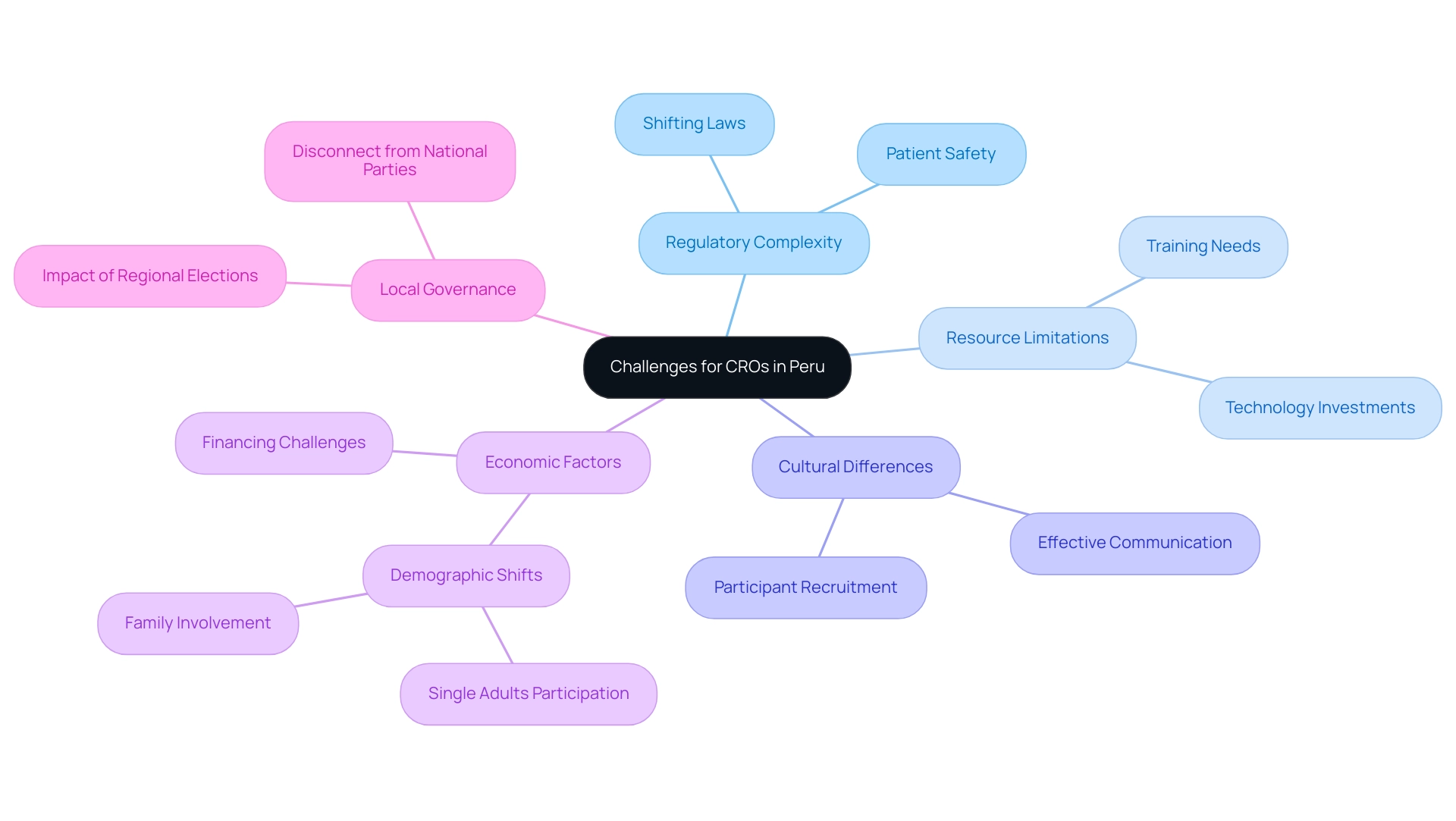
Organizations conducting research in Peru, particularly those partnered with a Medical Device CRO Peru, encounter various obstacles that greatly affect the efficiency and effectiveness of studies. One of the foremost issues is regulatory complexity. The continuously changing legal environment requires that Medical Device CRO Peru stay informed about shifting laws and requirements, complicating the start and execution of studies.
Navigating this regulatory maze is essential for maintaining compliance and ensuring patient safety in the realm of Medical Device CRO Peru, as highlighted by expert opinions.
Furthermore, numerous Medical Device CROs in Peru are struggling with restricted resources, which can impede both the quality and pace of research studies. Resource allocation remains a critical concern, affecting everything from staff training to technology investments. As one expert noted, 'That is the only viable way to guarantee rights while breaking out of this frustrating cycle of ever-harsher crackdowns,' emphasizing the urgency of addressing these limitations to protect research integrity and improve operational capabilities.
Cultural differences also pose significant challenges. Effective communication and participant recruitment in research studies conducted by a Medical Device CRO Peru necessitate a deep understanding of local cultural nuances. This sensitivity is essential for building trust with participants and ensuring adherence to research protocols.
Lastly, economic factors play a pivotal role in shaping the landscape for research related to Medical Device CRO Peru. Variations in the economy can directly impact financing and investments in research studies, posing risks to ongoing projects. In August 2024, statistics revealed that 62% of encounters with clinical trial participants involved single adults, indicating a demographic shift potentially influenced by economic instability.
Such economic pressures require Chief Research Officers at Medical Device CRO Peru to develop innovative strategies for securing funding and maintaining the momentum of crucial research initiatives.
Additionally, the case study on regional movements and local governance illustrates how local governance impacts the operations of Medical Device CRO Peru. The 2022 regional elections in Peru, where independent candidates secured over 50% of regional governorships, reflect a disconnect between national party structures and local needs. This disconnect can influence the regulatory environment and operational challenges faced by Medical Device CRO Peru, particularly regarding compliance reviews and the necessity for adaptive project management strategies.
By addressing these multifaceted challenges and leveraging comprehensive clinical study management services—including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and detailed reporting of study status, inventory, and adverse events—CROss can enhance their operational effectiveness and significantly contribute to the advancement of clinical research as a Medical Device CRO Peru.
Future Trends in Medical Device CRO Services in Peru
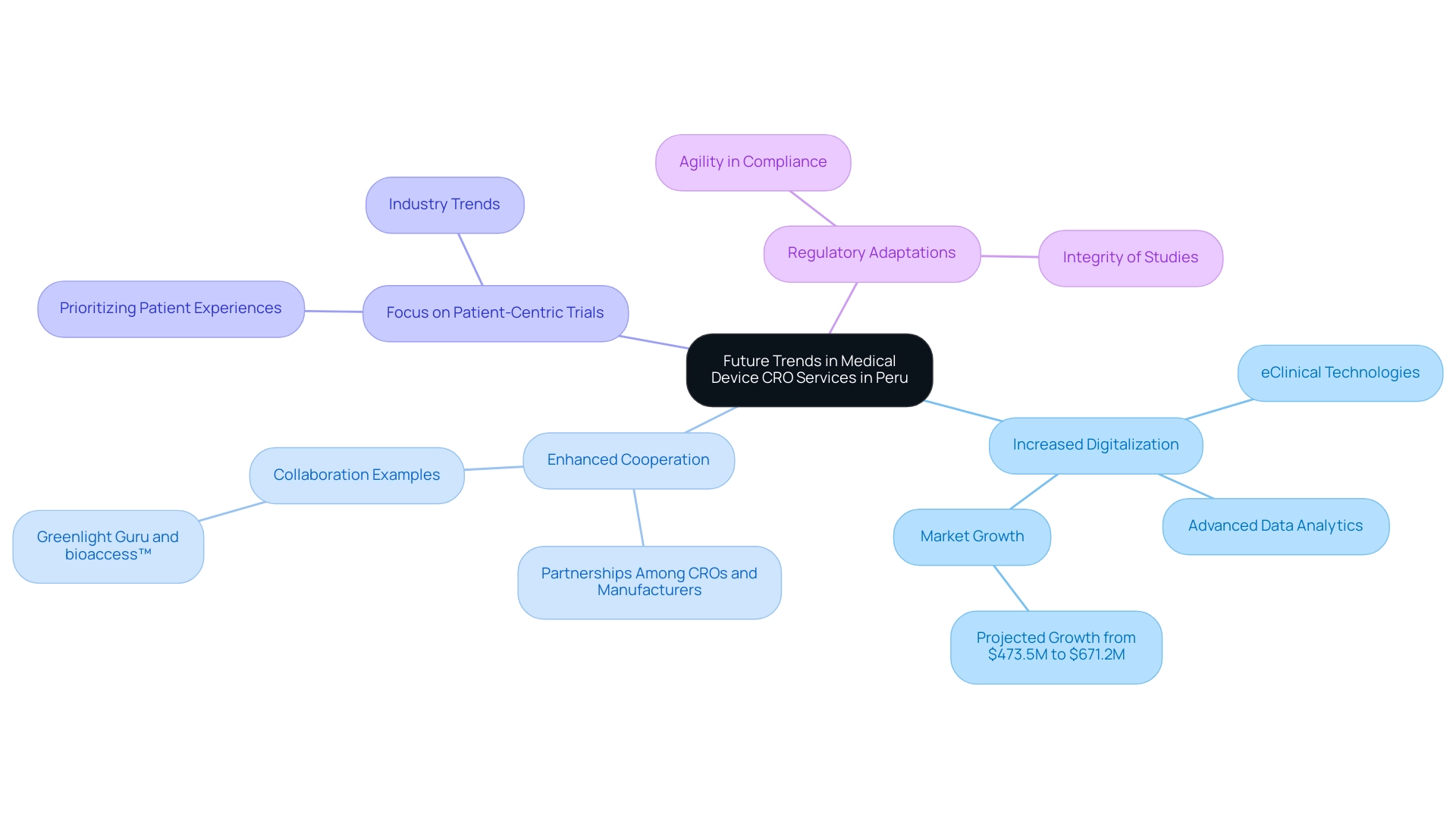
The landscape of Medical Device CRO Peru is poised for significant transformation in the upcoming years, driven by several key trends:
- Increased Digitalization: The integration of eClinical technologies and advanced data analytics is set to streamline study processes, enhancing data quality and operational efficiency. This shift is particularly crucial as the medical equipment market in Peru is projected to grow from $473.5 million in 2022 to $671.2 million by 2027, reflecting the steady economic growth that has led to increased healthcare spending and a favorable regulatory environment.
- Enhanced Cooperation: Strengthened partnerships among contract research organizations, device manufacturers, and regulatory bodies will create a more cohesive research environment, facilitating smoother project execution and compliance, particularly as highlighted by the collaboration between Greenlight Guru and bioaccess™ to accelerate Medtech innovations and studies in Latin America.
- Focus on Patient-Centric Trials: There is an increasing emphasis on designing trials that prioritize patient experiences and outcomes, reflecting a broader industry trend towards more humane and effective medical research.
- Regulatory Adaptations: As the regulatory landscape evolves, CROs will need to remain agile, adapting to new requirements while ensuring the integrity of their studies.
These adaptations will be essential for maintaining compliance and fostering trust in research outcomes conducted by the Medical Device CRO Peru. bioaccess® provides a variety of services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), which are essential for managing the intricacies of research studies. Additionally, the impact of Medtech clinical studies on local economies, such as job creation, economic growth, and healthcare enhancement, further underscores the importance of these developments in Peru's medical equipment sector.
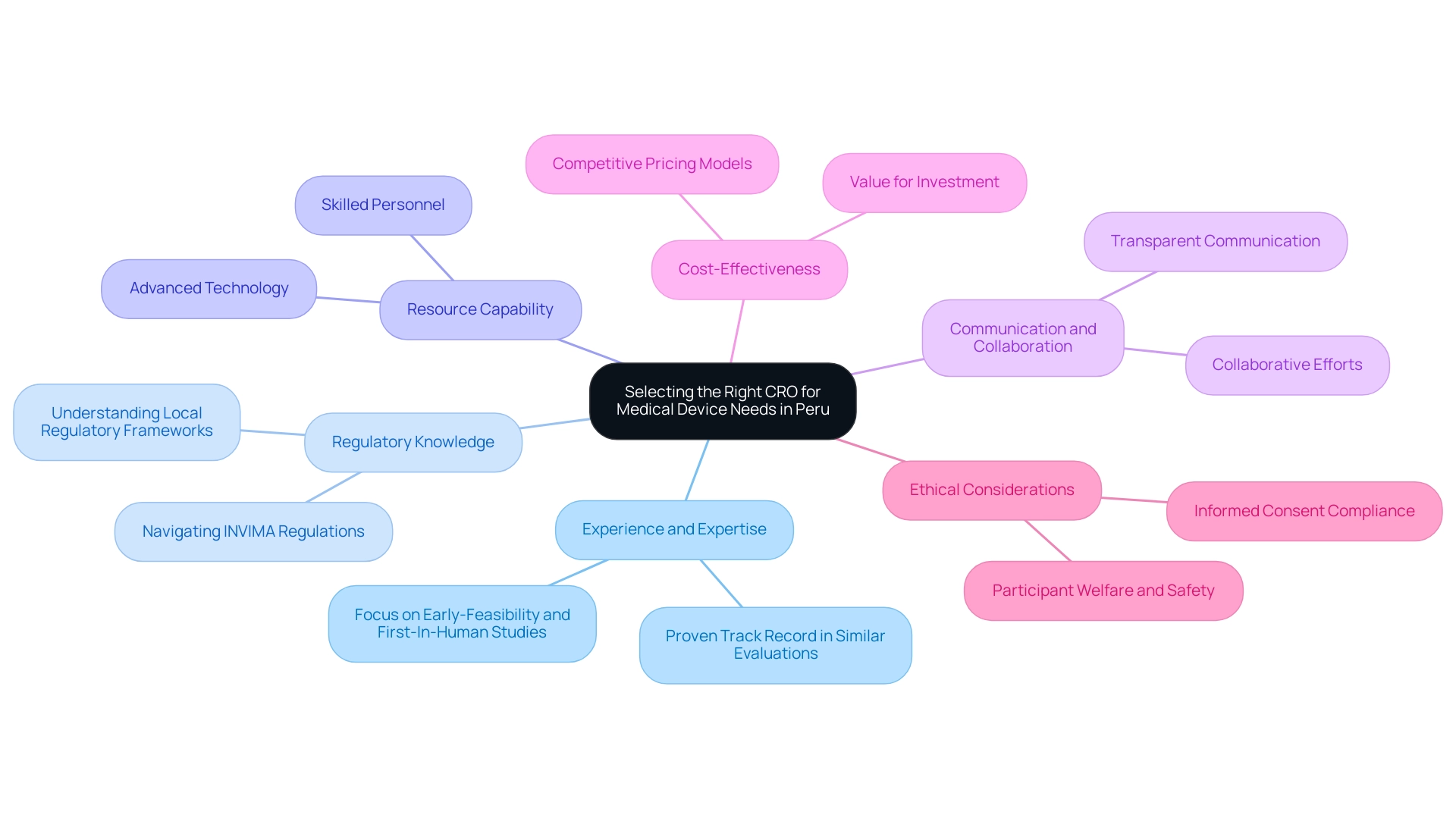
How to Select the Right CRO for Your Medical Device Needs in Peru
Choosing a contract research organization (CRO) for medical equipment studies in Peru necessitates a thorough evaluation of multiple essential factors:
- Experience and Expertise: Investigate the CRO's history in managing studies that align with your specific device category. A proven track record in similar evaluations, such as Early-Feasibility and First-In-Human studies, reflects their capability and reliability. For instance, bioaccess® boasts over 20 years of experience in Medtech as a leading Medical Device CRO Peru, focusing on accelerating clinical trials through comprehensive management services, including feasibility studies and site selection.
- Regulatory Knowledge: A robust understanding of local regulatory frameworks and compliance obligations is essential. Given the complex landscape of medical equipment regulations, this knowledge is crucial for ensuring adherence to guidelines. The Colombia National Food and Drug Surveillance Institute (INVIMA) serves as a Level 4 health authority, overseeing medical device classifications and regulatory functions, which your CRO must navigate effectively.
- Resource Capability: Evaluate whether the CRO possesses the necessary resources, including skilled personnel and advanced technology, to effectively support your study. Sufficient resources are essential for preserving the quality and integrity of the research, ensuring smooth setup and monitoring throughout the process.
- Communication and Collaboration: Opt for a CRO that fosters transparent communication and values collaborative efforts. This approach not only enhances the experiment experience but also helps in promptly addressing any challenges that may arise during the study, ensuring that all stakeholders are aligned.
- Cost-Effectiveness: Assess the CRO's pricing structure in relation to the quality of services offered. Ensuring that you receive good value for your investment is paramount, as it directly impacts the success and efficiency of your clinical studies. For example, bioaccess® offers competitive pricing models that align with the quality of their comprehensive services, ensuring that clients maximize their investment.
Additionally, it is important to consider the perspectives of study participants. As noted by G-ResEthics, participants in this study population may be persuaded to enter a study with the hope of obtaining benefits from their involvement in the research. Furthermore, the informed consent form (ICF) must comply with guidelines from relevant ethical review committees, underscoring the importance of ethical considerations in the CRO selection process. Bioaccess® also provides assistance in ensuring that ethical standards are met throughout the trial process.
Conclusion
The role of Contract Research Organizations (CROs) in the medical device sector in Peru is undeniably pivotal, serving as indispensable allies for manufacturers navigating the complexities of clinical trials and regulatory compliance. Through effective clinical trial management, adept handling of regulatory affairs, and thorough data analysis, CROs like bioaccess® are equipped to address the multifaceted challenges faced by Medtech startups. Their comprehensive services not only streamline the development process but also enhance the likelihood of successful market entry, which is crucial in a landscape where the demand for innovative medical technologies continues to grow.
However, the journey is not without its challenges. CROs must contend with a dynamic regulatory environment, limited resources, economic fluctuations, and cultural considerations that influence participant recruitment and trial execution. By recognizing and addressing these challenges, CROs can significantly improve their operational effectiveness and contribute to the advancement of clinical research in Peru.
Looking ahead, the future of CRO services in Peru is poised for transformation, marked by increased digitalization, greater collaboration, and a focus on patient-centric trials. These trends will not only enhance the efficiency and quality of clinical trials but will also foster a more cohesive and innovative research environment. Understanding the critical role of CROs in this evolving landscape is essential for manufacturers aiming to succeed in the competitive medical device market. As the industry continues to evolve, the strategic selection of a CRO will play a vital role in ensuring the success of medical device trials and ultimately improving healthcare outcomes for the population.
Frequently Asked Questions
What is the role of Medical Device CRO Peru in the medical equipment sector?
Medical Device CRO Peru provides specialized services to assist manufacturers throughout the complete research and development process of medical equipment, ensuring adherence to local and international regulations.
Why is effective medical equipment important in Peru?
With EsSalud serving 30 percent of the population, the need for effective medical equipment is paramount to meet the healthcare demands of the population.
What services does bioaccess® offer?
bioaccess® offers a complete range of services, including regulatory approval processes, research site activation, subject recruitment strategies, and data management.
How do Medical Device CROs help Medtech startups?
They tackle complex challenges such as regulatory obstacles and extended subject recruitment timelines, providing quick, economical, and high-quality medical data.
What is required under Decree 021-2017 regarding clinical research?
A procedures manual approved by the research institution is critical, outlining operational requirements and monitoring procedures.
What challenges have CROs faced due to COVID-19?
Administrative processes were halted, demonstrating difficulties in sustaining research operations, but bioaccess® adapted by implementing remote monitoring and flexible trial designs.
Why is understanding the role of Medical Device CRO Peru essential for manufacturers?
Their expertise streamlines the development process and enhances the likelihood of successful market entry for new medical products in a competitive environment.
What complexities exist in public procurement in Peru for U.S. suppliers?
The complexities can be arduous, making local partnerships beneficial for U.S. suppliers aiming to penetrate the Peruvian market.
What are the key services provided by Medical Device CRO Peru?
Key services include trial management, regulatory affairs, data management and analysis, site management, quality assurance, import permits and nationalization, and reporting.
How do CROs ensure compliance with ethical standards in research?
They implement rigorous monitoring and auditing practices and ensure that consent can be revoked at any moment without detriment to participants, as highlighted in Decree 021-2017.




