Introduction
The medical device landscape in Argentina is undergoing a transformative shift, characterized by a surge in clinical trials aimed at evaluating innovative technologies. This evolution is propelled by a convergence of local and international stakeholders eager to tap into the region's diverse patient demographics and favorable regulatory environment.
However, the journey is not without its challenges, including:
- Navigating complex regulatory frameworks
- Securing funding
- Overcoming communication barriers
As the demand for advanced healthcare solutions escalates, driven by an aging population and rising chronic disease prevalence, Argentina emerges as a pivotal player in the global medical device arena.
With a commitment from the government to streamline regulatory processes and a growing network of collaborations among academia, healthcare providers, and industry leaders, the country is poised to enhance its role in advancing medical device innovation and clinical research.
Current Landscape of Medical Device Trials in Argentina
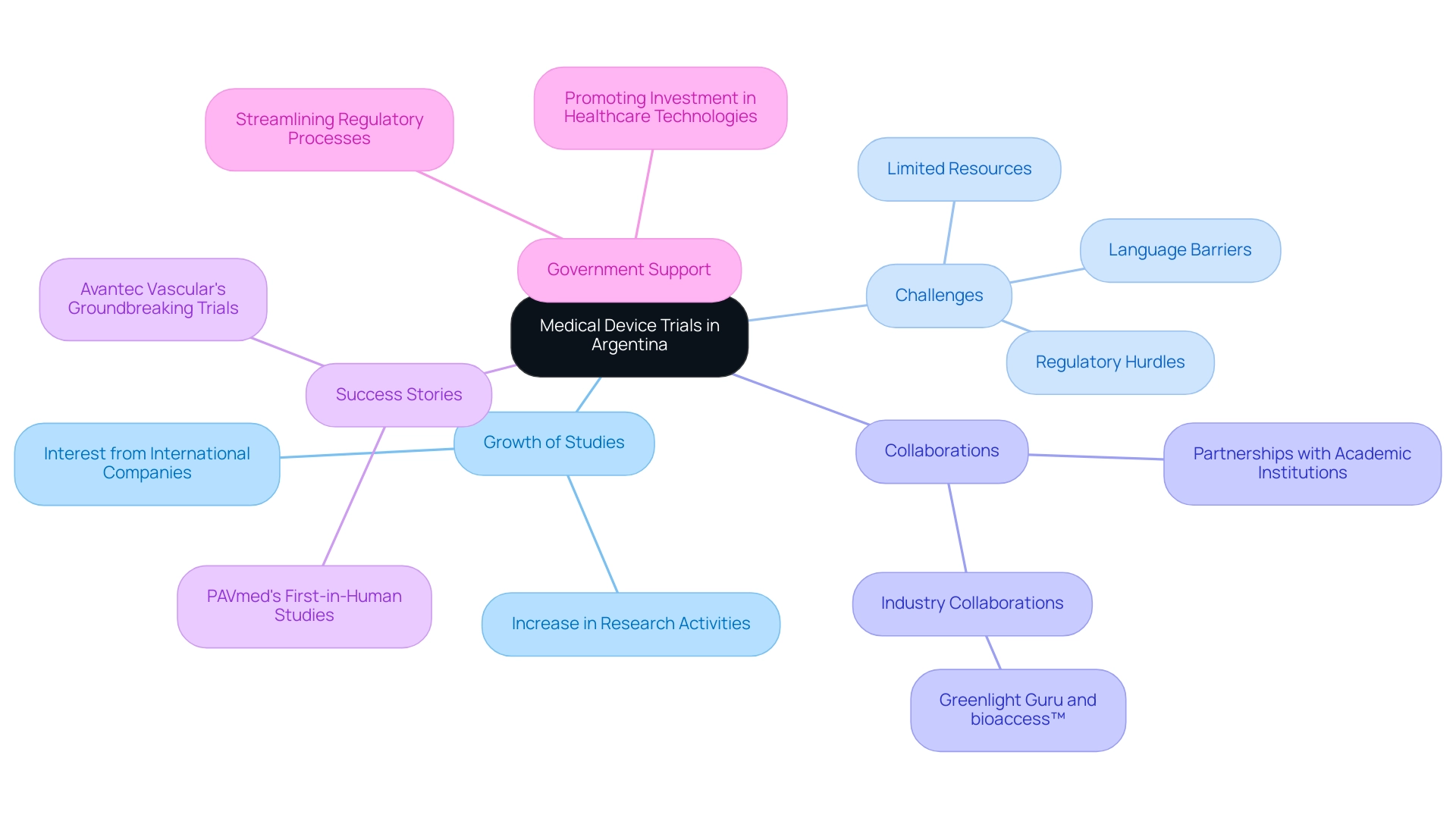
The scenery of healthcare equipment evaluations in Argentina is swiftly changing, with a growing number of studies in Medical Device Research Argentina being carried out to assess novel technologies. This growth in the field of Medical Device Research Argentina is driven by both local and international companies seeking to capitalize on the region’s unique patient demographics and regulatory incentives. However, Medtech companies face significant challenges in the context of Medical Device Research Argentina, including:
- Regulatory hurdles that can delay the approval process
- Limited financial resources that restrict research capabilities
- Language barriers that complicate communication with stakeholders
Recent years have seen a shift towards more collaborative Medical Device Research Argentina efforts, with partnerships forming between academic institutions, healthcare providers, and industry stakeholders, such as the collaboration between Greenlight Guru and bioaccess™ that aims to accelerate Medtech innovations. Successful first-in-human studies, such as PAVmed's in Colombia and Avantec Vascular's groundbreaking trials, illustrate the potential of Latin America as a location for medical research. Furthermore, the Argentine government has demonstrated a commitment to enhancing the research environment for Medical Device Research Argentina by streamlining regulatory processes and promoting investment in healthcare technologies, which is crucial for bolstering the country’s position in the global clinical research arena.
Navigating the Regulatory Framework for Medical Devices in Argentina
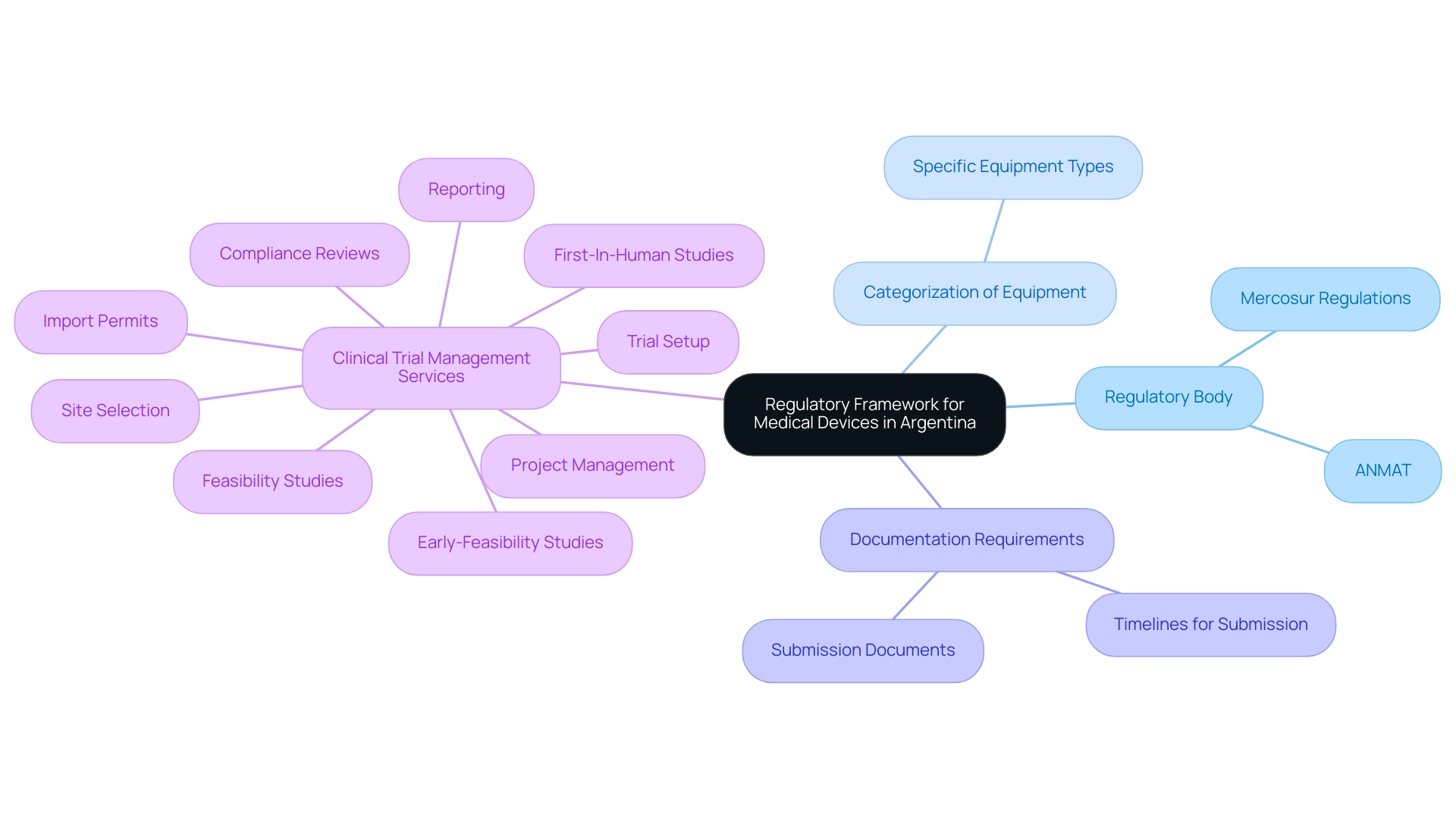
The regulatory framework for Medical Device Research Argentina is primarily governed by the National Administration of Drugs, Food and Medical Technology (ANMAT), which oversees the approval and registration of medical products to ensure compliance with safety and efficacy standards. Furthermore, Mercosur regulations play a significant role in harmonizing requirements across member countries, which is essential for medical device research Argentina, facilitating smoother processes for multinational trials. To navigate the complex landscape of Medical Device Research Argentina effectively, it is essential for researchers to comprehend:
- The specific categorization of their equipment
- The necessary documentation for submissions
- The timelines involved
Collaborating with seasoned regulatory consultants, like Katherine Ruiz, an acknowledged authority in regulatory matters for healthcare products and in vitro diagnostics in Colombia, can offer invaluable insights into enhancing the registration process. Furthermore, utilizing extensive clinical trial management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
is essential for addressing the challenges encountered by healthcare startups, such as regulatory hurdles, competition from established players, recruitment issues, and financial constraints. bioaccess® provides customized solutions aimed at tackling these challenges, ensuring that startups can progress their healthcare products through the regulatory process more efficiently.
Challenges and Opportunities in Argentina's Medical Device Market
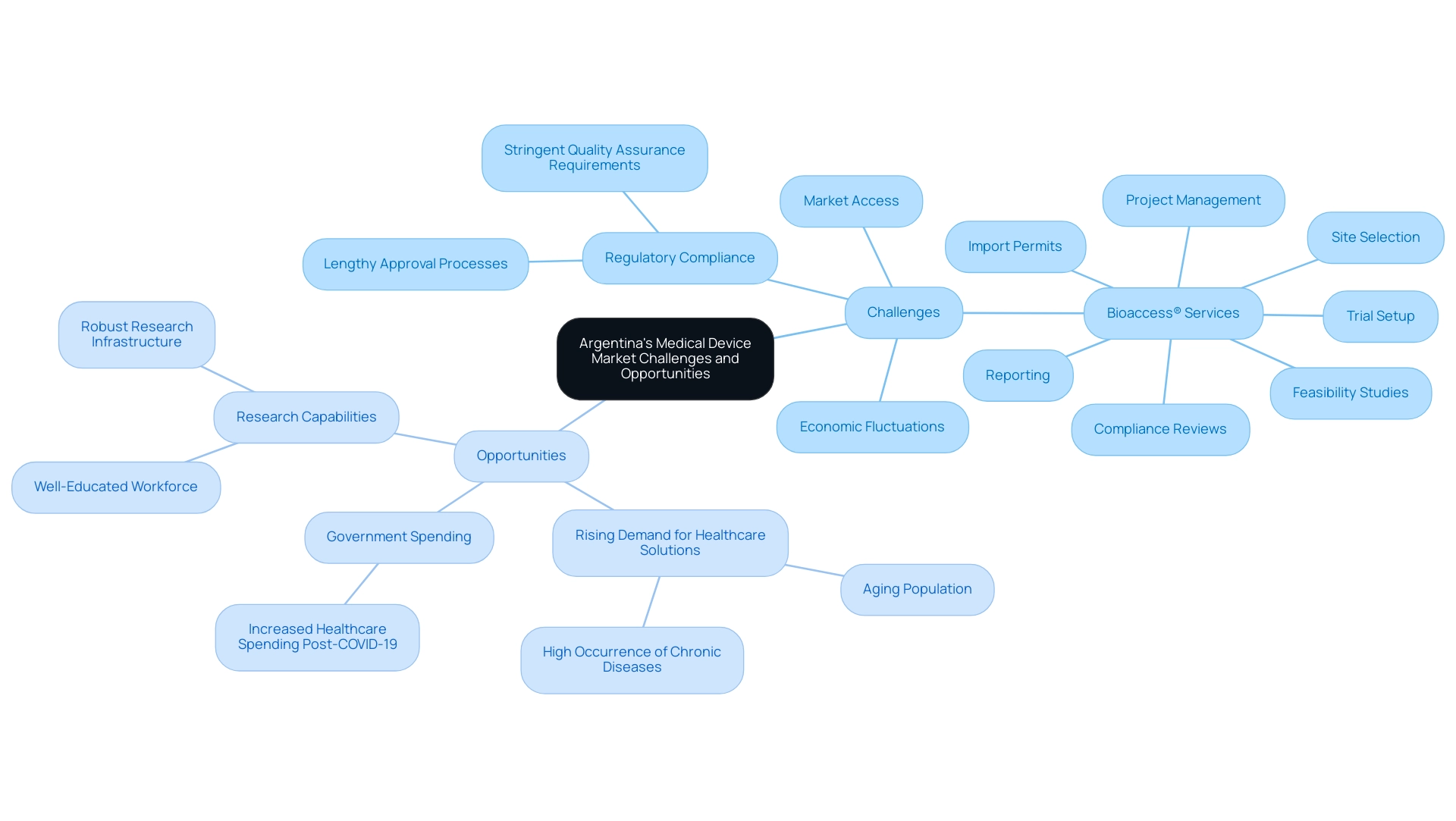
The landscape of regulatory hurdles, market access challenges, and economic fluctuations in Argentina's healthcare equipment market poses significant effects on funding and investment opportunities in Medical Device Research Argentina. Regulatory compliance remains a primary concern, as navigating the local legal frameworks can be daunting for newcomers. These regulations often involve lengthy approval processes and stringent quality assurance requirements that can hinder timely market entry.
However, amidst these challenges lies a promising horizon. The rising demand for innovative healthcare solutions is driven by an aging population and a high occurrence of chronic diseases, such as cardiovascular conditions and diabetes, which are especially common in the region.
Recent healthcare spending increases by the government in response to the COVID-19 pandemic have created a favorable environment for device manufacturers and suppliers looking to enter the market. According to Statista, 'Brand shares' refer to the value share of selected leading brands of the total market size of the chosen market in the chosen region, indicating the competitive dynamics at play.
Moreover, Argentina's robust research capabilities and its well-educated workforce offer a strong foundation for Medical Device Research Argentina as well as the development and testing of new healthcare technologies. Companies that effectively navigate the regulatory landscape and establish partnerships with local entities can leverage comprehensive trial management services offered by bioaccess®, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- project management
- reporting
This places them advantageously to take advantage of growth opportunities.
The presence of key participants in the technology sector, such as Medtronic, Johnson & Johnson, Abbott Laboratories, and Siemens Healthineers, underscores the competitive dynamics that stimulate innovation and revenue generation. Furthermore, the impact of clinical studies extends beyond individual companies; they contribute to job creation, promote economic growth, enhance healthcare quality, and foster international collaboration. For organizations willing to invest the necessary resources into overcoming these initial challenges, the potential for growth in healthcare solutions in Medical Device Research Argentina is significant.
It is essential to recognize that out-of-scope items encompass pharmaceuticals, treatment services, consumer technologies such as fitness trackers, and blood transplants, ensuring a concentrated discussion on healthcare tools.
Argentina's Role in Advancing Medical Device Innovation
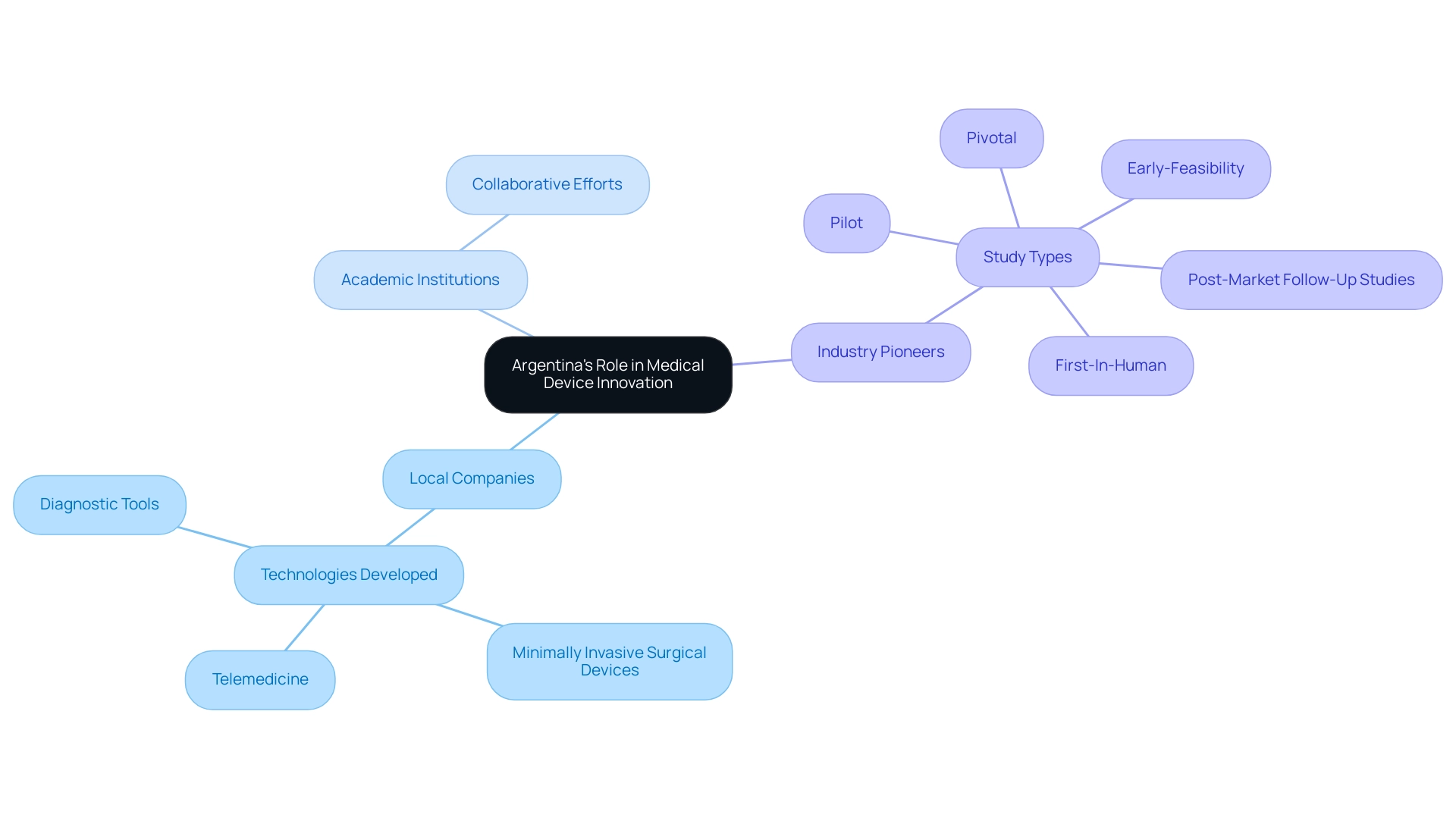
Argentina has positioned itself as a leader in promoting medical innovation through Medical Device Research Argentina, with numerous local companies developing cutting-edge technologies to address both national and global healthcare needs. The country's robust academic institutions are fostering Medical Device Research Argentina by collaborating with industry partners to bring new products to market. Notable advancements have been made in areas such as telemedicine, minimally invasive surgical devices, and diagnostic tools.
Moreover, insights from industry pioneers like Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, and Dr. John B. Simpson, CEO of Avinger, emphasize the successful implementation of studies, such as bioaccess® during its initial human study in Colombia. Bioaccess® specializes in extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Study setup
- Project management
Their expertise encompasses various study types, such as:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
These innovations not only improve patient care in Argentina but also establish the country as an appealing location for international studies in Medical Device Research Argentina, thereby fostering job creation and economic growth in the local economy through collaborative efforts with LATAM CRO specialists.
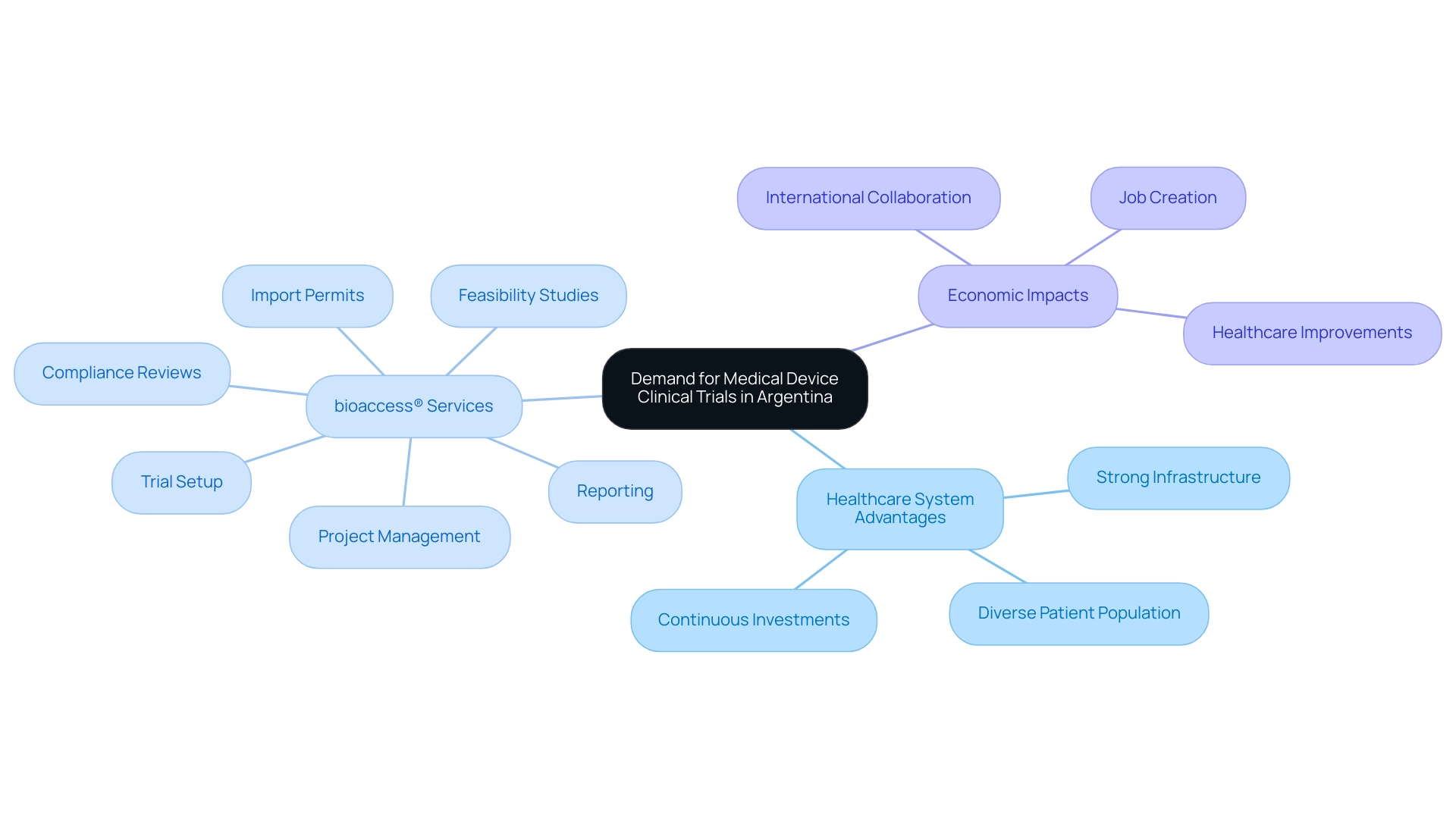
Demand for Medical Device Clinical Trials: Infrastructure and Healthcare System
The demand for medical device research in Argentina is supported by a strong healthcare system and a network of accredited hospitals able to conduct high-quality studies. With a diverse patient population, the country is particularly advantageous for studies that require varied demographics. Continuous investments in healthcare infrastructure further improve the research environment, providing researchers access to modern facilities and skilled medical professionals.
bioaccess® specializes in managing various aspects of trials, including:
- Feasibility studies
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are essential for navigating the complex regulatory environments inherent in conducting research studies. These factors, along with bioaccess®’s proficiency in handling Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up studies, position Argentina as a strategic center for Medical Device Research in the medical device sector.
Moreover, the impact of these Medtech studies extends beyond research, contributing to job creation, economic growth, and improvements in healthcare, thereby fostering international collaboration. For instance, clinical trials in the region have been linked to the creation of thousands of jobs and significant investments in local healthcare infrastructure.
Conclusion
The medical device landscape in Argentina presents a unique convergence of opportunity and challenge. As outlined, the country is experiencing a notable increase in clinical trials driven by both local and international stakeholders. This growth is fueled by Argentina's diverse patient demographics and a supportive regulatory environment, which has become increasingly favorable due to government initiatives aimed at streamlining processes.
However, navigating the complexities of regulatory frameworks, securing funding, and overcoming communication barriers remain significant hurdles for Medtech companies. Despite these challenges, the robust healthcare system, along with a well-educated workforce and ongoing investments in healthcare infrastructure, positions Argentina as a promising destination for innovative medical device trials. The commitment of local entities to foster collaborations further enhances the potential for growth in this sector.
Ultimately, Argentina's commitment to advancing medical device innovation, supported by collaborative efforts among academia, healthcare providers, and industry leaders, underscores its emerging role in the global medical device arena. The synergy between demand for advanced healthcare solutions and the capacity to conduct high-quality clinical research establishes a fertile ground for the development of cutting-edge technologies that can benefit both local and international markets. As this landscape continues to evolve, the potential for Argentina to become a key player in the global Medtech industry remains bright.




