Introduction
The landscape of medical device trials is intricate, marked by a series of meticulously structured phases that are essential for ensuring the safety and efficacy of innovative technologies. From the initial preclinical testing, which lays the groundwork for human applications, to the rigorous evaluations conducted in Phase I through Phase III trials, each stage plays a pivotal role in the development process. This article delves into the various phases of medical device trials, highlighting their specific objectives and the regulatory frameworks that govern them.
By examining the challenges faced and the evolving methodologies employed, a clearer understanding emerges of how these trials not only advance medical technology but also address the pressing needs of patient care in a rapidly changing healthcare environment.
Overview of Medical Device Trial Phases
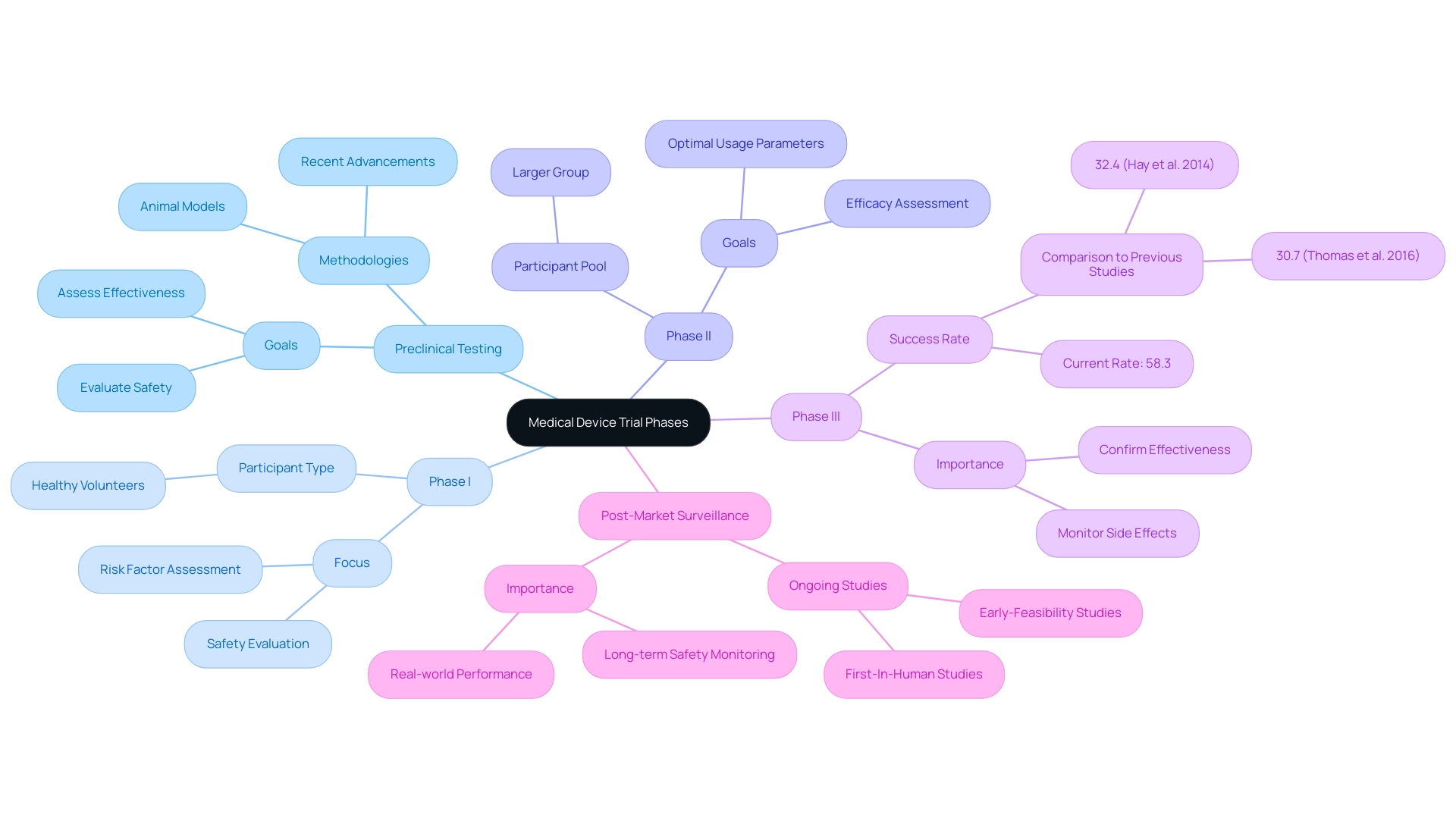
Studies of medical equipment are systematically organized into medical device trial phases, each intended to achieve particular goals essential to the development process. These phases include the various medical device trial phases:
1. Preclinical Testing: This initial phase utilizes animal models to evaluate the safety and effectiveness of the instrument prior to its application in human subjects.
Recent advancements in preclinical testing methodologies have improved the reliability of outcomes, contributing to more robust data for the medical device trial phases. The research team is currently focused on the medical device trial phases to ensure safety and efficacy.
- Phase I: Concentrated mainly on safety, Phase I trials evaluate the risk factors linked to the apparatus and confirm its proper operation.
Conducted with a small cohort of healthy volunteers, this phase is crucial within the medical device trial phases for identifying any immediate adverse effects. The medical device trial phases are crucial for ensuring the safety and efficacy of new products.
- Phase II: This phase broadens the participant pool to a larger group, enabling a comprehensive assessment of the instrument's efficacy and optimal usage parameters.
Here, researchers can gather critical data that informs future clinical applications within the medical device trial phases. The research will focus on the different medical device trial phases.
- Phase III: Conducted on an even larger population, Phase III studies are pivotal in confirming the device's effectiveness while monitoring side effects.
The present success rate for medical device trial phases, specifically in Phase 3 trials, is an impressive 58.3%, a significant rise compared to the 32.4% and 30.7% rates recorded in previous research by Hay and colleagues (2014) and Thomas and associates (2016). As noted by Thomas and others, 'The overall POS presented in this research, Hay and others (2014), and Thomas and others (2016) are much higher than the 1% to 3% that is colloquially seen as it is conditioned on the drug development program entering Phase 1.' These findings challenge the common perception that only 1% to 3% of instruments successfully progress through the medical device trial phases, emphasizing a more optimistic narrative for the medical equipment landscape.
-
The research will focus on the medical device trial phases.
-
Post-Market Surveillance: Following the market release of the product, ongoing studies, such as those managed by bioaccess®, including Early-Feasibility Studies and First-In-Human Studies, are conducted to monitor long-term safety and effectiveness within the broader population. This phase is vital for understanding the equipment's performance in real-world settings during the medical device trial phases and ensuring that any emerging issues are promptly addressed.
Furthermore, issues brought up by stakeholders about the possible workload for investigators and institutional review boards (IRBs) emphasize the difficulties in establishing a registry for studies in Latin America. Bioaccess® addresses these challenges by offering extensive research management services, including feasibility studies, site selection, and compliance reviews. Recent advancements indicate a positive trend in the FDA's approval of new technologies, suggesting a more favorable environment for medical innovation and patient care as we move into 2024.
For more insights and updates on ongoing clinical studies and successful outcomes achieved by bioaccess®, consider subscribing to our newsletter.
Purpose and Objectives of Each Trial Phase
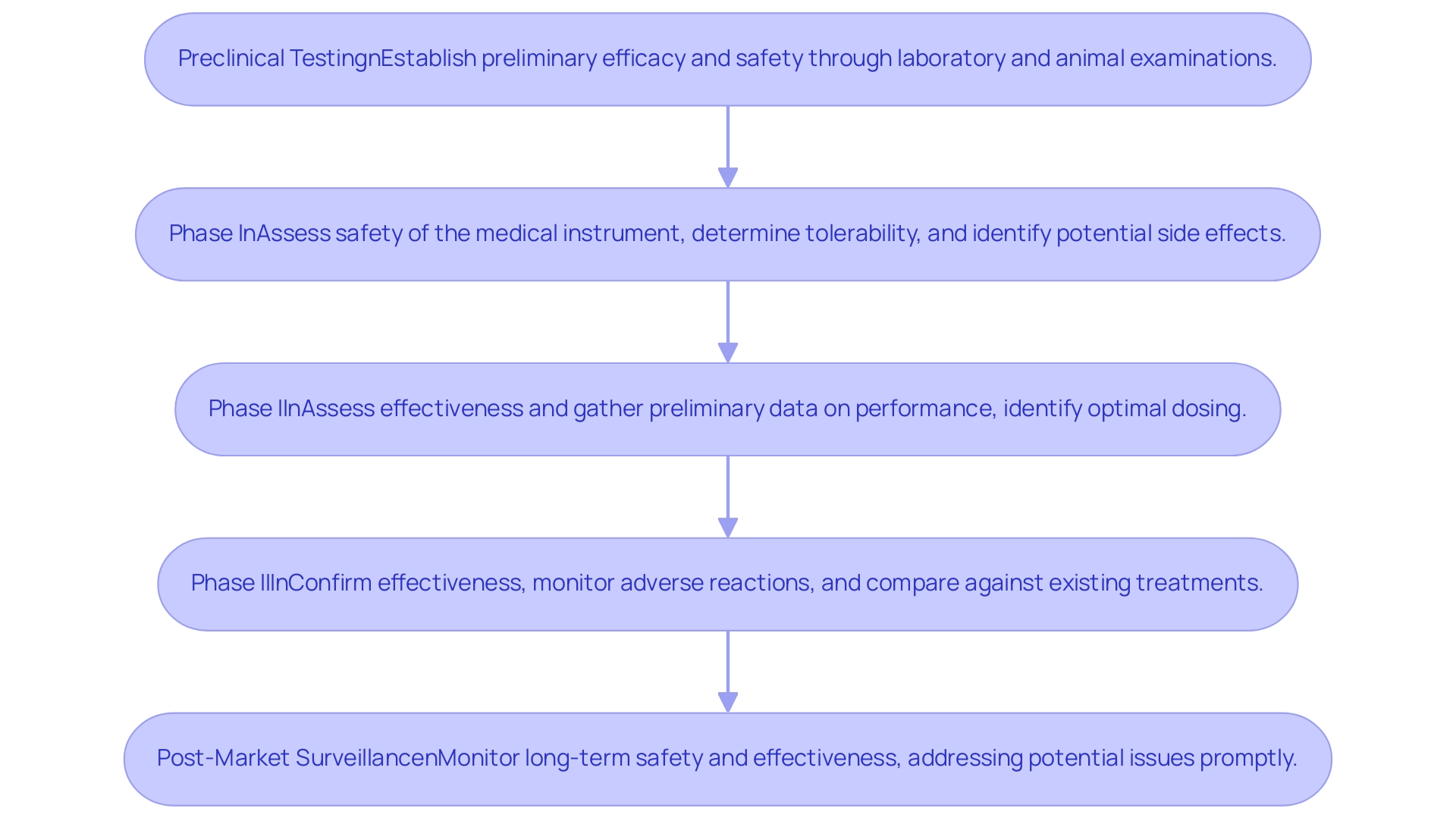
Each phase of medical device trial phases, expertly managed by bioaccess®, is designed with specific objectives, contributing to the comprehensive evaluation of safety and efficacy.
- Preclinical Testing: This initial phase establishes preliminary efficacy and safety through rigorous laboratory and animal examinations. It is essential that the same investigational cellular therapy (CT) product intended for therapeutic use is utilized in definitive preclinical studies.
Any variations between preclinical and clinical lots must be thoroughly discussed in the Investigational New Drug (IND) submission. The recommendations for general preclinical program design emphasize this point, ensuring that each lot of the investigational product is characterized appropriately.
- Phase I: The primary aim of this phase in the medical device trial phases is to assess the safety of the medical instrument.
This involves determining device tolerability and identifying potential side effects. Recent findings indicate that safety assessments in Phase I studies have become increasingly sophisticated, utilizing advanced methodologies, including multiple imputation to handle missing data, thereby enhancing data integrity and robustness in data collection. bioaccess® specializes in First-In-Human Studies (FIH) to ensure a thorough evaluation of safety in this critical phase, ultimately contributing to more reliable trial outcomes.
-
Phase II: The focus of this phase is on assessing the effectiveness of the system and gathering preliminary data on its performance within the targeted patient population. Objectives during this phase include identifying optimal dosing and understanding patient reactions to the equipment. bioaccess®'s expertise in Early-Feasibility Studies (EFS) supports informed decision-making during this phase, ensuring that the insights gained lead to actionable strategies for product development.
-
Phase III: In the medical device trial phases, the goal is to confirm the effectiveness of the apparatus while monitoring adverse reactions. This phase also compares the results against existing treatment options, providing critical insights into the system's relative performance. bioaccess®'s comprehensive project management ensures that pivotal research is conducted efficiently and effectively, with a strong focus on maintaining compliance with regulatory standards throughout the process.
-
Post-Market Surveillance: Following market introduction, this phase is essential for monitoring long-term safety and effectiveness, ensuring that any potential issues are addressed promptly. Techniques like matrixing and bracketing allow for the testing of various drug doses and device configurations, streamlining the evaluation process without the need for extensive analysis of every iteration. bioaccess® also excels in Post-Market Clinical Follow-Up Studies (PMCF), ensuring ongoing compliance and safety monitoring, which is crucial for maintaining trust with stakeholders and regulatory bodies.
As noted by industry expert Lewis,
This technique platform is now available coupled with microscopy and automated sample stage handling to provide mapping capability across a large area of the study sample.
Such advancements highlight the changing environment of studies and the ongoing enhancement of methods to improve safety and effectiveness assessments. Moreover, the significance of quality management in research studies is emphasized by the fact that Qualio is employed by hundreds of medical equipment companies, ensuring compliance and excellence throughout the phases. With more than 20 years of experience in Medtech, bioaccess® is uniquely equipped to manage the intricacies of research studies, providing outstanding results for our clients.
Regulatory Framework for Medical Device Trials
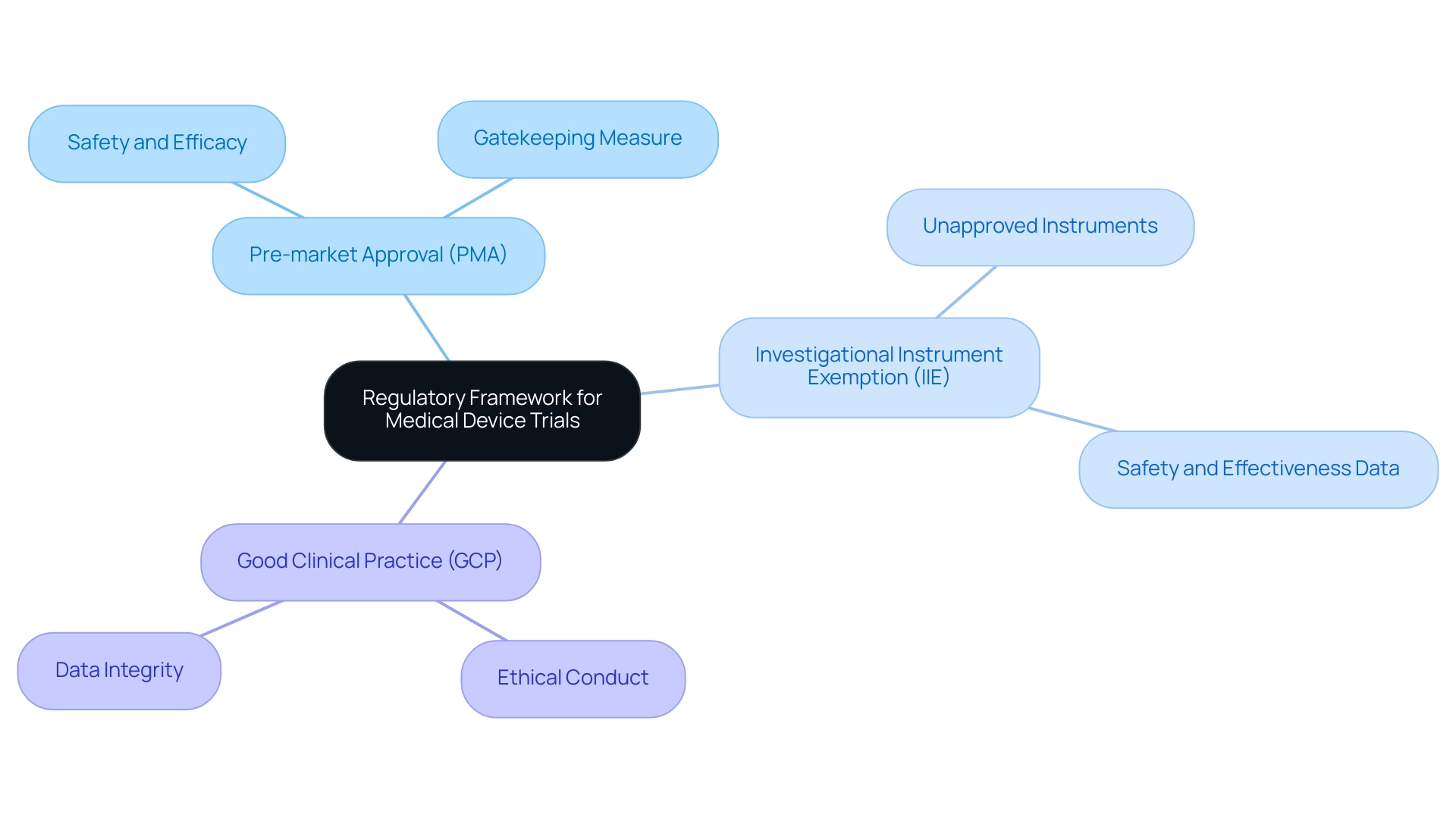
The regulatory framework governing clinical trials for medical products is anchored in both national and international standards, notably encapsulated in the FDA's 21 CFR Part 812 and ISO 14155. These regulations are crucial for ensuring the safety and efficacy of medical products prior to market entry. Key components of this framework include:
-
Pre-market Approval (PMA): This rigorous process demands comprehensive evidence demonstrating the safety and effectiveness of a product before it can be marketed. It functions as an essential gatekeeping measure to safeguard patients and guarantee high standards of medical instruments.
-
Investigational Instrument Exemption (IIE): The IIE provision allows the use of unapproved instruments in research studies, enabling investigators to gather vital safety and effectiveness data that are crucial for future regulatory submissions.
-
Compliance with Good Clinical Practice (GCP): Following GCP is vital for ensuring that research studies are conducted ethically and that the integrity of the data gathered is uncompromised.
In this context, bioaccess® stands out as a leading MedTech research organization in Latin America, concentrating on innovation and regulatory excellence. They provide extensive research management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and thorough reporting. A significant aspect of compliance is highlighted by the FDA's traceability requirements in §820.10(d), which are considered substantially similar to those found in the QS regulation.
This highlights the significance of keeping precise and accessible documentation throughout the research process.
Comprehending these regulatory obligations is essential for researchers navigating the complex realm of medical device trial phases. As noted by the FDA, adherence to these frameworks is paramount:
FDA considers this phrase to be substantially similar to the requirement in the QS regulation that records be 'reasonably accessible' and 'readily available.'
Furthermore, recent updates to regulations reflect the FDA's commitment to maintaining rigorous standards, as exemplified by the compassionate use system that allows access to unapproved products for patients with severe diseases.
This system, emphasized in the case study named 'Compassionate Clinical Trials Update,' seeks to balance risks and advantages while enabling access to investigational products, demonstrating the FDA's changing approach to medical equipment studies in 2024 and beyond.
To discover more about how bioaccess® can assist your research needs, we encourage you to SCHEDULE A MEETING with our team to discuss your specific requirements.
Comparing Medical Device Trials to Pharmaceutical Trials
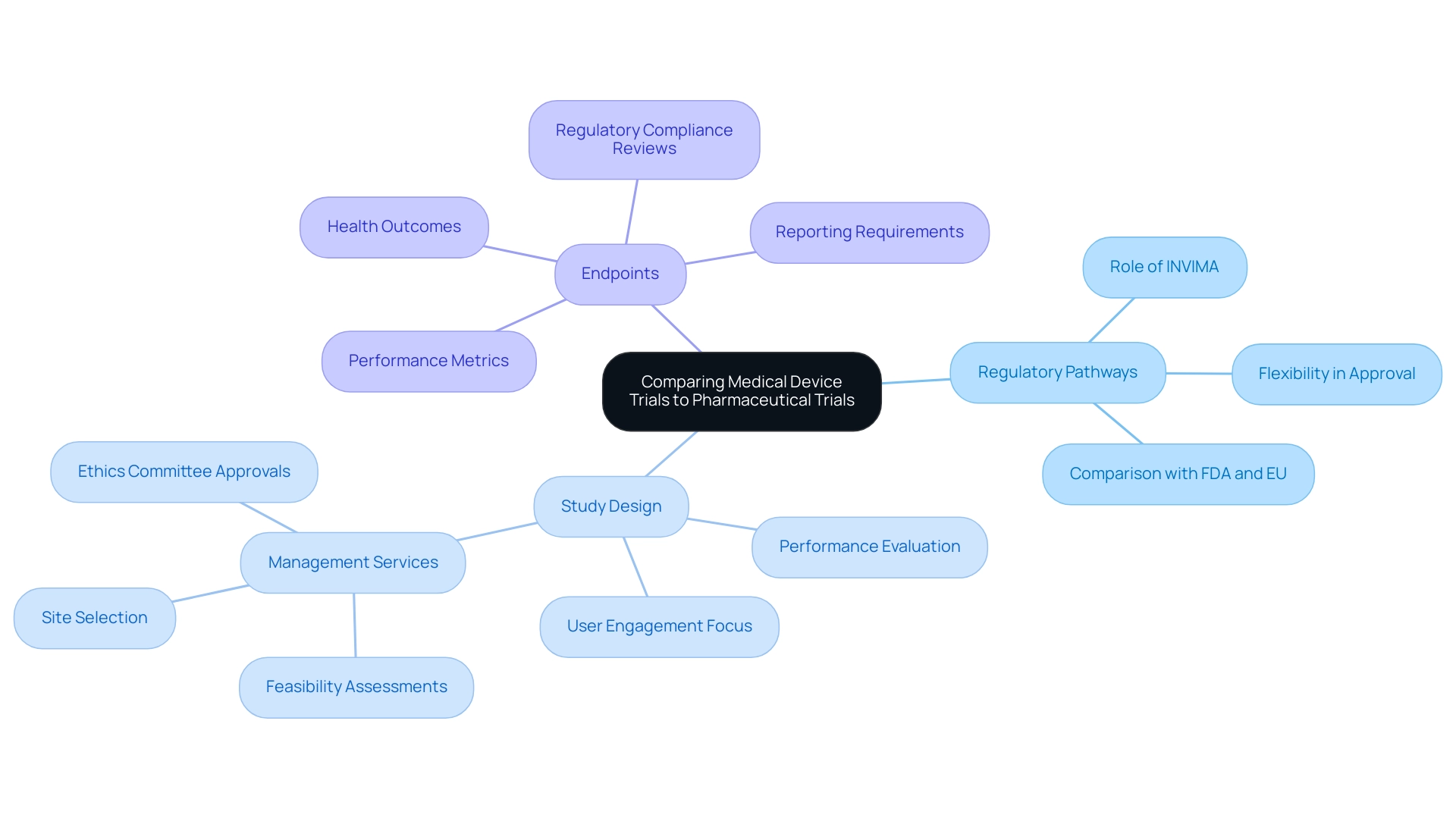
Medical equipment studies and pharmaceutical experiments show notable distinctions across multiple aspects:
- Regulatory Pathways: The regulatory environment for medical products is often more flexible than that for pharmaceuticals, which generally require extensive preclinical and clinical data prior to approval. In Colombia, the INVIMA (National Food and Drug Surveillance Institute) plays a crucial role, overseeing the regulatory functions and classification of medical instruments as a Level 4 health authority by PAHO/WHO. This adaptability permits a quicker reaction to advancements in the medical equipment industry.
- Study Design: Equipment assessments concentrate on user engagement and performance, evaluating how effectively a product functions in practical environments. In contrast, drug studies are centered on pharmacodynamics and pharmacokinetics, examining how drugs affect biological systems and how they are processed by the body. Thorough management services for research, including feasibility assessments, site selection, and setup involving ethics committee approvals, are crucial in ensuring that the medical device trial phases are organized efficiently.
- Endpoints: The primary outcomes measured in device evaluations frequently relate to performance metrics, such as safety and durability, while pharmaceutical assessments emphasize health outcomes like improvements and symptom relief. Regulatory compliance reviews and project management are essential in aligning these endpoints with industry standards. Reporting requirements, including study status and documentation of serious and non-serious adverse events, are also crucial components of the management process. Understanding these distinctions is essential for researchers as they tailor their study designs and regulatory submissions. As pointed out by Annetine C. Gelijns, the implications of these strategies significantly affect the efficiency with which biomedical research findings are converted into practical application. Moreover, perspectives from specialists such as Katherine Ruiz and Oswaldo Amaya, MD, highlight the importance of knowledge in regulatory matters and clinical research management, especially regarding the execution of medical assessments in Latin America. The analysis on the regulation of medical substances and instruments in the U.S. and EU emphasizes the challenges and benefits of the FDA's centralized method in contrast to the EU's network, demonstrating the practical effects of these regulatory routes on research design and implementation.
Challenges in Conducting Medical Device Trials
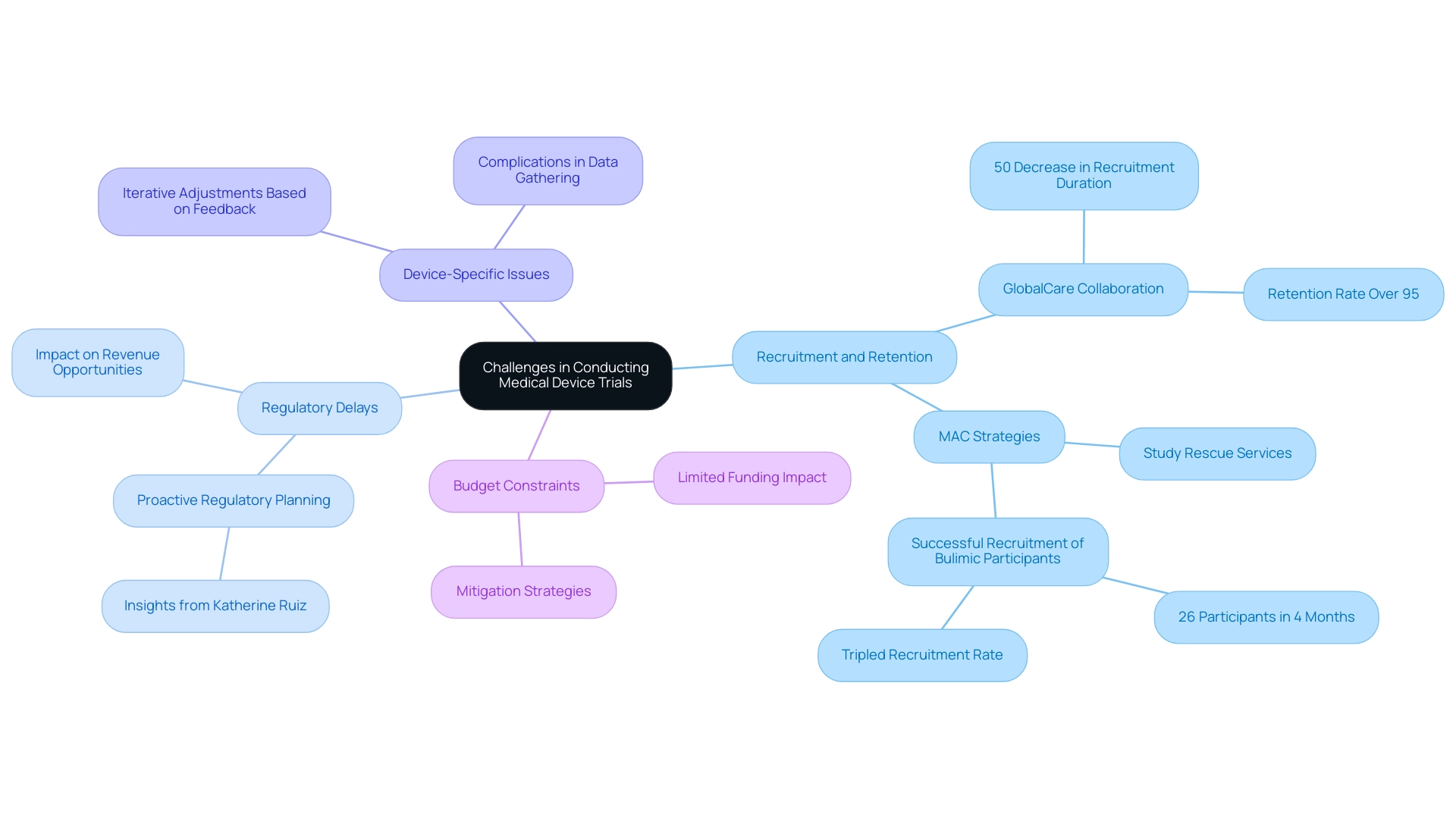
Conducting medical device evaluations involves navigating several complex challenges that can impact the overall success of the study. Key obstacles include:
-
Recruitment and Retention: Identifying qualified participants and maintaining their engagement throughout the study can pose significant difficulties. With the right strategies, however, organizations can enhance recruitment outcomes. For example, the collaboration between GlobalCare Clinical Trials and bioaccess® in Colombia has attained over a 50% decrease in subject recruitment duration and a retention rate surpassing 95%. Such tailored recruitment strategies can significantly enhance participant involvement, which is especially pertinent considering the obstacles encountered in medical research. Enhancing patient recruitment can conserve time, money, and resources in the clinical study process, ultimately resulting in more successful outcomes.
-
Regulatory Delays: Navigating the intricate regulatory landscape often leads to unexpected delays, hindering study initiation and progress. Such delays can lead to missed revenue opportunities for sponsors, as delayed market entry can significantly impact overall profitability. This highlights the necessity for proactive regulatory planning, as shown by professionals such as Katherine Ruiz, who specializes in regulatory matters for medical products and in vitro diagnostics in Colombia. Her insights are vital in reducing the risks linked to regulatory obstacles.
-
Device-Specific Issues: Unlike pharmaceuticals, medical devices often need iterative adjustments based on user feedback during testing. This necessity complicates data gathering and examination, as ongoing modifications may change research parameters.
-
Budget Constraints: Limited funding can restrict the scope of experiments, affecting the ability to conduct thorough investigations. Anticipating these challenges and developing effective mitigation strategies is crucial for successful management of the process. Organizations like MAC have recognized these hurdles and are committed to overcoming them. As Nicola Armitstead, Vice President of Site Clinical Operations at MAC, states,
At MAC, we are dedicated to tackling these challenges, and our thorough strategy assists in ensuring effective recruitment and retention, allowing us to fulfill and surpass the requirements of clinical investigations while speeding up timelines and delivering therapies to participants more quickly.
Their innovative research rescue services, which include targeted recruitment strategies and enhanced participant engagement techniques, demonstrate the potential for turning around recruitment challenges. For instance, in a recent study involving bulimic participants, they successfully recruited 26 individuals in just four months, tripling the recruitment rate after initiating their sites. This highlights the importance of strategic planning and adaptability in the face of recruitment challenges in medical device trial phases. Additionally, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring a comprehensive approach to clinical trial management.
Conclusion
The intricate phases of medical device trials play a crucial role in ensuring the safety and efficacy of innovative technologies. Each phase, from preclinical testing to post-market surveillance, is designed with specific objectives that collectively enhance the understanding and performance of medical devices. The progress through these phases is governed by a robust regulatory framework, ensuring that each trial adheres to the highest standards of safety and effectiveness.
Challenges such as participant recruitment, regulatory delays, and budget constraints can hinder the trial process, yet organizations like bioaccess® demonstrate that strategic planning and innovative solutions can overcome these obstacles. By leveraging advanced methodologies and comprehensive clinical trial management services, they contribute to a more streamlined and effective trial process.
Ultimately, the evolving landscape of medical device trials reflects a commitment to improving patient care through rigorous evaluation and adherence to regulatory standards. As the industry continues to advance, it is essential for stakeholders to remain informed and engaged in the trial process, ensuring that medical devices not only meet regulatory requirements but also fulfill the pressing needs of patients in a rapidly changing healthcare environment.




