Overview
The article focuses on the role and significance of ophthalmology contract research organizations (CROs) in clinical research, highlighting their essential functions such as trial design, patient recruitment, and regulatory compliance. It emphasizes that CROs are crucial for advancing ophthalmic therapies and improving patient outcomes, as they streamline research processes and facilitate innovative studies, which are increasingly important given the evolving landscape of ophthalmic medical research.
Introduction
In the rapidly evolving field of ophthalmology, Contract Research Organizations (CROs) play a pivotal role in advancing clinical trials and research initiatives tailored to eye care. These specialized entities not only manage the intricacies of trial design and patient recruitment but also ensure compliance with stringent regulatory standards, thereby enhancing operational efficiency for sponsors.
As the demand for innovative ophthalmic treatments grows, particularly in regions like Latin America, the expertise of CROs becomes increasingly vital. This article delves into the multifaceted services offered by ophthalmology CROs, the challenges they face, and the future trends shaping their impact on patient care and medical advancements.
With a spotlight on organizations like bioaccess®, the discussion highlights how these entities bridge the gap between clinical research and real-world application, ultimately striving to deliver effective therapies to patients in need.
Defining Ophthalmology Contract Research Organizations (CROs)
Ophthalmology contract research organizations (CROs) are specialized entities dedicated to supporting research projects and initiatives focused on ophthalmic products and therapies. Their responsibilities encompass a wide array of critical functions, including:
- Trial design
- Patient recruitment
- Data management
- Ensuring regulatory compliance
By entrusting these essential tasks to a CRO like bioaccess®, sponsors can tap into specialized expertise, boost operational efficiency, and maintain strict adherence to regulatory standards.
Bioaccess® distinguishes itself as a leader in Latin America, offering customized solutions that expedite the research process for Medtech startups, guaranteeing swift regulatory approval and effective project management, including thorough oversight of research status and inventory management. Additionally, bioaccess® assists with the import permit process and nationalization of investigational devices, ensuring that all regulatory requirements are met. According to market segmentation data, the North America ophthalmology market is experiencing significant growth, underscoring the importance of CROs in navigating this evolving landscape.
For example, Forbion, a life sciences firm, was involved in financing Aiolos Bio, Inc. for conducting phase 2 trials to evaluate the safety and efficacy of drug candidate AIO-001, indicated for treating asthma. This emphasizes the essential function that CROs, especially bioaccess®, have in promoting medical studies. Furthermore, input from the Business Development Manager at Renaissance Lakewood LLC concerning the CENTRAL PRECOCIOUS PUBERTY (CPP) TREATMENT report demonstrates the practical impact and effectiveness of CROs in research studies.
The importance of an ophthalmology contract research organization goes beyond simple logistics; it serves as a vital intermediary that connects the gap between scientific studies and practical application, ensuring that innovative therapies are provided to patients in an effective and timely manner. With the constantly changing field of ophthalmic research, the role of the ophthalmology contract research organization has become increasingly essential, especially as they adapt to the latest advancements in study methodologies and regulatory frameworks. To discover more about how bioaccess® can assist your research studies, SCHEDULE A MEETING today.
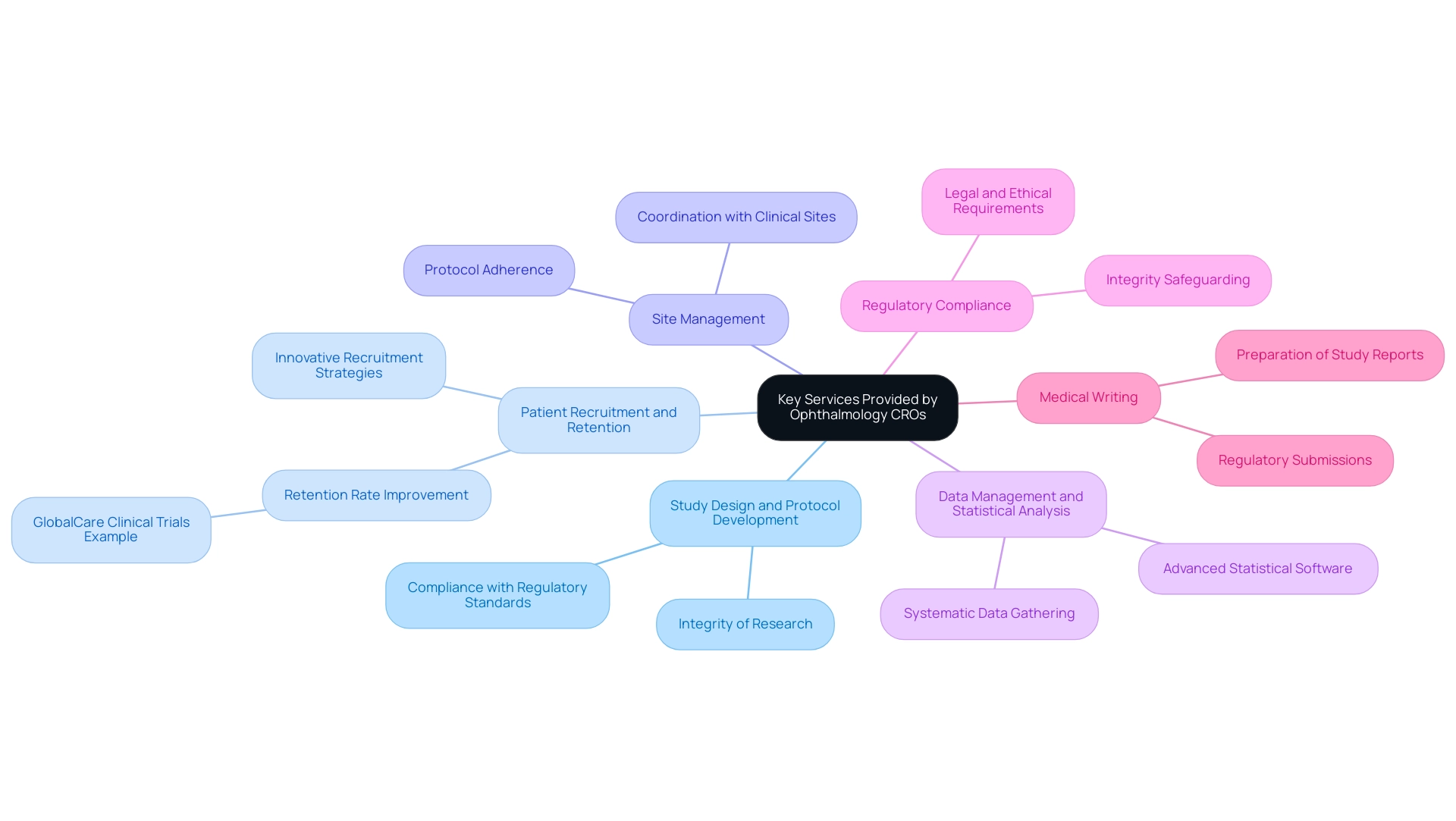
Key Services Provided by Ophthalmology CROs
Ophthalmology contract research organizations (CROs) provide a comprehensive range of services essential for the successful completion of research studies. These services include:
- Study Design and Protocol Development: Creating solid study protocols that comply with both local and international regulatory standards is essential for the integrity of research.
- Patient Recruitment and Retention: Effective patient recruitment strategies are essential, particularly in light of recent trends showing a substantial rise in research study registrations, with Southeast Asia alone documenting 11,030 studies in 2022, representing a 48.5% increase since 2019. CROs utilize innovative approaches to attract and retain the right participants, enhancing study outcomes. A significant example is GlobalCare Clinical Trials, which, in collaboration with bioaccess™, accomplished over a 50% decrease in trial subject recruitment time and an impressive retention rate of over 95% in Colombia.
- Site Management: Coordinating with clinical sites to ensure strict adherence to protocols and timelines is a core function that guarantees operational efficiency and compliance throughout the research.
- Data Management and Statistical Analysis: Gathering, managing, and analyzing data systematically allows CROs to extract meaningful insights, which are critical for the evaluation of study hypotheses and outcomes. This frequently entails the use of advanced statistical software and methodologies customized to specific study needs.
- Regulatory Compliance: A stringent focus on regulatory compliance helps safeguard integrity, ensuring that all activities meet the necessary legal and ethical requirements.
- Medical Writing: The preparation of essential documents, including study reports and regulatory submissions, is facilitated by expert medical writers, ensuring clarity and precision in communication with stakeholders.
These services not only streamline the research process for the ophthalmology contract research organization but also address the evolving needs of patients and healthcare providers, enhancing overall satisfaction and outcomes in ophthalmology healthcare services. Further research is recommended to explore patients' needs and improve these services. Additionally, IDx Technologies' collaboration with bioaccess™ to identify Latin American ophthalmology centers for AI-based disease detection exemplifies the innovative partnerships shaping the future of clinical trials.
As noted by Laboratory Corporation of America Holdings following their acquisition of Toxikon Corporation, the integration of comprehensive services can significantly impact patient care. A case assessment evaluating patient satisfaction in ophthalmology clinics in the Makkah Region found that 75% of participants expressed satisfaction with the services provided, highlighting the importance of CROs in optimizing patient outcomes.
The Importance of Clinical Research in Ophthalmology
Clinical investigations in ophthalmology play a crucial role in enhancing patient care and medical knowledge for several key reasons:
- Advancement of Medical Knowledge: Investigative efforts deepen our understanding of various eye diseases and conditions, paving the way for innovative diagnostic and therapeutic modalities. For instance, ongoing investigations into diabetic macular edema (DME) are exploring novel intravitreal medications and innovative drug delivery systems, such as implants for sustained release, which may significantly enhance treatment effectiveness and reduce the frequency of injections needed for DME management. Additionally, eyeFlow, Inc. recently received INVIMA approval for an 18-month pilot project in Barranquilla, Colombia, focused on a revolutionary glaucoma treatment. This study intends to enlist 60 participants, with the goal of assessing the safety and effectiveness of this groundbreaking therapy, highlighting the dedication to innovative studies in ophthalmology.
- Enhancing Patient Outcomes: Through experimental procedures, investigators carefully evaluate the safety and efficiency of new treatments. A notable statistic from Medina et al. indicates that a handheld fundus camera demonstrates a remarkable 96.9% sensitivity and 94.8% specificity for identifying diabetic retinopathy, underscoring the potential of innovative technologies in improving patient outcomes.
- Regulatory Approval of New Therapies: Clinical studies are vital for securing regulatory approvals for innovative ophthalmic products, ensuring they meet stringent safety and efficacy standards prior to market release. Organizations such as ProMedica International (PMI), established in 1985 and located in Orange County, California, serve as an ophthalmology contract research organization specializing in overseeing ophthalmic studies, providing crucial regulatory strategy advice and product commercialization that aids this process.
- Evidence-Based Practice: The results from medical investigations offer compelling evidence that informs treatment guidelines, ensuring ophthalmologists are equipped with the best available therapies for their patients. As emphasized by Michael Young, Co-Founder of Lindus Health, > If you're seeking to bring your ophthalmology research from concept to reality with ease and precision, look no further than Lindus Health <. Lindus Health offers comprehensive services to streamline ophthalmology clinical trials from concept to reality, reinforcing the commitment to integrating research findings into practice, which is essential in driving advancements in patient care and treatment efficacy.
As we move toward 2024, the importance of clinical research in ophthalmology will only continue to grow, reinforcing its critical position in enhancing patient care and outcomes.
Challenges in Ophthalmology Clinical Research
Clinical researchers associated with an ophthalmology contract research organization face numerous challenges that can greatly affect the success of their investigations. One primary concern is the Recruitment of Qualified Personnel. The intricacies of inclusion criteria often complicate participant recruitment, especially in niche ophthalmic research where targeted populations may be limited.
This issue is underscored by ongoing recruitment difficulties highlighted in recent articles examining clinical trials statistics for 2024. For instance, the VIP-HIP research demonstrated that uncorrected moderate hyperopia was linked to diminished near function and attention issues, highlighting the significance of precisely identifying and recruiting the appropriate participants to tackle such conditions.
Complex Research Protocols present another significant hurdle. The multifaceted nature of ophthalmic conditions can lead to intricate research designs, making adherence challenging for participants. These complexities are further exacerbated when researchers must balance Managing Multiple Projects; however, leveraging comprehensive clinical study management services from an ophthalmology contract research organization can help streamline this process.
Services such as feasibility and selection of investigation locations and lead investigators, along with trial setup and project management, can alleviate the burden on teams, allowing for more efficient coordination of concurrent projects. Additionally, Compliance Reviews ensure that documentation complies with country requirements, facilitating smoother approvals and minimizing delays. Furthermore, Regulatory Hurdles contribute to the complexities that ophthalmology contract research organizations face in conducting ophthalmologic investigations.
Navigating the regulatory landscape demands considerable time and a thorough understanding of compliance requirements. This is where an ophthalmology contract research organization can provide expert support in compliance reviews and obtaining necessary Import Permits to facilitate smoother research initiation and continuity. The nationalization of investigational devices is also crucial in ensuring compliance with local regulations.
Lastly, Funding Limitations remain a critical barrier to innovative research.
Securing adequate resources for research is often fraught with challenges, restricting the potential for groundbreaking discoveries. As noted in case analyses, such as those examining Diabetic Macular Edema (DME), the pursuit of innovative treatment methods—like novel intravitreal medications and advanced delivery systems—can be significantly hampered by these funding obstacles. As Dr. Malloy remarked concerning an initial examination on clinically active TED, tackling these recruitment challenges is vital to advance progress in ophthalmology clinical trials.
Additionally, the 'Essentials in Ophthalmology' series highlights recent developments in various subspecialties, further underscoring the importance of overcoming these challenges to enhance the transfer of knowledge into daily practice. Effective reporting on research status, inventory, and adverse events is also vital for maintaining transparency and ensuring ongoing funding and support.
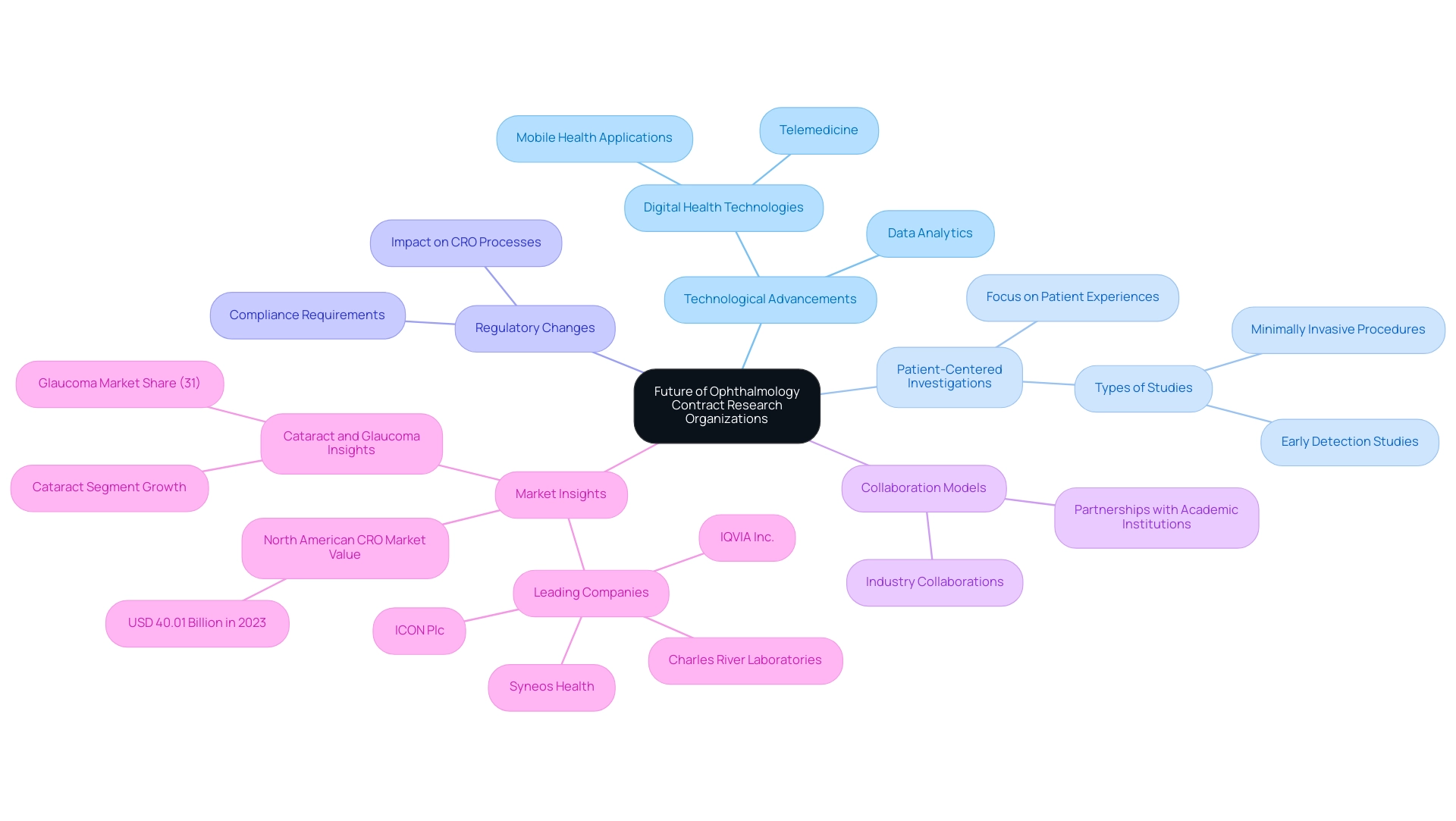
The Future of Ophthalmology Contract Research Organizations
The future of ophthalmology contract research organizations (CROs) is set for transformation, directed by several crucial trends. The North American CRO services market, valued at USD 40.01 billion in 2023, underscores the substantial investments in this sector. One of the most significant drivers is the technological advancements that are reshaping the landscape.
The adoption of digital health technologies, including telemedicine and mobile health applications, is anticipated to revolutionize patient recruitment and enhance management efficiency. As Denise Barnes insightfully notes, the impact of regulatory changes on healthcare requires CROs to be agile in their approaches, particularly in integrating advanced technologies that can streamline processes and improve patient outcomes.
A pronounced focus on patient-centered investigations is also emerging, with CROs increasingly designing studies centered around patient experiences and outcomes. This shift not only aligns with regulatory expectations but also aims to foster more relevant and impactful studies. Moreover, the collaboration and partnership models are expected to grow, as CROs seek to leverage shared expertise with academic institutions and industry partners to enhance research quality and efficiency.
Leading companies in this field, such as ICON Plc, Charles River Laboratories, Syneos Health, and IQVIA Inc., are at the forefront of these advancements, establishing benchmarks for innovation and effectiveness.
The extensive service capabilities of CROs encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Detailed reporting on study status
- Adverse events (both serious and non-serious)
All essential for successful research studies. This holistic approach not only enhances operational efficiency but also contributes to local economies through job creation and improved healthcare outcomes. The evolution of regulations will necessitate CROs to adapt their processes to ensure compliance while keeping operational efficiency intact.
In tandem, the utilization of data analytics is set to expand, empowering CROs to extract deeper insights from research trials. This analytical capability will play a crucial role in improving decision-making and research outcomes, crucial for advancing patient care in ophthalmology.
Additionally, news from Reichert, Inc. regarding its revamped refractometers and analytical instruments website highlights the ongoing technological advancements in the field, promising a significantly enhanced user experience that could further support clinical research efforts.
As the cataract segment is projected to grow rapidly due to an aging population and advancements in surgical techniques, it is evident that the emphasis on early detection and minimally invasive procedures will significantly enhance patient management in the field, particularly in conditions such as glaucoma, which held a 31% market share in 2023. This convergence of technology and patient-centered approaches heralds a promising future for the ophthalmology contract research organization sector, driving global health improvement through international collaboration and innovation in Medtech.
Conclusion
The role of ophthalmology Contract Research Organizations (CROs) is increasingly pivotal in advancing clinical trials and enhancing patient care. By providing specialized services such as:
- Study design
- Patient recruitment
- Regulatory compliance
- Data management
CROs like bioaccess® ensure the efficient execution of clinical research tailored to the ophthalmic field. The significance of these organizations is underscored by their ability to navigate the complexities of clinical trials while maintaining rigorous compliance with regulatory standards, ultimately bridging the gap between innovative research and real-world application.
As the demand for innovative ophthalmic treatments continues to grow, particularly in emerging markets such as Latin America, the future of CROs appears bright. Technological advancements, a focus on patient-centric research, and collaborative partnerships are set to transform the landscape of ophthalmology clinical trials. These trends not only promise to enhance operational efficiency but also aim to improve patient outcomes significantly. The integration of advanced technologies and data analytics will empower CROs to refine their processes, leading to more effective and timely delivery of new therapies.
In summary, the evolution of ophthalmology CROs is essential for driving forward the field of eye care. By overcoming challenges such as:
- Recruitment difficulties
- Funding limitations
- Adapting to regulatory changes
These organizations play a crucial role in the advancement of medical knowledge and patient care. As clinical research in ophthalmology continues to progress, the contributions of CROs will be instrumental in ensuring that innovative treatments reach those in need, ultimately enhancing the quality of life for patients worldwide.
Frequently Asked Questions
What are ophthalmology contract research organizations (CROs)?
Ophthalmology CROs are specialized entities that support research projects focused on ophthalmic products and therapies, handling critical functions such as trial design, patient recruitment, data management, and ensuring regulatory compliance.
What services do ophthalmology CROs provide?
They offer a range of services including study design and protocol development, patient recruitment and retention, site management, data management and statistical analysis, regulatory compliance, and medical writing.
How do CROs assist with patient recruitment?
CROs implement effective recruitment strategies to attract and retain participants, which is crucial given the rising number of research studies. For example, GlobalCare Clinical Trials, in collaboration with bioaccess™, achieved over a 50% decrease in trial subject recruitment time in Colombia.
Why is regulatory compliance important for CROs?
Regulatory compliance ensures that all research activities meet necessary legal and ethical standards, safeguarding the integrity of the study and protecting patient safety.
What role does bioaccess® play in the ophthalmology CRO landscape?
Bioaccess® is a leading CRO in Latin America that provides customized solutions to expedite research processes for Medtech startups, ensuring swift regulatory approvals and effective project management.
How do CROs contribute to the success of research studies?
By managing essential tasks such as trial design, patient recruitment, and data management, CROs enhance operational efficiency and help ensure that innovative therapies reach patients effectively and on time.
What is the significance of the North American ophthalmology market?
The North American ophthalmology market is experiencing significant growth, highlighting the importance of CROs in navigating the evolving landscape of ophthalmic research.
Can you provide an example of a successful collaboration involving a CRO?
IDx Technologies collaborated with bioaccess® to identify Latin American ophthalmology centers for AI-based disease detection, showcasing innovative partnerships that shape the future of clinical trials.
How do CROs impact patient satisfaction in ophthalmology?
Research indicates high patient satisfaction rates with the services provided by CROs, such as a case assessment in the Makkah Region where 75% of participants expressed satisfaction with ophthalmology clinic services.




