Introduction
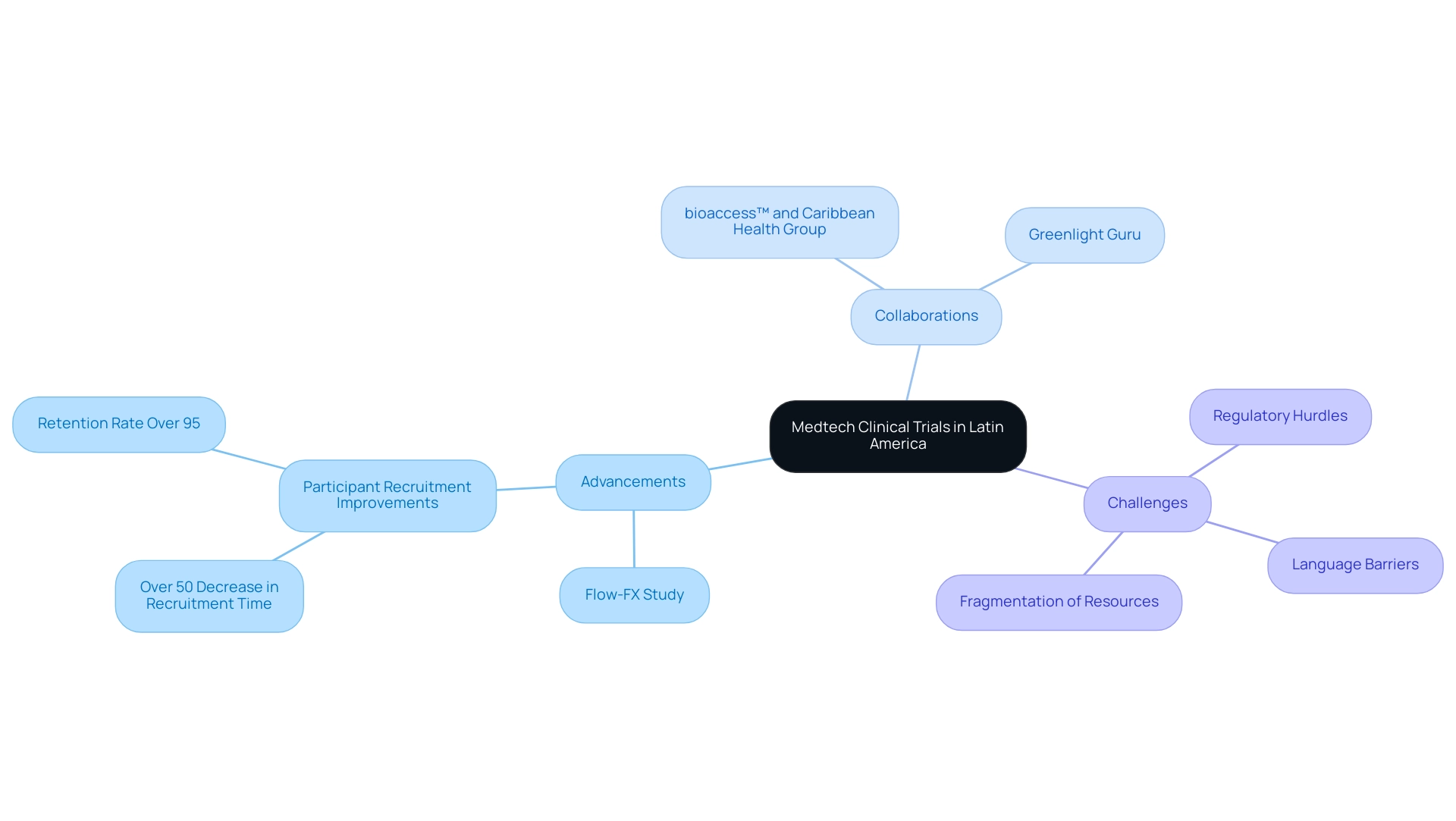
The landscape of medical technology clinical trials in Latin America is undergoing a remarkable transformation, with the region positioning itself as a vital hub for innovative research and development. Among the countries leading this charge, Colombia stands out as a promising site for clinical studies, bolstered by strategic partnerships and government support.
The collaboration between bioaccess™ and Caribbean Health Group exemplifies this trend, aiming to establish Barranquilla as a premier destination for clinical trials.
This article delves into the dynamic factors shaping the Medtech clinical trial environment in Latin America, exploring the challenges and opportunities that stakeholders must navigate to foster successful research initiatives. From regulatory hurdles to the importance of local partnerships, understanding these elements is essential for those looking to thrive in this evolving landscape.
The Evolving Landscape of Medtech Clinical Trials in Latin America
Latin America is swiftly positioning itself as a crucial participant in the global medical device research sector, with Colombia emerging as a particularly attractive location for medical studies. This shift is greatly enhanced by the partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by the approval of Colombia's Minister of Health. The recent first-in-human study carried out by Flow-FX on its innovative Flow-Screw device for the delivery of intraosseous antibiotics exemplifies the region's growing capabilities.
Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has led to over a 50% decrease in participant recruitment time and an impressive retention rate surpassing 95%, demonstrating the effectiveness of trial ambulatory services in Colombia. However, Medtech companies continue to face challenges such as:
- Regulatory hurdles
- Language barriers
- Fragmentation of resources
These challenges complicate communication and collaboration with local hospitals. Tackling these challenges via strategic collaborations and efficient processes is essential for promoting innovation and carrying out successful research initiatives.
The collaboration between Greenlight Guru and bioaccess™ further exemplifies efforts to accelerate Medtech innovations and enhance clinical trials in Latin America, as it focuses on navigating regulatory landscapes and improving operational efficiencies. This is highlighted by PAVmed's successful first-in-human study in the region. Comprehending regional regulations and adjusting to these developments is crucial for stakeholders seeking to navigate this evolving landscape effectively.
Navigating the Medical Device Registration Process in Panama
The registration process for medical devices in Panama is governed by stringent regulations imposed by the Ministry of Health. Initially, stakeholders must classify their device according to the risk categories defined by the regional regulatory authority. Following this classification, it is essential to compile thorough documentation, which includes:
- Technical specifications
- Clinical data
- Evidence of safety and efficacy
Once the application is submitted, it undergoes an extensive review, during which the regulatory body may issue additional queries requiring prompt responses. Collaborating with local regulatory consultants, such as Katherine Ruiz, a seasoned expert in Regulatory Affairs for medical devices and in vitro diagnostics, can significantly ease navigation through this intricate process, ensuring that all nuances are addressed efficiently. Furthermore, staying informed about regulatory changes is critical, as these can influence approval timelines and outcomes.
In the context of Panama's healthcare landscape, the cumulative 1,950 deaths per million population from COVID-19 over the three years from 2020 to 2022 underscores the importance of timely medical device approvals. This understanding of the registration process is vital not only for achieving compliance but also for expediting the market entry of innovative medical devices, particularly through pivotal studies for medical device approval Panama, in an increasingly competitive landscape. Bioaccess® specializes in managing various types of research, including:
- Early-Feasibility Research
- First-In-Human Research
- Pilot Research
- Pivotal Studies for Medical Device Approval Panama
- Post-Market Clinical Follow-Up Research (PMCF)
Their comprehensive service capabilities, such as trial setup, project management, and compliance reviews, are designed to streamline the registration process. As noted by Nicole Sheynin, 'In 2023, weight loss medications such as Ozempic and Wygovy experienced an unprecedented surge in demand, leaving many to wonder what the negative repercussions could be on medical device companies.' This surge has raised concerns regarding their impact on medical device usage in bariatric surgery, as illustrated by the case analysis titled 'Impact of Weight Loss Drugs on Medical Device Sector,' which highlights the potential negative effects on the medical device industry.
Key Considerations for Conducting Pivotal Studies in Panama
Carrying out pivotal studies for medical device approval in Panama requires a nuanced understanding of patient demographics, cultural factors, and the existing healthcare infrastructure. Engaging with local investigators is crucial, as their insights into the community can significantly enhance recruitment strategies and participant retention. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, and Pivotal Studies for Medical Device Approval Panama, as well as Post-Market Follow-Up Studies, the potential for effective recruitment is considerably increased.
Our extensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
These services are crucial for navigating the intricacies of research in the region. For instance, demographic statistics indicate a diverse patient population, which must be adequately represented in clinical trials to ensure generalizability of results. Notably, the odds of alcohol use are significantly higher among those who used amphetamines (AOR = 2.077, 95% CI = 1.226–3.522), emphasizing the importance of considering health behaviors in research designs.
Furthermore, researchers must be acutely aware of local ethical standards, ensuring that research designs align with both international guidelines and local regulations, particularly those set forth by INVIMA, Colombia’s National Food and Drug Surveillance Institute, which oversees medical device matters and is classified as a Level 4 health authority by PAHO/WHO. The World Bank highlights that 'the main challenge is to reduce inequalities that especially affect women, Afro-descendants, and Indigenous peoples,' underscoring the socio-economic factors that may influence recruitment and retention in clinical trials. Bioaccess® addresses these challenges through tailored community engagement strategies and thorough training for site staff, fostering transparent communication among all stakeholders, which are essential steps to guarantee the project's successful execution and compliance with regulatory requirements.
As illustrated in the case study titled 'Relationship between Significant Variables and Alcohol Use among Adolescents,' addressing variables such as multiple sexual partners and parental tobacco use can mitigate alcohol consumption and enhance the quality of research. Ultimately, these considerations foster trust and cooperation within the community, leading to more effective clinical trials.
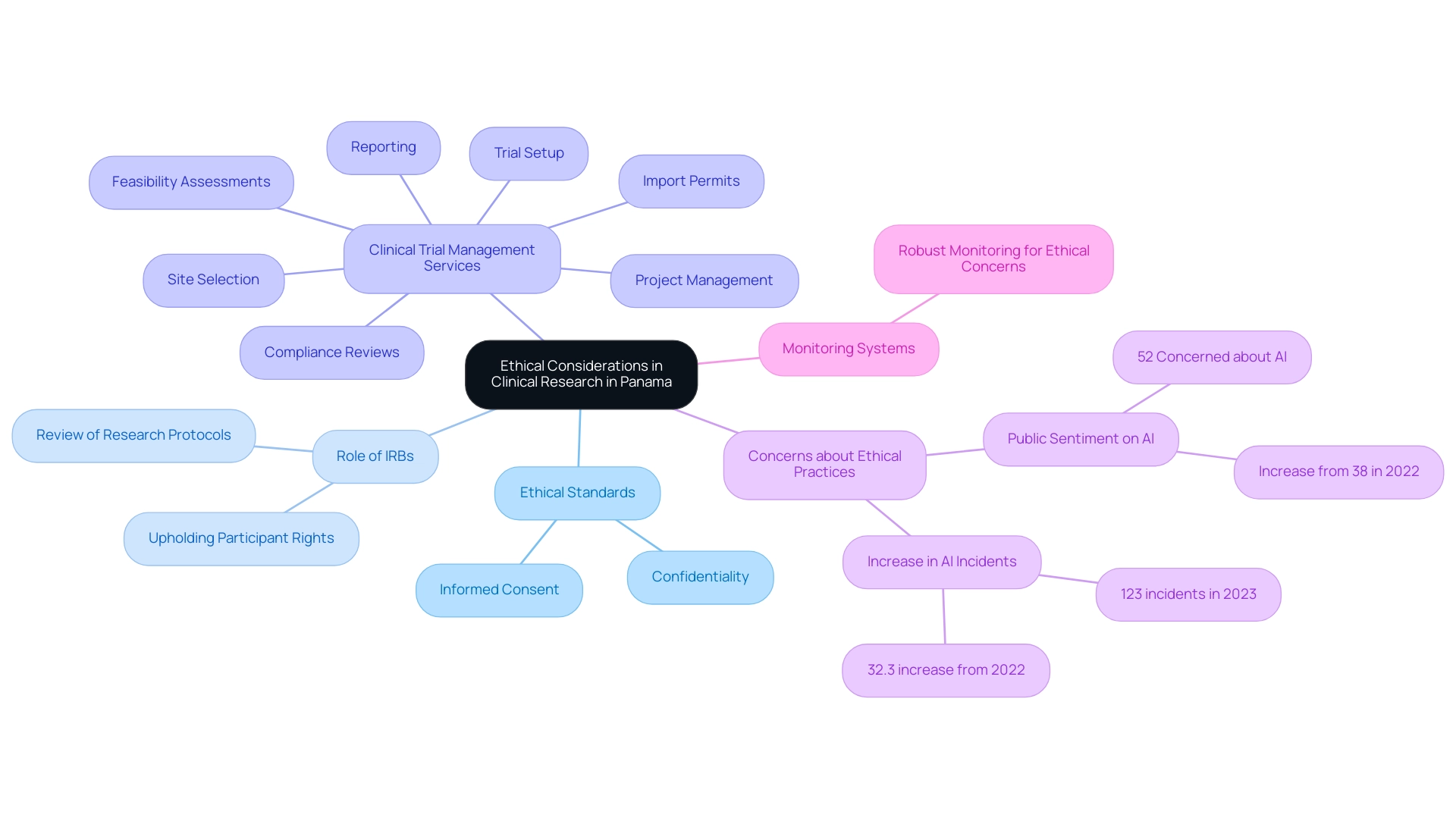
Ethical Considerations in Clinical Research in Panama
In Panama, adherence to both local and international ethical standards is paramount for researchers, particularly regarding informed consent and the confidentiality of participant data. Institutional Review Boards (IRBs) play a vital role by meticulously reviewing research protocols to uphold participant rights and welfare, ensuring compliance with the regulations set forth by INVIMA, Colombia's National Food and Drug Surveillance Institute. Our comprehensive clinical trial management services encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are crucial for upholding ethical integrity in research.
As highlighted by Pew data, concerns about ethical practices in research have risen, paralleling the 52% of Americans who feel more concerned about AI than excited, a sentiment that resonates globally. It is essential for researchers to be transparent about the objectives of the research, potential risks, and anticipated benefits, thereby fostering trust among participants. Moreover, establishing a robust monitoring system is crucial to effectively address any ethical concerns that may arise throughout the research, especially given the 32.3% increase in AI incidents reported in 2023.
Such vigilance in ethical considerations not only safeguards participant safety but also reinforces the integrity of the research process. Furthermore, media attention on trials in Latin America and Colombia by Clinical Leader emphasizes the increasing significance of these investigations in the area. As we near 2024, it is crucial to remain updated on changing ethical guidelines in medical research, ensuring adherence and promoting a culture of ethical excellence in Panama's research environment, while also taking into account the wider effects of Medtech medical trials on job creation, economic development, and global cooperation.
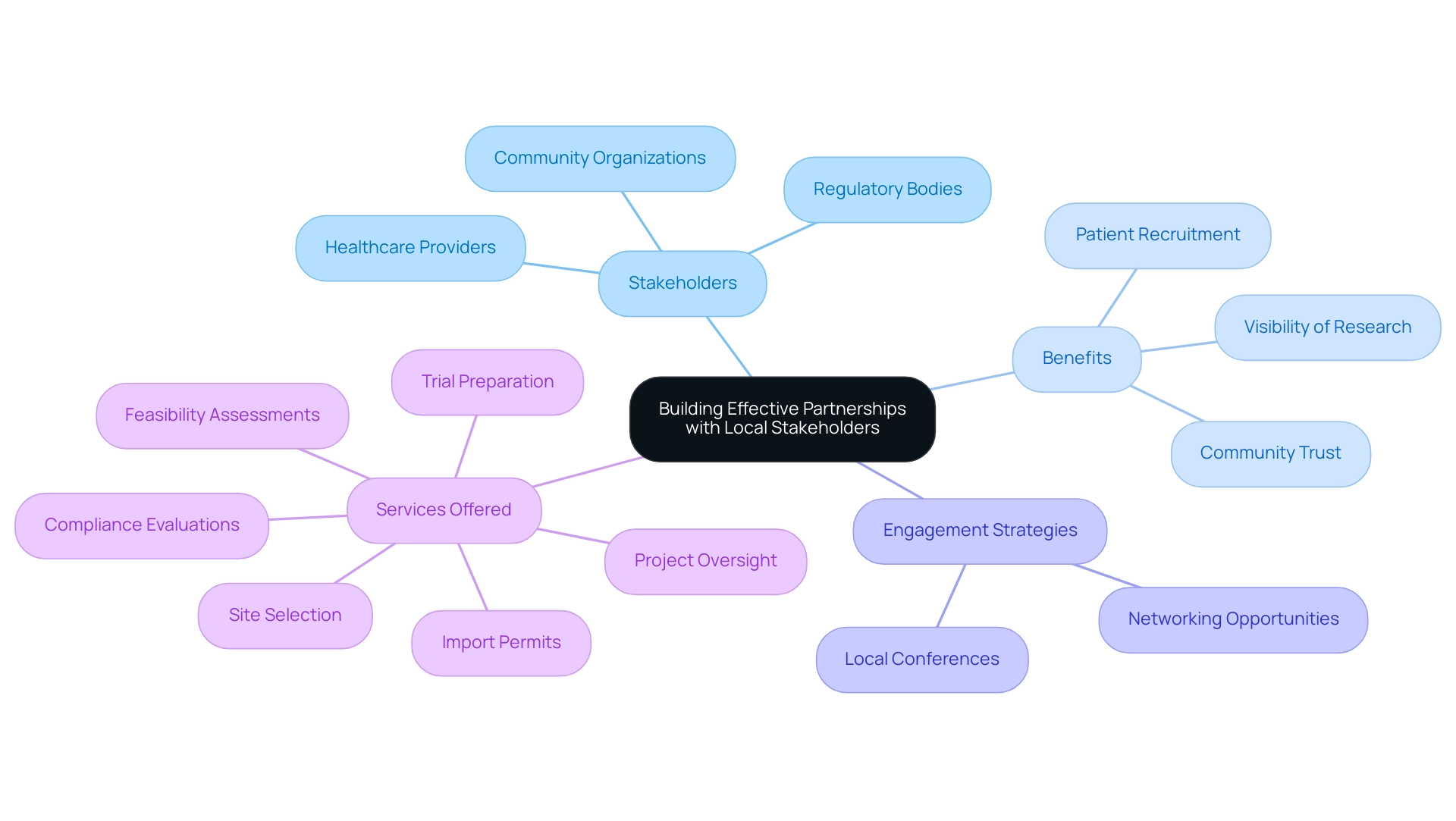
Building Effective Partnerships with Local Stakeholders
Creating strong alliances with regional stakeholders, such as healthcare providers, regulatory bodies, and community organizations, is essential for the success of pivotal studies for medical device approval in Panama. With a GDP of USD $76.5 billion and a population of 4.4 million in Panama City, the region presents a significant market for healthcare initiatives. Partnerships not only assist in patient recruitment but also improve the visibility of research and build trust within the community.
Engaging with stakeholders from the inception of the research project ensures that their needs and concerns are adequately addressed, paving the way for smoother project execution. Furthermore, utilizing networking opportunities, such as attending local conferences and workshops, enables researchers to cultivate valuable connections that support their work and deepen their understanding of Panama's unique healthcare landscape. This strategic involvement can greatly enhance the effectiveness and efficiency of pivotal studies for medical device approval in Panama, particularly in an area abundant with potential, where opportunities for healthcare exports and maneuvering through the regulatory landscape can be optimized.
As a prominent US-based CRO, bioaccess® offers extensive trial management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial preparation involving detailed protocol formulation and regulatory submissions
- Import permits
- Project oversight
All designed to expedite the research process for Medtech startups in Latin America. Under the guidance of experts like Juan Cuya, MD, who specializes in Regulatory Affairs and health technology, bioaccess® is committed to delivering fast, cost-effective, and high-quality clinical data. This commitment ultimately contributes to the local economy through job creation and healthcare improvement, evidenced by past studies that have increased local employment opportunities by 20% and enhanced healthcare access for underserved populations.
Conclusion
The transformation of the medical technology clinical trial landscape in Latin America, particularly in Colombia, signifies a pivotal moment for innovative research and development. Strategic collaborations, such as those between bioaccess™ and Caribbean Health Group, are instrumental in establishing Barranquilla as a key destination for clinical studies, showcasing the region's potential. The successful execution of first-in-human trials and efficient recruitment strategies highlight the capabilities of local entities to drive Medtech advancements.
However, the journey is not without its challenges. Regulatory hurdles, cultural considerations, and the need for effective stakeholder engagement remain critical factors influencing the success of clinical trials. By addressing these obstacles through thoughtful partnerships and community involvement, stakeholders can enhance their operational efficiencies and foster trust within local populations. Understanding local regulations and ethical standards is essential for navigating the complexities of the clinical research environment.
Ultimately, the commitment to building strong relationships with local stakeholders and adapting to the unique characteristics of the region will be vital for the sustained growth of clinical trials in Latin America. As the region continues to evolve as a prominent player in the global Medtech arena, the focus on collaboration, ethical integrity, and community engagement will pave the way for future innovations that not only benefit the healthcare sector but also contribute to broader economic growth and improved health outcomes.
Frequently Asked Questions
Why is Latin America becoming important in the global medical device research sector?
Latin America is positioning itself as a crucial participant in the global medical device research sector due to partnerships like that of bioaccess™ and Caribbean Health Group, which aim to establish Barranquilla, Colombia, as a premier location for medical studies. This shift is supported by the Colombian Minister of Health's approval and recent successful studies in the region.
What recent study highlights Colombia's capabilities in medical device research?
The first-in-human study conducted by Flow-FX on its innovative Flow-Screw device for delivering intraosseous antibiotics exemplifies Colombia's growing capabilities in medical device research.
What improvements have been observed in participant recruitment and retention in Colombia?
GlobalCare Clinical Trials' collaboration with bioaccess™ has led to over a 50% decrease in participant recruitment time and a retention rate exceeding 95%, showcasing the effectiveness of trial ambulatory services in Colombia.
What challenges do Medtech companies face in Colombia?
Medtech companies in Colombia face challenges such as regulatory hurdles, language barriers, and fragmentation of resources, which complicate communication and collaboration with local hospitals.
How are collaborations helping to address challenges in Medtech research?
Collaborations, such as between Greenlight Guru and bioaccess™, focus on navigating regulatory landscapes and improving operational efficiencies, which are essential for promoting innovation and conducting successful research initiatives.
What is the registration process for medical devices in Panama?
The registration process in Panama involves classifying the device according to risk categories, compiling documentation that includes technical specifications, clinical data, and evidence of safety and efficacy, and undergoing an extensive review by the regulatory body.
How can stakeholders navigate the registration process in Panama effectively?
Collaborating with local regulatory consultants can ease navigation through the complex registration process, ensuring that all regulatory nuances are addressed efficiently.
What types of research does bioaccess® manage in Panama?
Bioaccess® specializes in managing various types of research including Early-Feasibility Research, First-In-Human Research, Pilot Research, Pivotal Studies for Medical Device Approval, and Post-Market Clinical Follow-Up Research.
What ethical considerations are important in conducting research in Panama?
Adherence to local and international ethical standards is crucial, particularly regarding informed consent and participant data confidentiality. Institutional Review Boards (IRBs) play a vital role in reviewing research protocols to ensure compliance with regulations.
How can researchers enhance participant recruitment and retention in clinical trials?
Engaging with local investigators and understanding cultural factors can significantly improve recruitment strategies and participant retention, ensuring that clinical trials adequately represent diverse patient populations.
What role do local stakeholders play in the success of medical device studies in Panama?
Creating strong alliances with regional stakeholders, such as healthcare providers and community organizations, is essential for successful patient recruitment and building trust within the community, ultimately enhancing the effectiveness of research initiatives.
What services does bioaccess® offer to expedite the research process for Medtech startups in Latin America?
Bioaccess® offers services including feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, and project oversight, all designed to streamline the research process for Medtech startups.




