Introduction
The medical device approval landscape in Paraguay is undergoing a transformative evolution, characterized by heightened regulatory scrutiny and an increasing emphasis on compliance. As stakeholders prepare to navigate the intricate regulations established by the Ministry of Public Health and Social Welfare, the significance of early feasibility studies has never been more pronounced. These studies not only serve as critical tools for assessing device viability but also play a pivotal role in addressing potential regulatory hurdles before full-scale clinical trials commence.
With notable partnerships driving Medtech innovations in Latin America, understanding the regulatory framework and the advantages of preliminary evaluations becomes essential for companies aiming to thrive in this dynamic market. As Paraguay witnesses a surge in medical device approvals, the intersection of robust compliance, ethical standards, and strategic collaboration emerges as a cornerstone for enhancing healthcare outcomes in the region.
Overview of Paraguay's Medical Device Approval Landscape
The approval environment for medical equipment in Paraguay is increasingly defined by a strict emphasis on adherence to standards and reliability, crucial for pivotal studies for medical device approval Paraguay. In 2024, stakeholders must navigate a complex array of regulations established by the Ministry of Public Health and Social Welfare (MSPBS), which are part of the pivotal studies for medical device approval Paraguay, crucial for ensuring safety and efficacy of the equipment. Preliminary assessments have become crucial components of the approval framework, enabling companies to assess the practicality of their products before large-scale trials and recognize potential regulatory hurdles early in the process.
Significantly, collaborations such as that between Greenlight Guru and bioaccess™ are crucial in speeding up Medtech innovations and trials in Latin America, as shown by:
- PAVmed's first-in-human trial
- ReGelTec's successful early feasibility assessment on HYDRAFIL™ for treating chronic low back pain
- Flow-FX's initial human trial of the Flow-Screw apparatus for intraosseous antibiotic delivery in Colombia
These examples illustrate the practical use of these investigations. By successfully implementing the pivotal studies for medical device approval Paraguay, medical manufacturers can enhance the strength of their market authorization applications, increasing the likelihood of approval.
However, US Medtech companies face significant challenges when entering the Latin American market, including:
- Professionalism
- Language barriers
- Fragmentation of resources
These challenges can hinder effective collaboration with local hospitals. Addressing these challenges is essential for improving patient outcomes, as the collaborations between entities like Greenlight Guru and bioaccess™ not only facilitate regulatory navigation but also enhance the overall quality of clinical trials. Recent updates indicate that the Paraguayan market is evolving, with an increasing number of medical equipment approvals reflecting a commitment to enhancing healthcare standards.
Grasping the nuances of these regulations, the significance of early feasibility analyses, and the intense scrutiny encountered by the medical device sector due to the need for robust compliance and ESG programs is crucial for companies aiming to capitalize on the growing opportunities presented by pivotal studies for medical device approval Paraguay.
Regulatory Framework for Early Feasibility Studies in Paraguay
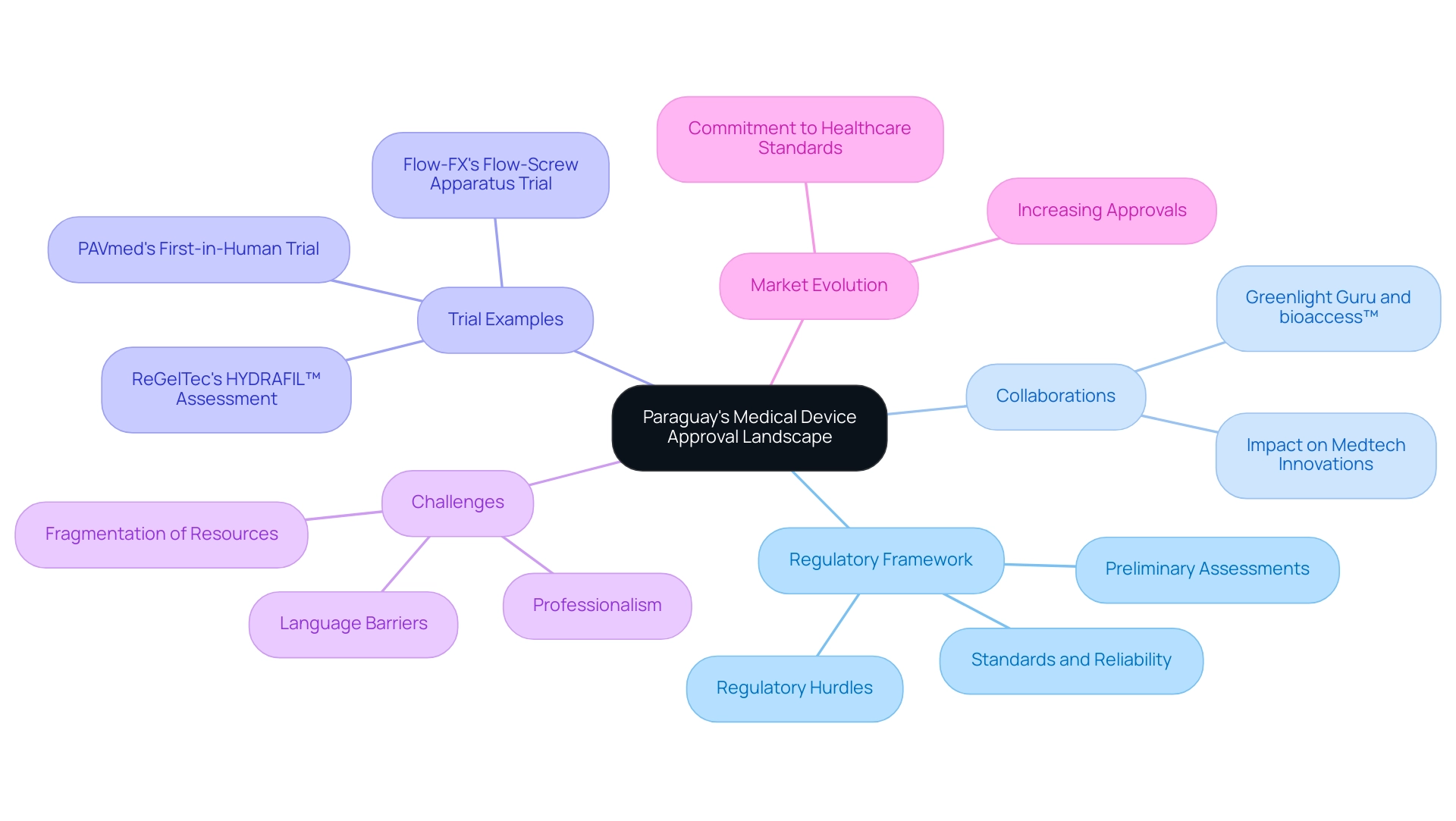
In Paraguay, pivotal studies for medical device approval are critical for navigating the regulatory framework for early feasibility assessments, primarily governed by the Ministry of Public Health and Social Welfare (MSPBS). Companies seeking to initiate research must submit a comprehensive protocol detailing the objectives, methodologies, and adherence to ethical standards. Notably, approval from an ethics committee is mandatory before proceeding with pivotal studies for medical device approval, ensuring that participant welfare is prioritized and ethical guidelines are followed.
Adherence to Good Clinical Practice (GCP) is paramount, as these regulations form the foundation for conducting ethical research. Furthermore, companies must maintain vigilance concerning the reporting requirements for adverse events that may arise during the research. Transparency throughout the research process is essential for regulatory compliance and fostering stakeholder trust.
Recent insights from Elizabeth Barrios, Director of Research and Strategic Studies at the Ministry of Health, highlight the ongoing evolution of these processes, particularly in the context of pivotal studies for medical device approval in Paraguay, especially regarding the establishment of robust ethical standards for clinical trials. Barrios emphasized the process leading to the National Health Research Ethics Policy, which is crucial for pivotal studies for medical device approval and reflects the importance of thorough ethical oversight. Moreover, a recent investigation demonstrated an AUC of 0.92 for the plasma host signature differentiating PTB patients from those cured of TB, with a sensitivity of 94% and specificity of 79%.
This quantitative evidence highlights the critical role of compliance in monitoring tuberculosis treatment effectively. Furthermore, the case analysis titled 'Characterization of Immune Responses in PTB Patients' illustrates the practical implications of these regulations, showing how C1q and IL-13 levels could serve as promising biomarkers for TB treatment monitoring, thereby demonstrating the intersection of regulatory frameworks and medical research. At bioaccess®, we provide extensive clinical trial management services, including pivotal studies for medical device approval, feasibility assessments, selection of research sites, compliance reviews, trial setup, import permits, project management, and reporting.
With over 20 years of experience in Medtech, our team is equipped with the expertise needed to navigate these complex processes effectively.
Advantages of Early Feasibility Studies for Medical Device Companies
Preliminary feasibility assessments are essential for medical equipment firms, providing numerous benefits that greatly influence project success. One primary benefit is their ability to facilitate early risk assessment, allowing organizations to identify potential challenges related to functionality and regulatory compliance at the outset. This proactive approach is supported by resources such as ISO 14971 and ISO 10993-1, which guide effective risk assessment methodologies.
Notably, probability values for risk assessments can range from as low as 1 x 10^-6 to as high as 100%, emphasizing the critical nature of these evaluations. Additionally, these research efforts help to strengthen investor assurance; by showcasing initial data that illustrates the product's feasibility, companies can more efficiently secure financing. Research indicates that the results of pivotal studies for medical device approval in Paraguay positively influence investor confidence statistics in medical device funding in regions like Paraguay.
Additionally, early feasibility assessments simplify the subsequent phases of clinical trials, a process expertly managed by bioaccess®. With more than 20 years of experience in the Medtech sector, bioaccess® not only emphasizes early feasibility assessments but also excels in managing:
- First-In-Human Trials
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Trials
By refining research designs and enhancing regulatory submissions through insights gained during the feasibility phase, organizations can navigate the complex regulatory landscape more efficiently.
Our services include:
- Feasibility and selection of research sites and principal investigators
- Compliance reviews
- Trial setup
- Project management
Techniques such as HAZOP, FMEA, and FTA are increasingly being employed during design reviews to evaluate human errors and potential hazards, further supporting this refinement process. As Ganesh Kumar Palanichamy, a Senior Project Engineer, aptly noted, '(Design Of Things to Come: Medical equipment companies weave new business models to provide value-added services,' which highlights the innovative shifts within the industry and aligns with the objectives of early feasibility assessments.
Ultimately, these assessments represent a cost-effective strategy for validating concepts before committing to larger-scale trials. The rationale behind clarifying aspects such as extraction ratios, as discussed in the case analysis titled 'Understanding Extraction Ratios,' illustrates the complexity involved in biocompatibility assessments and the importance of thorough preliminary evaluations. This case analysis highlights that comprehending extraction ratios is not simple and is crucial for precise biocompatibility evaluations in medical equipment assessments.
The successful outcomes from recent early feasibility studies underscore their critical role in ensuring not only regulatory compliance but also the overall success of medical device innovations, which are essential components of the Pivotal Studies for Medical Device Approval in Paraguay.
Key Regulations and Documentation for Clinical Trials
Conducting pivotal studies for medical device approval Paraguay entails strict adherence to key regulations and meticulous documentation, supported by comprehensive trial management services. Recent statistics indicate a 15% increase in Clinical Trial Applications submitted in Paraguay over the past year, highlighting the role of pivotal studies for medical device approval Paraguay and reflecting a growing interest in clinical research within the region. Central to this process is the preparation of a Clinical Trial Application (CTA), which must encompass comprehensive research protocols, investigator brochures, and informed consent forms.
A recent case study involving a multinational pharmaceutical company demonstrated the critical importance of compliance with local legislation concerning patient privacy and data protection, thereby safeguarding participants' rights throughout the study. Furthermore, the documentation must include:
- Robust safety monitoring plans
- Clearly defined reporting mechanisms for any adverse events
- Thorough procedures for obtaining import permits for investigational devices
Notably, the key regulations for clinical trials in Paraguay, particularly those related to pivotal studies for medical device approval Paraguay, have been updated for 2024 to include stricter guidelines on data management and participant recruitment.
Given the frequent audits and inspections carried out by oversight bodies, it is crucial for organizations to maintain precise records and uphold the highest ethical standards throughout the trial process. This diligent approach not only ensures regulatory compliance but also fosters trust and integrity within the research community, contributing positively to local economies by creating jobs, promoting healthcare improvements, and enhancing international collaboration in Medtech. The expertise of our team members, including Trial Manager Oswaldo Amaya and Trial Associate Juan Cuya, further strengthens our commitment to navigating the complexities of trials effectively.
Future Trends in Paraguay's Clinical Research for Medical Devices
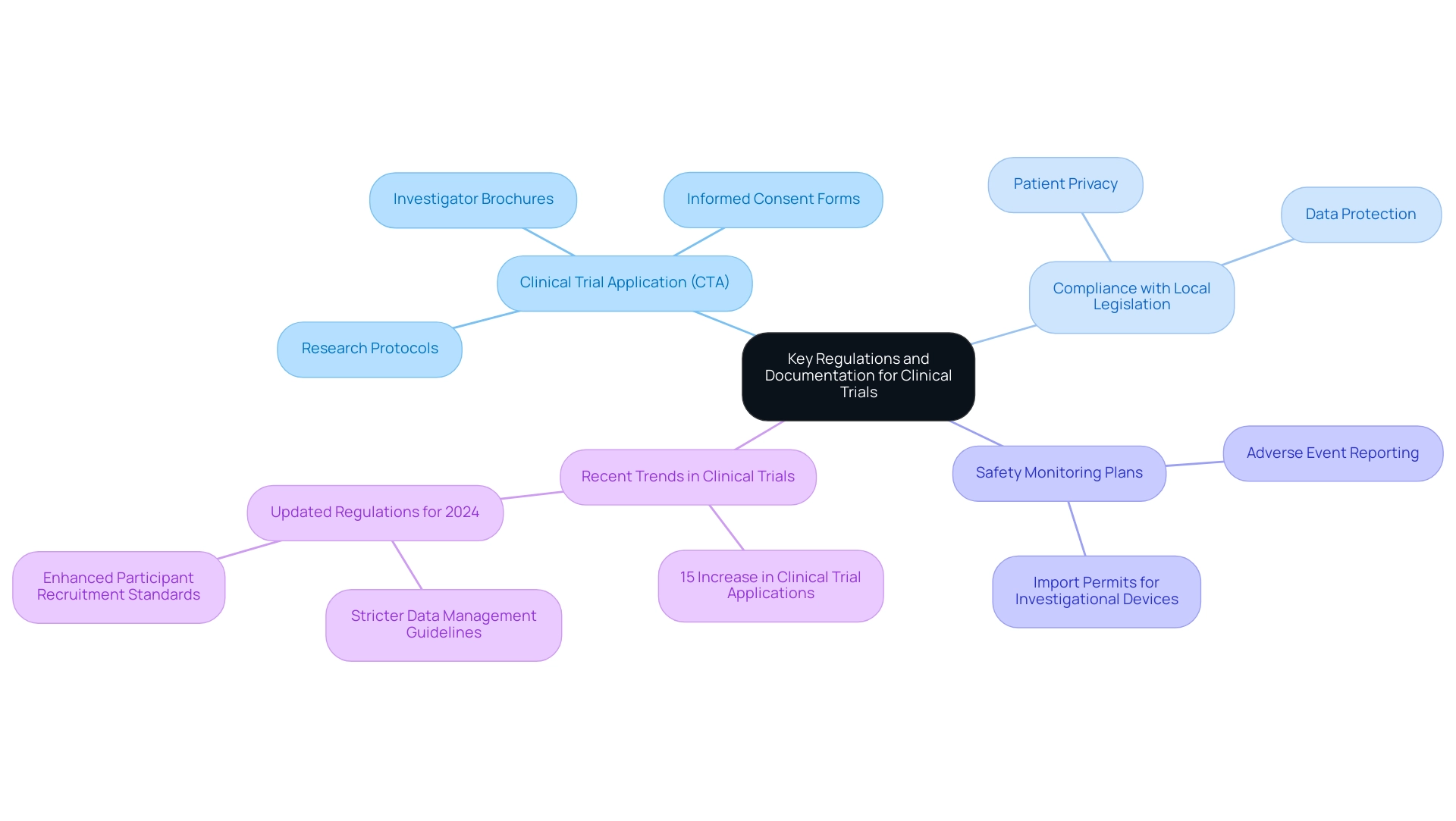
The trajectory of clinical research for medical devices in Paraguay is poised to be significantly influenced by several emerging trends, including pivotal studies for medical device approval in Paraguay. A key development is the anticipated increase in collaboration between oversight authorities and industry stakeholders, likely resulting in pivotal studies for medical device approval in Paraguay and creating a more streamlined approval process. This collaboration will promote innovation while ensuring patient safety remains a top priority, as highlighted by BBC’s Emily Maitlis, who emphasized that clear rules are essential in any regulatory framework.
Moreover, the services provided by bioaccess®, including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Trials (FIH)
- Pilot tests
- Pivotal trials
- Post-market follow-up assessments (PMCF)
- Feasibility evaluations
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Comprehensive project management
will be crucial in navigating these changes associated with pivotal studies for medical device approval in Paraguay. With over 20 years of experience in Medtech, bioaccess® brings a wealth of expertise to the table. Advancements in digital health technology and the rise of telemedicine are expected to transform clinical trial designs.
A survey of 234 digital leaders from 43 nations indicates a growing awareness of the challenges and opportunities presented by these technologies, which facilitate more flexible recruitment strategies and enhance monitoring capabilities, thereby improving patient engagement throughout the research process. As the regulatory framework continues to evolve, it is imperative for companies to remain vigilant regarding potential guideline changes and to actively incorporate new technologies that can refine their research methodologies. Moreover, the increasing focus on patient-centered research is poised to promote the adoption of more inclusive study designs, aligning research practices more closely with patient needs and expectations.
Drawing parallels with the New York Times' strategic investment in audio storytelling, clinical research in Paraguay can similarly benefit from investments in technology to enhance methodologies and patient engagement.
Conclusion
The evolving landscape of medical device approval in Paraguay underscores the critical role of regulatory compliance and the importance of early feasibility studies. As companies navigate the complexities established by the Ministry of Public Health and Social Welfare, these studies emerge as vital tools for assessing device viability and identifying potential regulatory challenges. The increasing number of medical device approvals in the country reflects a commitment to enhancing healthcare standards, driven by strategic collaborations and a focus on ethical practices.
Understanding the regulatory framework is essential for stakeholders aiming to successfully launch medical devices in this dynamic market. The rigorous protocols governing early feasibility studies ensure participant welfare and adherence to ethical standards, thereby fostering trust among stakeholders. Moreover, the proactive approach of conducting early feasibility studies not only mitigates risks but also enhances investor confidence, ultimately leading to more robust market authorization applications.
Looking ahead, the anticipated collaboration between regulatory authorities and industry stakeholders suggests a more streamlined approval process, fostering innovation while prioritizing patient safety. As advancements in digital health technologies and patient-centered research methodologies gain traction, medical device companies must remain agile, adapting to evolving regulations and leveraging new tools for improved clinical trial designs. The future of clinical research in Paraguay is bright, with the potential to significantly enhance healthcare outcomes through strategic compliance and innovation.
Frequently Asked Questions
What is the approval environment for medical equipment in Paraguay?
The approval environment in Paraguay emphasizes strict adherence to standards and reliability, governed by regulations established by the Ministry of Public Health and Social Welfare (MSPBS). This is crucial for pivotal studies necessary for medical device approval, ensuring the safety and efficacy of medical equipment.
What are pivotal studies for medical device approval in Paraguay?
Pivotal studies are essential evaluations that companies must conduct to demonstrate the safety and effectiveness of their medical devices. These studies involve submitting comprehensive protocols and obtaining ethics committee approval to prioritize participant welfare and adhere to ethical guidelines.
What role do preliminary assessments play in the approval process?
Preliminary assessments are critical as they allow companies to evaluate the practicality of their products before large-scale trials. This helps identify potential regulatory hurdles early, facilitating more efficient navigation of the approval process.
What challenges do US Medtech companies face in the Latin American market?
US Medtech companies encounter challenges such as professionalism, language barriers, and fragmentation of resources, which can hinder effective collaboration with local hospitals and impact patient outcomes.
How are collaborations influencing Medtech innovations in Paraguay?
Collaborations, such as those between Greenlight Guru and bioaccess™, are essential for accelerating Medtech innovations and trials in Latin America, enhancing the quality of clinical trials and regulatory navigation.
What are the key regulations for conducting clinical trials in Paraguay?
Key regulations include adherence to Good Clinical Practice (GCP), compliance with local legislation regarding patient privacy, and maintaining robust safety monitoring plans and reporting mechanisms for adverse events.
What benefits do early feasibility assessments provide?
Early feasibility assessments help in risk assessment, strengthen investor confidence by showcasing product feasibility, and simplify subsequent phases of clinical trials. They are a cost-effective strategy for validating concepts before larger-scale trials.
What documentation is required for Clinical Trial Applications (CTA) in Paraguay?
A CTA must include comprehensive research protocols, investigator brochures, informed consent forms, safety monitoring plans, and detailed reporting mechanisms for adverse events.
How is the regulatory landscape for medical devices in Paraguay evolving?
The Paraguayan regulatory landscape is evolving with stricter guidelines on data management and participant recruitment, as well as an increase in collaboration between oversight authorities and industry stakeholders to streamline the approval process.
What services does bioaccess® provide to support medical device approval?
Bioaccess® offers a range of services including early-feasibility assessments, first-in-human trials, pilot tests, pivotal trials, post-market follow-up assessments, site selection, compliance reviews, trial setup, and comprehensive project management.




