Overview
The article focuses on the importance of pivotal studies in the medical device approval process in Peru, emphasizing their role in demonstrating safety and efficacy to meet regulatory standards. It highlights how these studies, characterized by rigorous methodologies and compliance with Good Clinical Practice guidelines, enhance transparency, improve patient safety, and facilitate successful market entry for medical devices in a rapidly evolving regulatory environment.
Introduction
The approval process for medical devices is a critical aspect of ensuring patient safety and efficacy in healthcare. Pivotal studies serve as the backbone of this process, providing essential data that regulators rely on to make informed decisions.
In Peru, where the medical device landscape is rapidly evolving, understanding the intricacies of these studies and the regulatory framework is paramount for stakeholders. As technological advancements reshape the industry, the importance of compliance with established standards becomes increasingly clear.
This article delves into the pivotal role of clinical trials, the regulatory environment in Peru, and the future trends that are set to influence medical device approval, highlighting the necessity for strategic navigation through these complex waters.
The Role of Pivotal Studies in Medical Device Approval
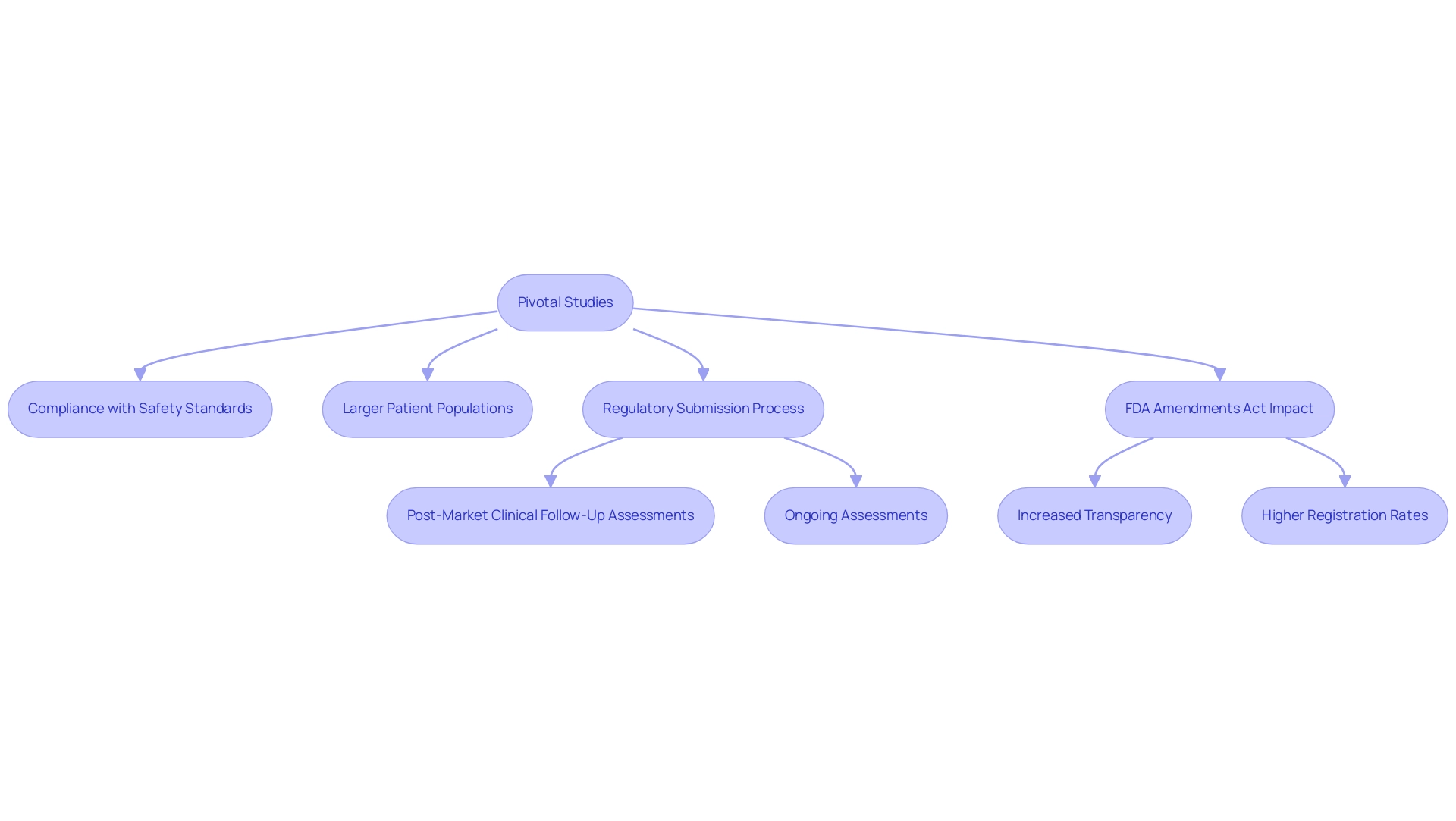
Essential research contributes significantly to offering conclusive proof concerning the safety and efficacy of medical products before market authorization. Characterized by larger patient populations and adherence to rigorous regulatory requirements, this research is fundamental to the regulatory submission process in Peru, acting as Pivotal Studies for Medical Device Approval Peru that demonstrate compliance with established safety and performance standards. Significantly, progress in vascular medicine was highlighted recently when Dr. Jorge Hernando Ulloa presented one-year data from the first-in-human VenoValve® trial at the prestigious Charing Cross International Symposium, emphasizing the importance of such research in the medical equipment sector.
Moreover, the FDA Amendments Act (FDAAA) has notably enhanced transparency in the registration and reporting of clinical studies, particularly for high-risk medical equipment. This includes a requirement for at least one post-approval examination for 11 instruments, illustrating the oversight emphasis on ongoing assessment and safety assurance. The case analysis titled 'Trial Registration, Result Reporting, and Publication Rates' highlights this shift, showing that post-FDAAA experiments exhibited significantly higher rates of registration, result reporting, and publication compared to pre-FDAAA experiments, thereby indicating improved transparency.
This regulatory framework highlights the significance of pivotal studies for medical device approval in Peru for stakeholders in the medical equipment sector, as they not only aid in successful product approvals but also guarantee improved patient safety. Furthermore, the latest analyses indicate a marked improvement in trial registration and result reporting rates following the FDAAA, reflecting a greater commitment to transparency and accountability in clinical research. As James L Johnston notes, 'As a library, NLM provides access to scientific literature.
Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.' This viewpoint highlights the crucial importance of pivotal studies for medical device approval Peru in developing market entry strategies and enhancing the integrity of the medical product approval process. bioaccess® focuses on overseeing different kinds of research, including Early-Feasibility Assessments, First-In-Human Trials, Pilot Trials, and Pivotal Studies for Medical Device Approval Peru, along with Post-Market Clinical Follow-Up Assessments, ensuring adherence to the changing compliance framework.
Navigating Peru's Medical Device Regulatory Framework
In Peru, the oversight for medical devices is managed by the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID), which plays a crucial role in establishing comprehensive guidelines for medical device approval. These guidelines mandate that manufacturers prepare and submit thorough documentation, including:
- Clinical data
- Risk assessments
- Detailed design specifications
particularly for pivotal studies for medical device approval in Peru. Recent reports suggest that the expanding healthcare infrastructure in Peru is driving demand for advanced diagnostic equipment, necessitating efficient navigation of these compliance pathways.
Notably, statistics reveal that 60% of the 10 most commonly used mHealth apps in Peru did not mention the source of the information they provided, highlighting the importance of compliance and credibility in medical applications. Furthermore, as stated in Law No. 29459, any computer application that declares any of these functions as intended use will be considered a medical device, underscoring the need for manufacturers to adhere strictly to compliance standards.
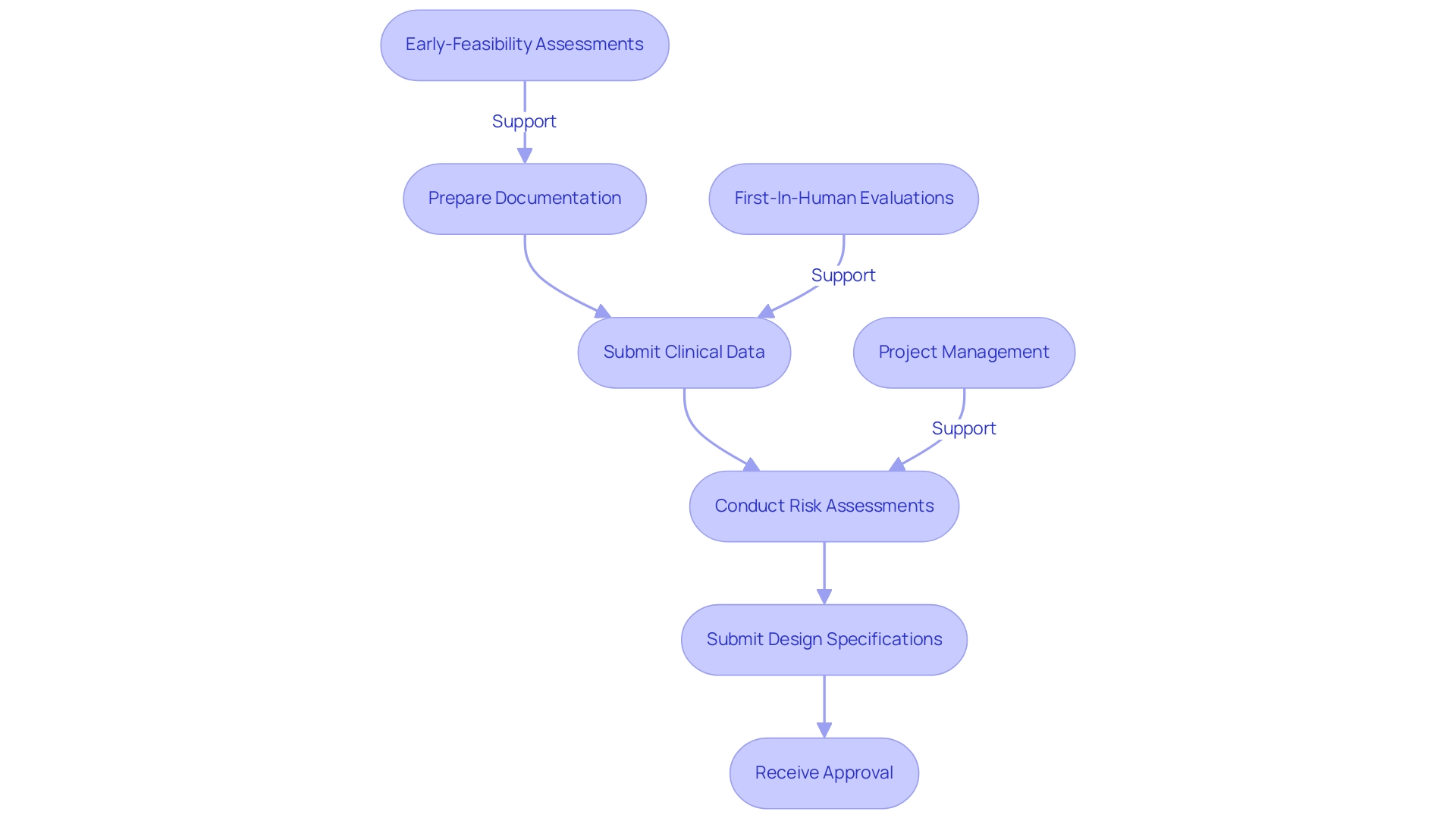
Additionally, Peru's collaboration within the Pacific Alliance with Chile, Colombia, and Mexico fosters a more internationally open, business-friendly, competitive, and productive economy, influencing the regulatory environment. By utilizing bioaccess®'s expertise in comprehensive clinical research management services—including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Evaluations (FIH)
- Feasibility analyses
- Site selection
- Project setup
- Project management
stakeholders can navigate DIGEMID's regulations effectively, facilitating smoother approval processes and significantly reducing the risks of delays or rejections, thereby enhancing the overall efficiency of their clinical research.
Understanding Pivotal Clinical Trials for Medical Devices
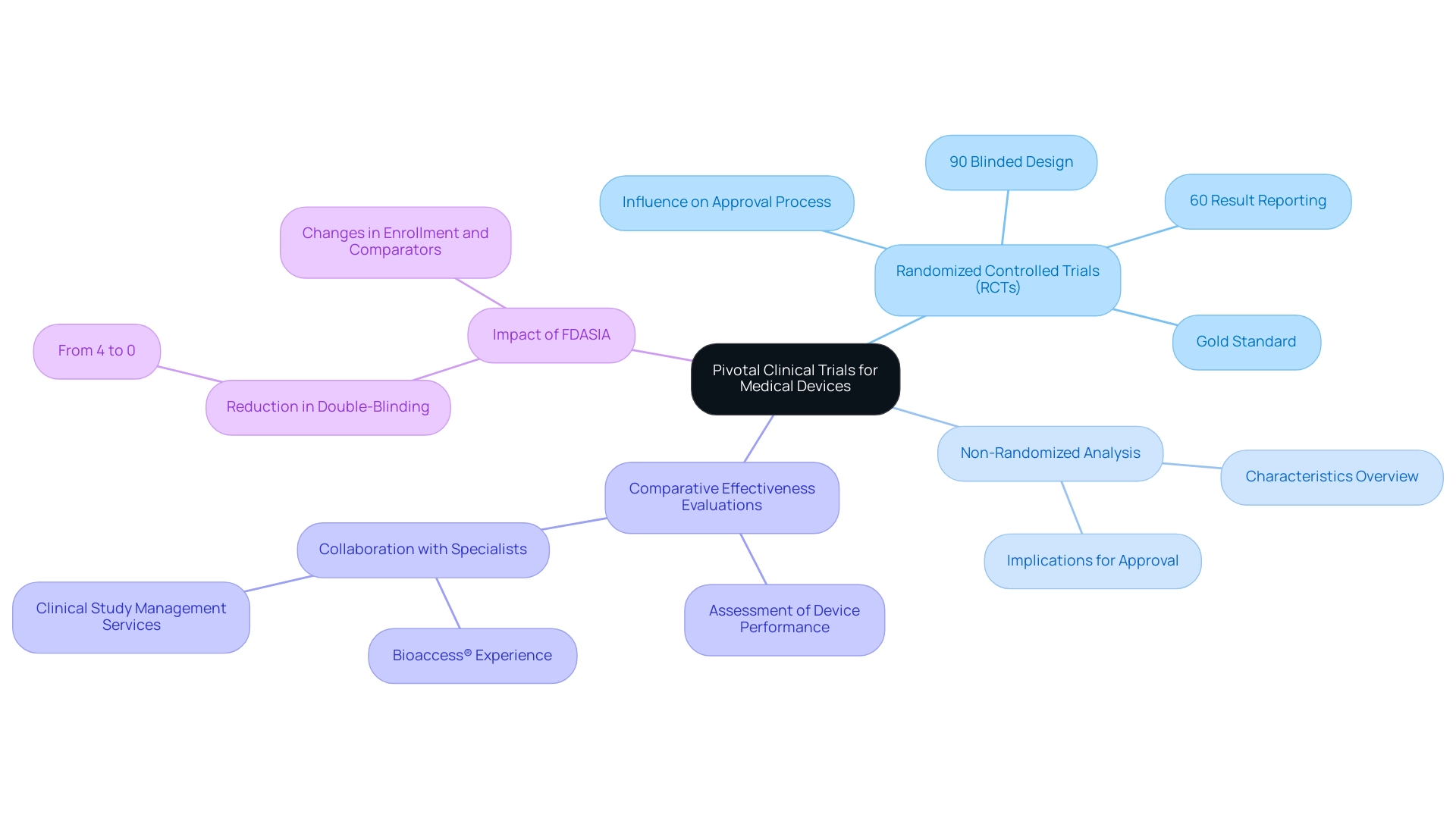
Pivotal Studies for [[[[[[[Medical Device Approval Peru](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide)](https://blog.bioaccessla.com/how-to-conduct-pivotal-studies-for-medical-device-approval-in-colombia-a-comprehensive-guide) include various classifications, such as randomized controlled trials (RCTs), non-randomized analysis, and comparative effectiveness evaluations. Each type plays a crucial role in assessing the performance of medical devices. Among these, RCTs are widely recognized as the gold standard due to their ability to minimize bias and yield robust data on both safety and efficacy.
Recent data indicates that 43 studies, or 90%, utilized a blinded design, with 26 of these (60%) successfully reporting their results. Such rigorous design is essential for researchers and manufacturers alike, as it ensures adherence to standards while delivering clear and actionable outcomes that can significantly influence pivotal studies for medical device approval in Peru. According to Meri Beckwith, a prominent co-founder in the field, understanding the nature of data aids in choosing the suitable statistical methods for analysis and ensuring the validity and reliability of results.
This understanding not only prepares stakeholders for the complexities of the approval process but also enhances the overall integrity of clinical evaluations. Moreover, pivotal studies for medical device approval in Peru should guide upcoming policy and oversight initiatives to ensure clarity and impartial documentation of clinical evaluations for high-risk medical instruments. For example, a case examination assessing the effect of the Food and Drug Administration Safety and Innovation Act (FDASIA) on endovascular device assessments revealed significant alterations in design and results.
Specifically, it was found that FDASIA reduced masking requirements, leading to a decrease in double-blinding from 4% to 0%, while also affecting enrollment and comparator usage. By thoroughly understanding the various kinds of pivotal studies for medical device approval Peru, stakeholders can equip themselves to maneuver through the regulatory environment efficiently and enhance their research designs. In Latin America, collaborating with specialists like bioaccess®, who possess over 20 years of experience in Medtech, can further enhance study efficiency through comprehensive clinical study management services, including feasibility assessments, site selection, compliance reviews, and project management.
Their flexibility and specialized knowledge ensure that assessments are customized to meet specific needs, maximizing the chances of success. For compliance purposes, please refer to the document's docket number: FDA-2011-D-0567.
Ensuring Compliance with Regulatory Standards
Ensuring compliance with regulatory standards necessitates a rigorous adherence to Good Clinical Practice (GCP) guidelines, which are fundamental to the ethical conduct of clinical studies. This involves:
- Obtaining informed consent from participants
- Maintaining meticulous and complete records
- Ensuring that research is executed ethically
Direct contact with study subjects or their guardians requires careful consideration of privacy and confidentiality, as outlined in GCP and clinical research requirements; ethics committees must review and approve such contacts.
Manufacturers must conduct comprehensive risk assessments and report any adverse events, both serious and non-serious, that arise during the trials, as mandated by GCP. Notably, a well-structured monitoring plan should be developed in accordance with ICH-GCP (R2) §5.18.7, reinforcing the commitment to quality assurance. Compliance not only streamlines the approval process for medical devices, including pivotal studies for medical device approval in Peru, but also fosters trust among stakeholders and the public, ultimately leading to improved patient outcomes.
By incorporating compliance into the research culture, organizations can significantly improve the quality and reliability of their essential investigations, thereby aligning with the ethical standards expected in the industry. Furthermore, the clinical research report must detail the use of productivity applications and any specific measures taken for quality assurance. As underscored by industry experts, 'The contract should permit the contract giver to audit outsourced activities performed by the contract acceptor or his mutually agreed subcontractors,' highlighting the need for transparency and accountability in all phases of clinical research.
Comprehensive clinical trial management services offered by bioaccess® include:
- Feasibility studies
- Site selection
- Trial setup
- Import permits
- Project management
- Reporting of serious and non-serious adverse events
All directed by experts like Katherine Ruiz, ensuring successful compliance and innovation in Latin America, particularly for pivotal studies for medical device approval in Peru.

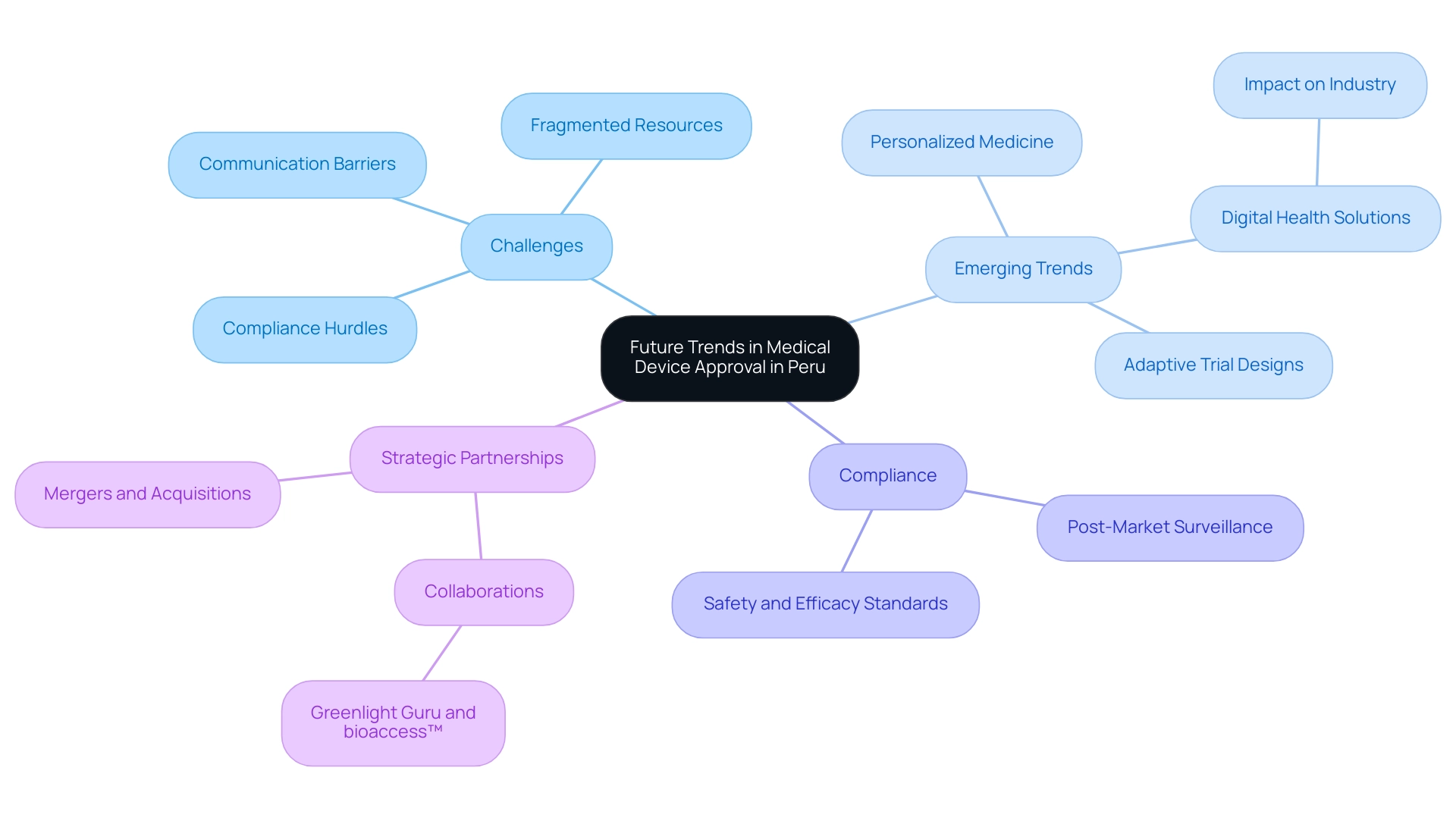
Future Trends in Medical Device Approval in Peru
The medical device approval environment in Peru is set for significant change, propelled by technological advancements and pivotal studies for medical device approval Peru. However, Medtech companies encounter multifaceted challenges, including compliance hurdles, fragmented resources, and significant communication barriers with Latin American hospitals. These issues necessitate a solution-driven approach to foster better collaboration.
Analysts note that trends such as the integration of digital health solutions and the adoption of adaptive trial designs are increasingly prevalent, enhancing certainty for companies and investors alike. A recent survey of Medtech leaders revealed that 63% believe digital therapeutics will significantly impact the industry over the next decade, underscoring the importance of these advancements. As the regulatory landscape changes, it is vital for stakeholders, including experts like Katherine Ruiz in Regulatory Affairs for medical equipment, to remain informed and adjust their strategies accordingly.
The growing focus on post-market surveillance is reshaping compliance expectations, ensuring that devices meet safety and efficacy standards even after reaching the market. Furthermore, the rise of personalized medicine poses a pivotal moment for the design and execution of pivotal studies, pushing the industry towards more patient-centered methodologies. As quoted by EY, 'At EY, our purpose is building a better working world,' reflecting the industry's commitment to creating long-term value through innovation and regulatory practices.
Additionally, the evolving landscape encourages strategic partnerships and collaborations, such as between Greenlight Guru and bioaccess™, to accelerate Medtech innovations and clinical trials in Latin America. By proactively engaging with these emerging trends and addressing the challenges of communication and professionalism, researchers and manufacturers can navigate the complexities of pivotal studies for medical device approval in Peru in 2024 and beyond.
Conclusion
The approval process for medical devices in Peru is intricately tied to the outcomes of pivotal studies, which serve as a cornerstone for demonstrating safety and efficacy. These studies, characterized by their rigorous methodologies and comprehensive data collection, are essential for navigating the regulatory landscape governed by DIGEMID. As highlighted, the increasing transparency mandated by regulations such as the FDAAA has elevated the importance of clinical trials, ensuring that stakeholders not only comply with safety standards but also contribute to enhanced patient outcomes.
Furthermore, understanding the nuances of pivotal clinical trials, including the various study designs and compliance requirements, equips manufacturers and researchers with the tools necessary to optimize their submission strategies. The emphasis on Good Clinical Practice (GCP) underscores the ethical obligations inherent in clinical research, reinforcing the need for meticulous documentation and adherence to regulatory expectations.
As the medical device sector in Peru evolves, emerging trends such as digital health solutions and personalized medicine present both challenges and opportunities. Stakeholders must remain vigilant and adaptable, leveraging strategic partnerships and innovative approaches to overcome potential barriers in communication and regulatory compliance. By embracing these shifts and prioritizing compliance, the future of medical device approval in Peru can foster a more efficient and patient-centered healthcare landscape.
Frequently Asked Questions
What is the role of essential research in medical product approval in Peru?
Essential research is crucial for providing conclusive proof of the safety and efficacy of medical products before market authorization. It involves larger patient populations and strict regulatory compliance, serving as pivotal studies for medical device approval in Peru.
What recent advancements were highlighted in vascular medicine?
Dr. Jorge Hernando Ulloa presented one-year data from the first-in-human VenoValve® trial at the Charing Cross International Symposium, showcasing significant progress in vascular medicine and the importance of research in the medical equipment sector.
How has the FDA Amendments Act (FDAAA) impacted clinical study transparency?
The FDAAA has improved transparency in the registration and reporting of clinical studies, especially for high-risk medical equipment, requiring at least one post-approval examination for 11 instruments, thus enhancing oversight and safety assurance.
What improvements have been observed in clinical trial registration and reporting since the FDAAA?
Post-FDAAA experiments have shown significantly higher rates of registration, result reporting, and publication compared to pre-FDAAA experiments, indicating a shift towards improved transparency in clinical research.
What is the significance of pivotal studies for medical device approval in Peru?
Pivotal studies are essential for successful product approvals and ensuring improved patient safety, as they help stakeholders navigate the regulatory framework effectively.
What documentation is required for medical device approval in Peru?
Manufacturers must submit thorough documentation, including clinical data, risk assessments, and detailed design specifications, particularly for pivotal studies.
What recent trends are driving the demand for advanced diagnostic equipment in Peru?
The expanding healthcare infrastructure in Peru is increasing the demand for advanced diagnostic equipment, necessitating efficient navigation of compliance pathways.
What is the importance of compliance in mHealth apps in Peru?
Statistics show that 60% of the most commonly used mHealth apps in Peru did not disclose their information sources, highlighting the need for compliance and credibility in medical applications.
How does the Pacific Alliance influence regulatory practices in Peru?
Peru's collaboration within the Pacific Alliance with Chile, Colombia, and Mexico promotes a more open and competitive economy, positively impacting the regulatory environment for medical device approval.
What services does bioaccess® provide to help navigate DIGEMID regulations?
Bioaccess® offers comprehensive clinical research management services, including Early-Feasibility Assessments, First-In-Human Evaluations, feasibility analyses, site selection, project setup, and project management, to facilitate smoother approval processes.




