Introduction
Parts Manufacturer Approval (PMA) is a critical certification issued by the Federal Aviation Administration (FAA) and the Food and Drug Administration (FDA) that ensures the safety, quality, and efficacy of aircraft parts and medical devices, respectively. In the aviation industry, PMA allows manufacturers to produce and sell aircraft parts as alternatives to those made by original equipment manufacturers (OEMs). Similarly, in the healthcare industry, PMA is essential for the assessment and authorization of certain medical devices before they can enter the market.
With over 10,000 types of medical devices acknowledged by the World Health Organization, the FDA's PMA process ensures that each device meets stringent safety and efficacy standards. This article explores the history and evolution of PMA, the methods of obtaining PMA approval, the role of regulatory bodies, the types of PMA approvals, the benefits of PMA parts, the importance of traceability and documentation, and the impact of PMA on business and healthcare. By adhering to rigorous standards and regulations, PMA plays a pivotal role in ensuring safety, innovation, and quality in both industries.
What is Parts Manufacturer Approval (PMA)?
Parts Manufacturer Approval (PMA) is a critical certification issued by the Federal Aviation Administration (FAA) that empowers manufacturers to produce and sell aircraft parts as suitable alternatives to those made by the original equipment manufacturers (OEMs). In the complex and multifaceted realm of healthcare, PMA takes on a different meaning as Pre-Market Approval. Under the purview of the Food and Drug Administration (FDA), this rigorous process is essential for the assessment and authorization of certain medical devices before they can enter the market.
Medical devices span a vast array of applications, from the simplicity of spectacles and bandages to the intricacies of MRI machines and pacemakers. The World Health Organization acknowledges the existence of over 10,000 distinct types of medical devices. This immense variety is attributed to human factors diversity—acknowledging the uniqueness of individual needs—and device factors diversity, which encompasses the mechanical nature and the variety of effects these devices can have on the human body.
These technologies leverage a wide spectrum of scientific and engineering disciplines, including materials science, bioengineering, electronics, and information technology. Given this diversity, the FDA's PMA process is designed to ensure that each medical device meets stringent safety and efficacy standards before reaching consumers.
On the aviation side, recent scrutiny of Boeing's manufacturing compliance has prompted the FAA to expand oversight and enforce stringent quality controls. The FAA's review process has included thorough document examinations and interviews, underscoring the importance of traceability and compliance in the production of aircraft parts. This increased oversight parallels the rigorous PMA standards in healthcare, ensuring safety and reliability are paramount.
In both industries, the concept of 'production' plays a pivotal role. Whether it's an aircraft owner managing the production of a part or a medical device manufacturer navigating the PMA process, the emphasis is on meeting established requirements, design specifications, and ensuring that each component conforms to the highest standards of quality and safety. The FAA guidelines for owner-produced parts similarly demand that replacements match the approved parts without alteration, reflecting the regulatory rigor mirrored in the FDA's approach to medical devices.
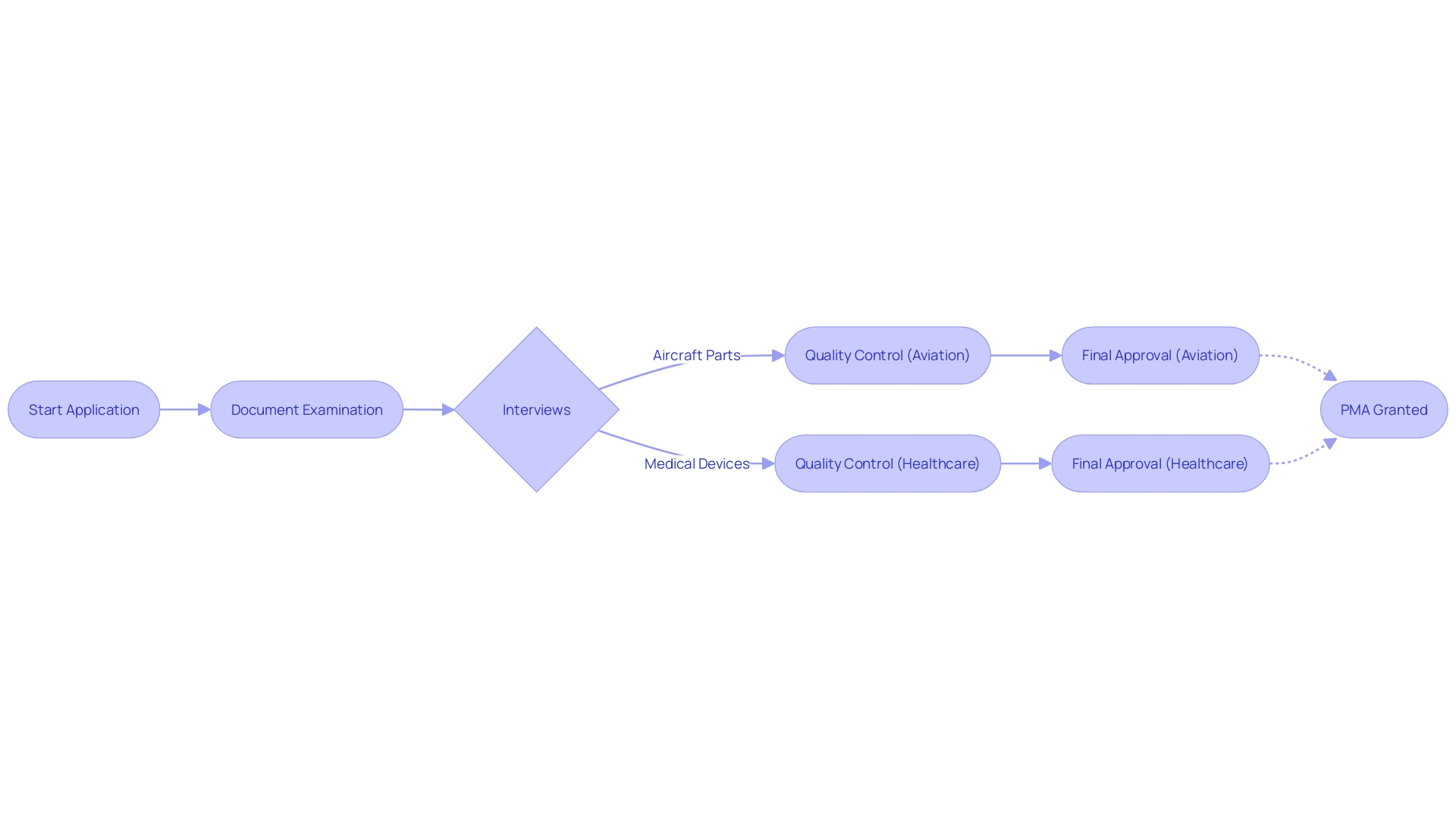
History and Evolution of PMA
The concept of Parts Manufacturer Approval (PMA) has a storied history, initially emerging in the 1950s within the aviation sector to offer cost-effective alternatives to parts produced by Original Equipment Manufacturers (OEMs). As the demands of the industry evolved, the PMA process underwent significant refinement, leading to a robust regulatory framework. This framework ensures that PMA components adhere to the rigorous safety and performance standards mandated by aviation authorities.
Transitioning into the healthcare space, PMA has taken on a critical role in the medical device industry. It is now a vital process for the thorough evaluation and authorization of medical devices prior to their market release, ensuring that the devices meet the highest standards of safety and effectiveness for patient care. The importance of PMA in healthcare is underscored by the meticulous involvement of biomedical engineers like Chris, with over a decade of experience in managing clinical studies for Class III medical devices, underscoring the meticulous nature of PMA studies and the level of scrutiny applied to medical devices.
Moreover, the application of PMA in business decisions within healthcare is highlighted by the experience of a steel-recycling company that utilized reference-based pricing to revamp its employee healthcare strategy. By delving into insurance claims data and severing traditional ties with provider networks, the company exemplifies how a data-driven approach to healthcare can lead to significant cost savings and efficiency gains.
In the broader context of market dynamics, PMA's impact extends to the aviation industry's supply chain, where current disruptions have led to increased turnaround times and component costs, ultimately affecting the bottom line. Additionally, the advent of smartphone apps and on-demand services has altered consumer access to aviation services, with the rapid expansion of uncrewed aircraft systems for professional use cases. These developments reflect the growing interdependence between PMA's regulatory rigor and the innovative technologies shaping the future of both business and healthcare.
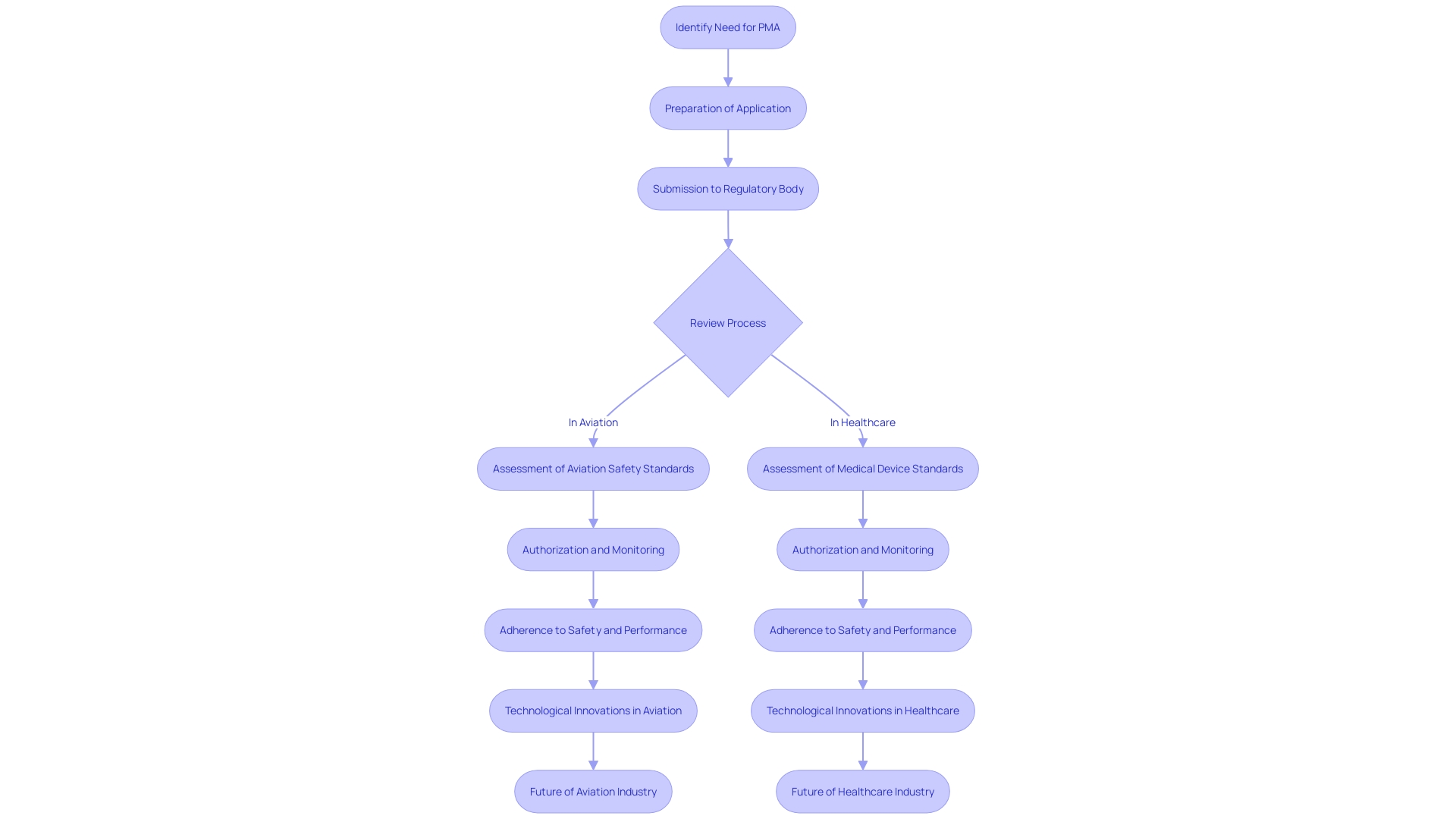
Methods of Obtaining PMA Approval
PMA (Premarket Approval) is a rigorous process in both the aviation and healthcare sectors, ensuring the highest standards of safety and effectiveness for products entering the market. In aviation, the focus is often on components such as aircraft seats, which must be approved to technical standard orders (TSO) and meet the Federal Aviation Administration (FAA) regulations. For instance, electronic components integrated into aircraft seats are assessed for compliance and do not cover those installed independently.
The industry recognizes the importance of detailed knowledge about an aircraft, including its maintenance history and components, to ensure safety beyond just the mandatory Airworthiness Directives or service bulletins.
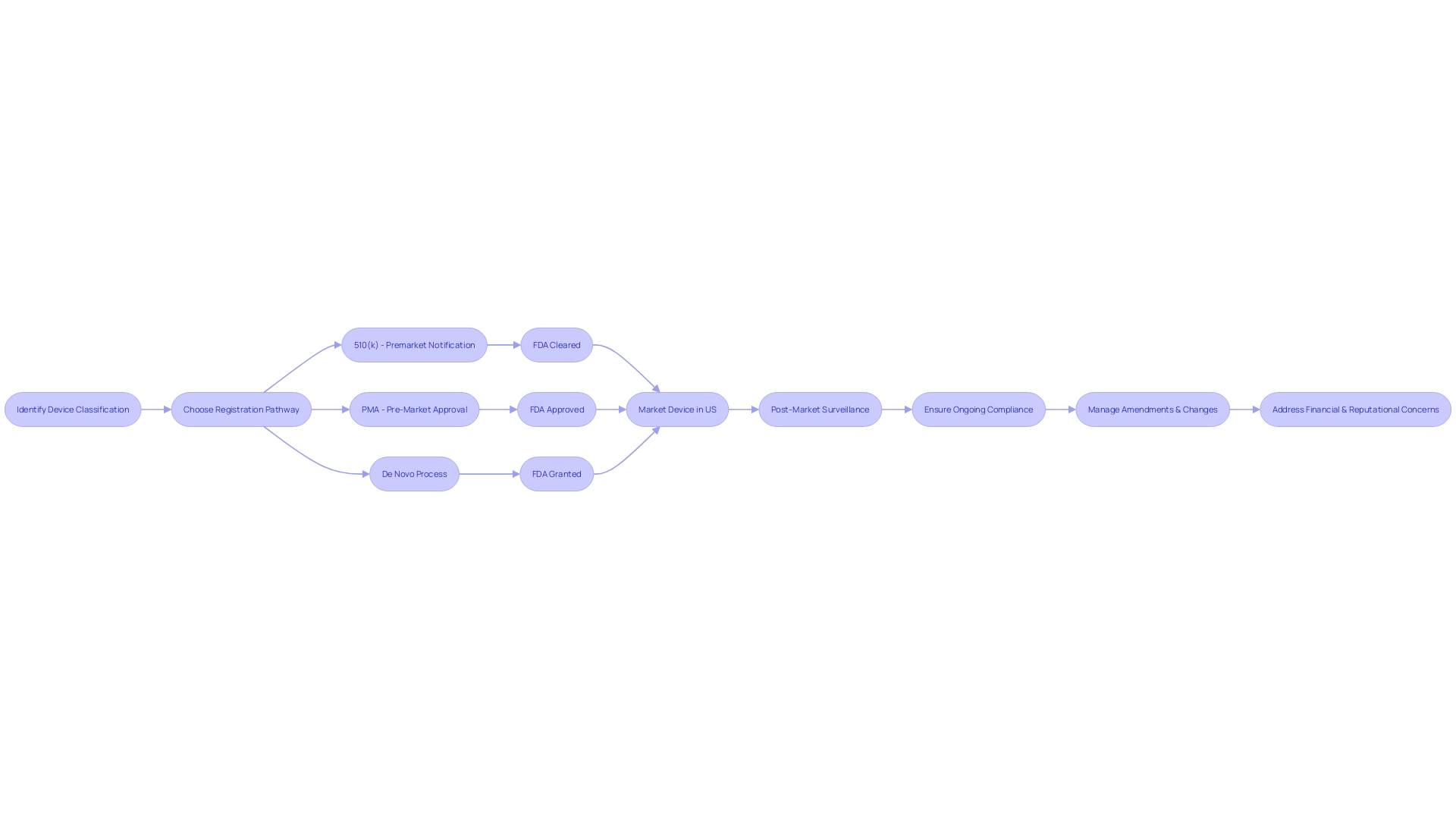
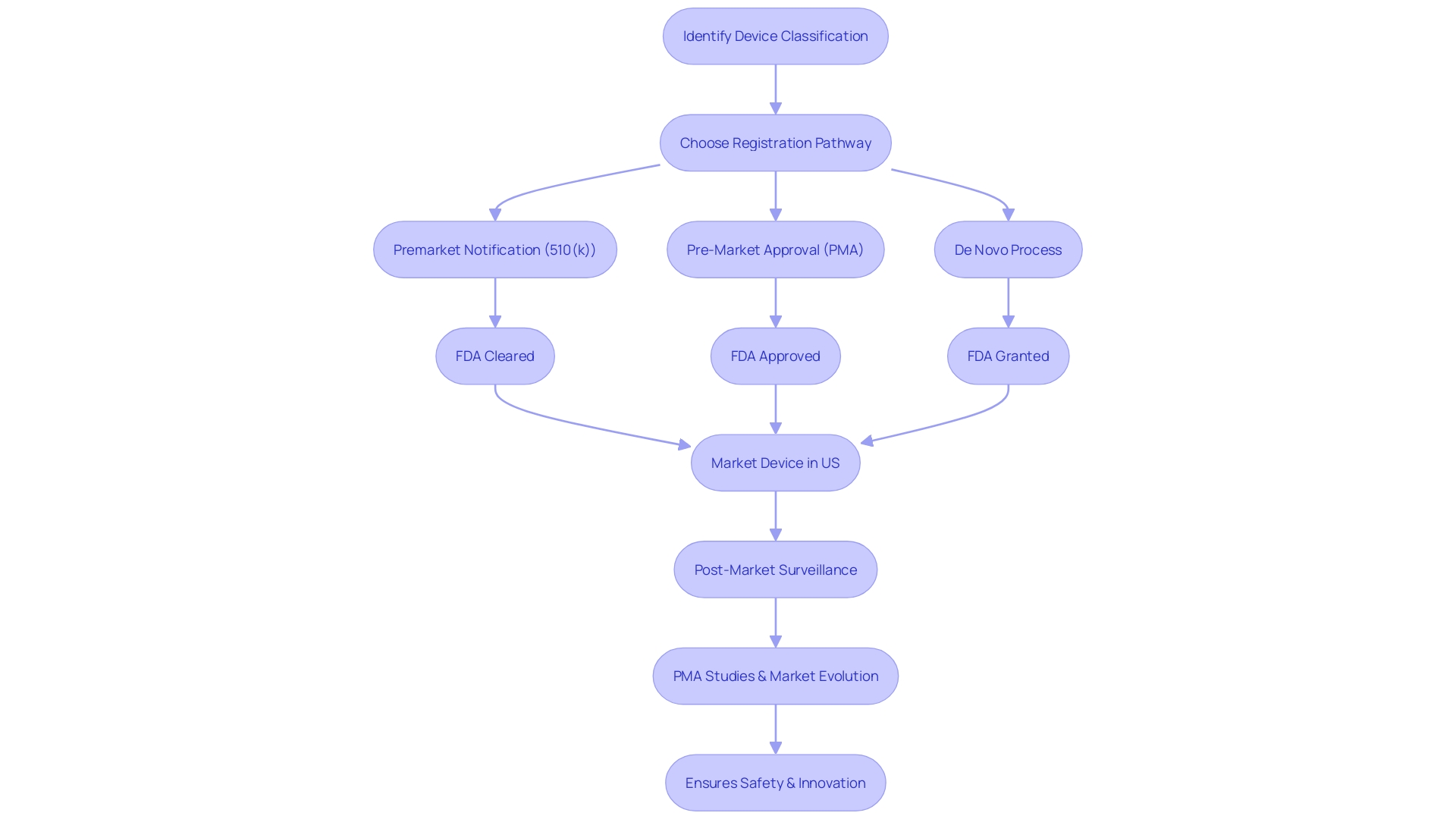
In the healthcare industry, the classification of a medical device determines the pathway for approval, which could be a 510(k) notification, PMA, or De Novo process. These classifications are based on the patient risk associated with the device. For example, Class III devices, which pose the highest risk, often require PMA approval, a process that includes extensive clinical trials and data analysis to prove safety and effectiveness before they can be marketed.
The difference between being FDA 'Cleared,' 'Approved,' or 'Granted' is significant, reflecting the level of scrutiny and evidence required.
The importance of this approval process is underscored by case studies such as the work of Chris, a biomedical engineer with over a decade of experience in managing clinical studies for Class III devices. Similarly, academic medical centers like one in Florida have shown the complexity of integrating healthcare operations with academic services, ensuring compliance and efficiency in their processes.
The business implications of PMA are also evident in industries like steel recycling, where companies have had to delve into insurance claims data and adopt new pricing models based on Medicare rates to manage healthcare costs effectively. Such strategic moves demonstrate the intricate relationship between regulatory compliance, product quality, and business outcomes.
The significance of PMA in ensuring only high-quality products reach the market is echoed by the aviation industry's safety records. For example, the International Air Transport Association (IATA) reported no fatal accidents or hull losses for jet aircraft in 2023, with a fatality risk rate of 0.03 per million sectors, highlighting the success of strict compliance and safety measures.
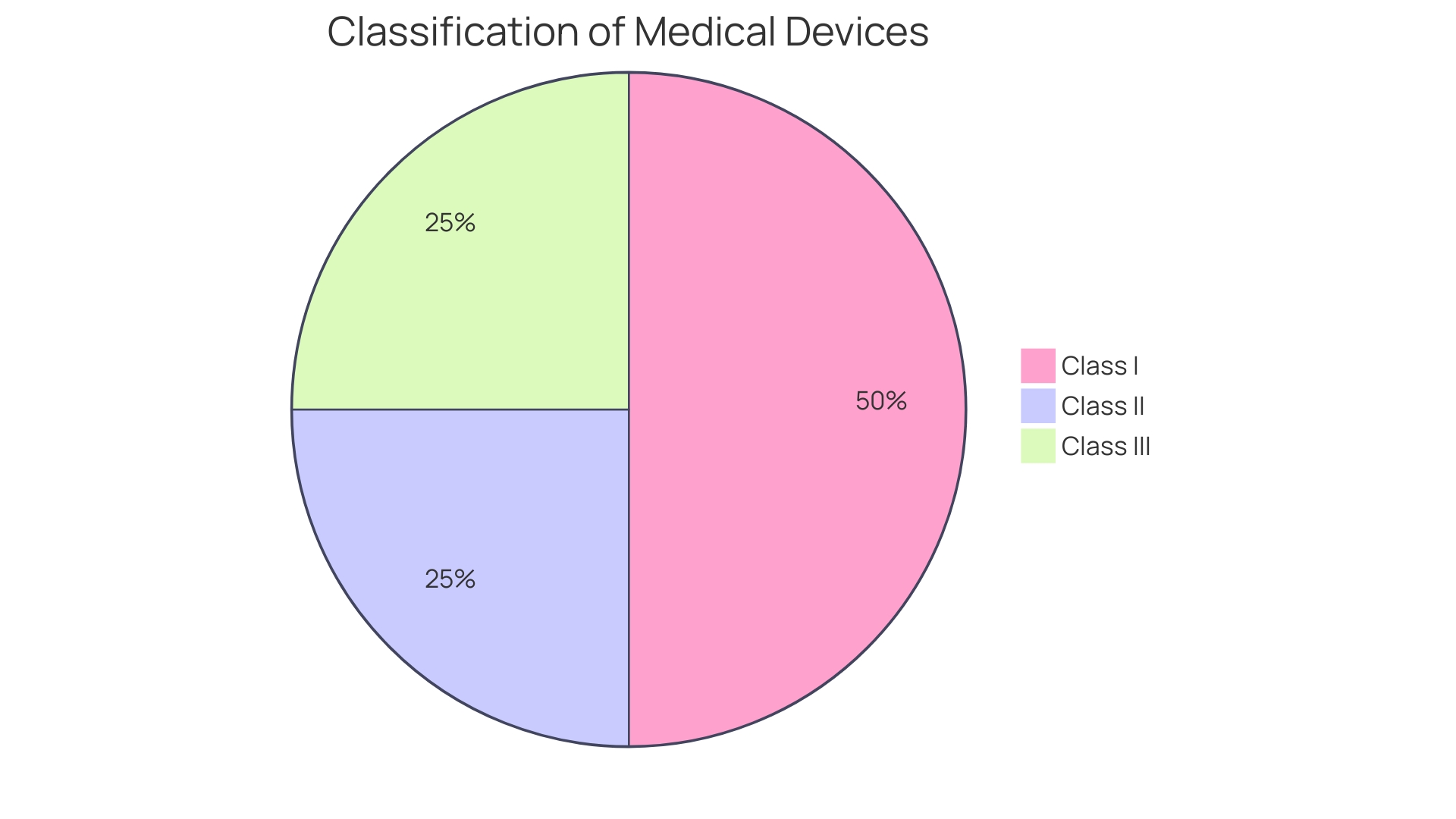
PMA Approval Process: Key Steps and Requirements
Navigating the Pre-Market Approval (PMA) process for medical devices is a nuanced journey, particularly for those devices classified as high-risk, such as life-sustaining implantables. It is essential to first determine the device's correct FDA classification, as this affects the regulatory pathway chosen—be it a 510(k) notification, PMA, or the De Novo process. For the approximately 10% of devices that fall under class three, demonstrating compliance with stringent safety, quality, and performance standards is a prerequisite for FDA approval.
The PMA pathway entails a meticulous evaluation of detailed submissions, including engineering schematics, manufacturing protocols, and robust testing outcomes. It's a comprehensive review that not only scrutinizes documentation but also includes rigorous inspections. This ensures that every PMA-approved device adheres to the highest standards of safety and efficacy.
The importance of this process is underscored by the growing complexity and market value of medical devices, with sectors like the corneal implant market projected to reach nearly $600 million by 2033.
While the process is demanding, it is essential for safeguarding public health. Post-market surveillance (PMS) continues beyond approval, with ongoing monitoring to track real-world device performance and safety—a crucial step for maintaining the highest standards over a device's lifecycle. The collaboration between regulatory bodies and industry stakeholders is key in streamlining these processes, as seen with recent efforts to expedite approvals in rapidly evolving fields like digital health.
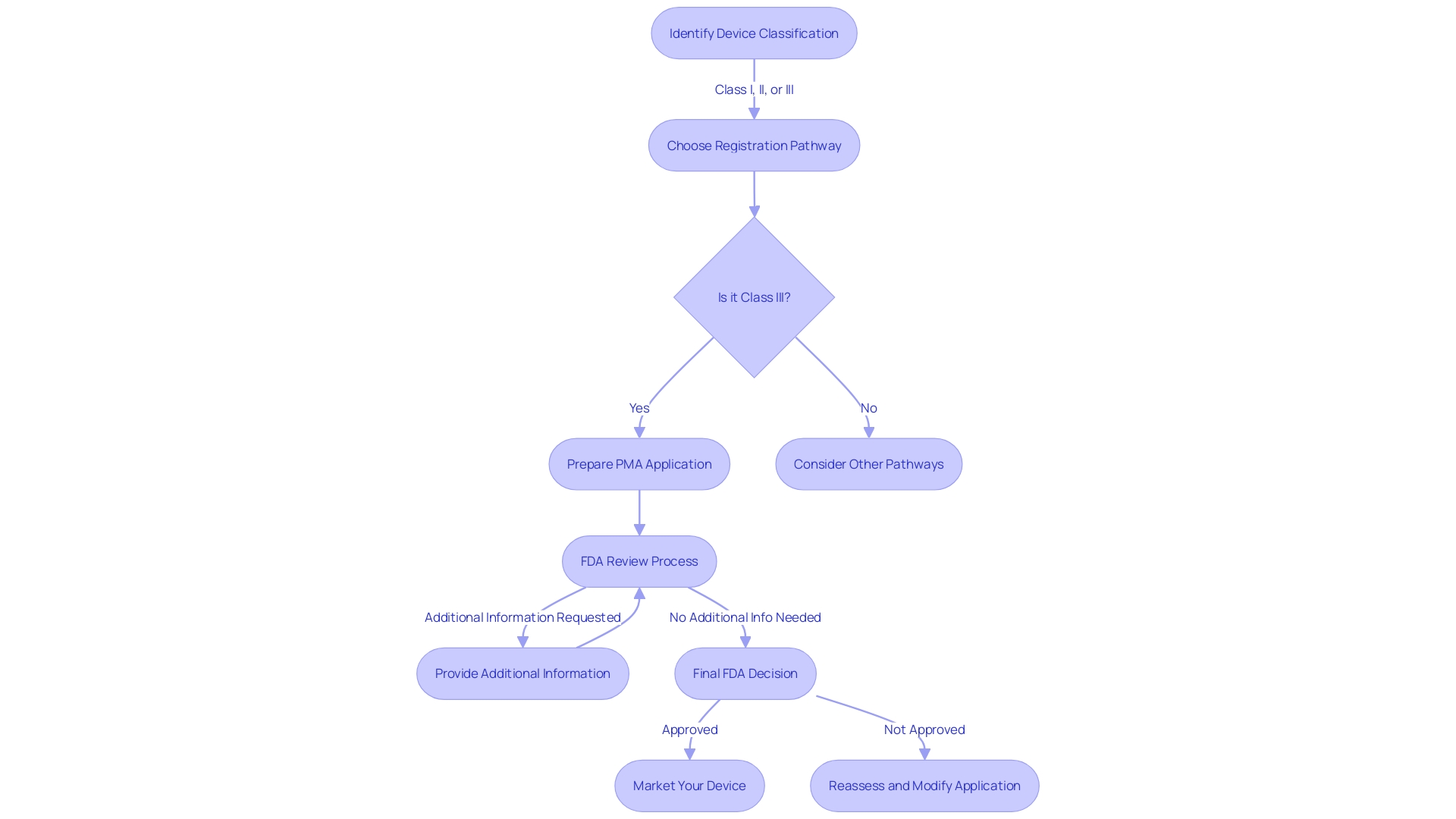
Role of FAA in PMA Approval
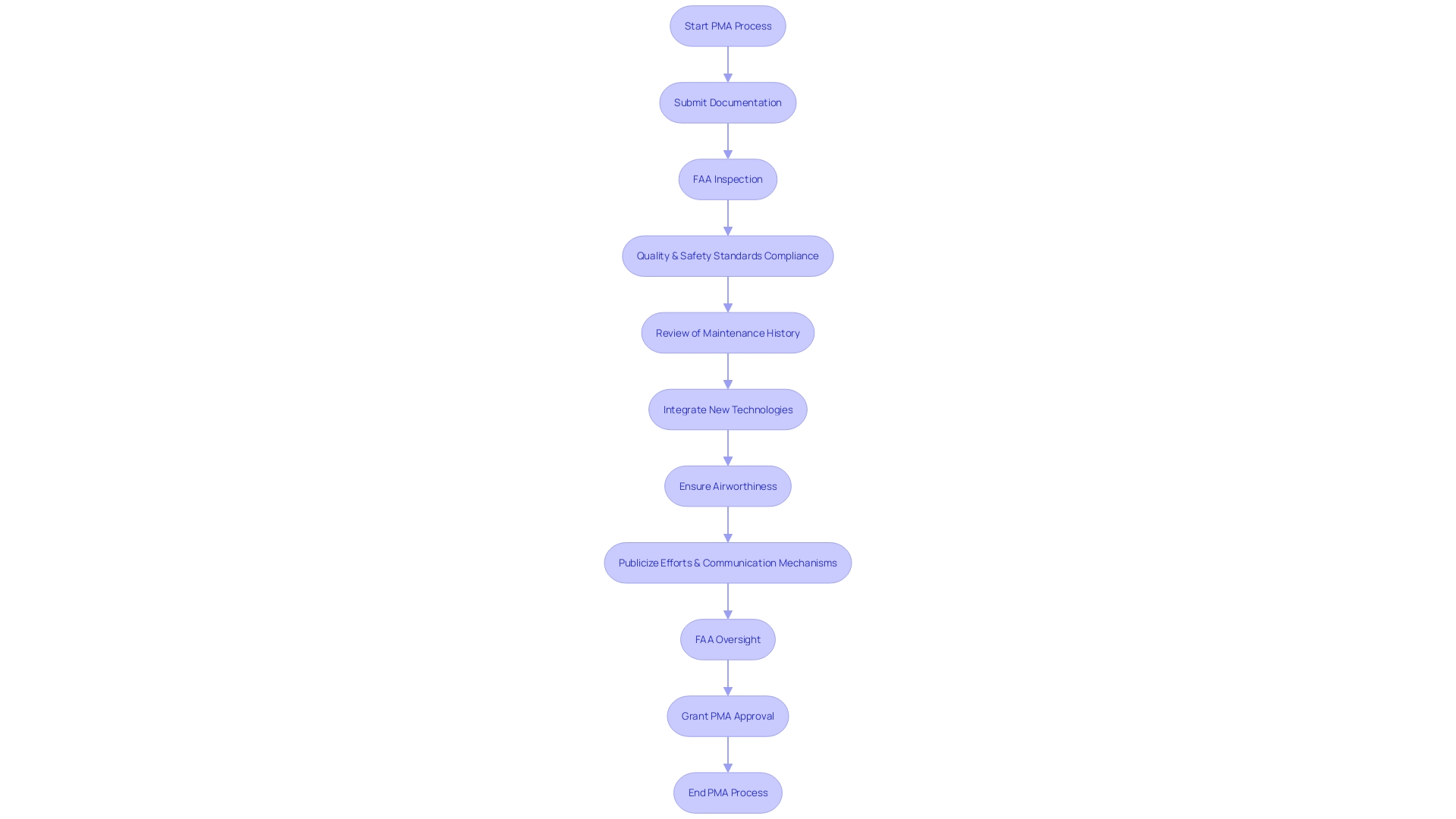
The FAA's rigorous oversight is indispensable in ensuring the airworthiness and safety of aircraft through the Parts Manufacturer Approval (PMA) process. This process is not just about checking boxes; it requires a comprehensive understanding of the aircraft's systems and the integration of new technologies. Manufacturers seeking PMA must demonstrate through detailed documentation and inspections that their parts meet the stringent standards for quality and safety.
Recognizing potential risks, such as those from alcohol abuse, the FAA also emphasizes the difference between its evaluations and routine clinical assessments to maintain the highest levels of safety in the National Airspace System (NAS).
In the realm of aviation innovation, the FAA's commitment to safety is unwavering, even as it confronts the challenges posed by the integration of advanced technologies like vertical launches and battery power. The authority takes a cautious approach without succumbing to industry pressures to compromise on safety protocols. As aviation evolves, the FAA continues to invest significantly in research and development to minimize environmental impacts, evidenced by allocating over $230 million towards the development of sustainable technologies and airport electrification.
These efforts align with the ambitious target set by the U.S. Aviation Climate Action Plan to achieve net-zero greenhouse gas emissions by 2050.
The FAA's approach extends beyond traditional regulatory frameworks, recognizing the importance of third-party service providers in supporting autonomous and advanced air mobility operations. This acknowledgment underscores the necessity for comprehensive guidelines to ensure the seamless integration of these services into the broader aviation ecosystem, which is still an area awaiting further legislative clarity.
Through a blend of meticulous inspections, adherence to safety regulations, and forward-thinking environmental commitments, the FAA's role is pivotal in fostering the next era of aviation, characterized by safety, sustainability, and innovation.
Types of PMA Approvals: License, Test and Computation, Identicality, and STC
Premarket Approval (PMA) is a crucial process within the medical and aviation industries for ensuring the safety and efficacy of novel or significantly modified devices and parts. In the realm of medical devices, particularly those classified as Class III, PMA is an elaborate and stringent FDA process designed to evaluate safety and effectiveness before these devices can reach the market. Chris, a biomedical engineer with 13 years of experience, has contributed significantly to this field by managing pivotal clinical studies for Class III devices, demonstrating the intricate balance between innovation and compliance.
In aviation, PMA covers a variety of approvals such as License, Test and Computation, Identicality, and Supplemental Type Certificate (STC). Newly designed and produced parts receive License approvals, validating their introduction into the market. Test and Computation approvals are reserved for parts that, upon thorough evaluation, are found to be equivalent to previously approved ones, ensuring consistency in quality and performance.
Identicality approvals are provided for parts that are exact replicas of original equipment manufacturer (OEM) parts, preserving the integrity of the original design. STC approvals are particularly important for modifications or alterations to existing aircraft, a process that is not only about innovation but also about maintaining the highest safety standards.
The meticulous nature of the PMA process is underscored by the commitment to safety in commercial aviation, which remains one of the most secure forms of transport. This safety-first approach is deeply ingrained in the industry, as evidenced by the continuous efforts towards sustainability and the comprehensive preflight practices that include a detailed review of an aircraft's maintenance history.
Moreover, the commercial aviation sector's safety record in 2023, with no fatal jet accidents and a fatality risk rate of only 0.03 per million sectors, reflects the effectiveness of such regulatory frameworks. The integration of rigorous conformity assessments and adherence to consensus standards, as highlighted by DSCA, plays an integral role in this success. These standards facilitate patient access to novel devices and contribute to the quality and efficiency of regulatory reviews by promoting the appropriate use of standards in regulatory processes.
Benefits of PMA Parts: Cost Savings and Quality Assurance
Parts Manufacturer Approval (PMA) stands as a significant regulatory endorsement in both the aviation and healthcare sectors, denoting a rigorous approval process for manufacturing spare and replacement parts. The advantages of PMA parts are multifaceted, with cost-effectiveness being a standout benefit. These parts are typically more economical than their Original Equipment Manufacturer (OEM) counterparts, which enables organizations to economize without sacrificing the integrity of safety or quality standards.
The reliability of PMA parts is backed by stringent testing and adherence to FAA standards for quality. For instance, the meticulous process involves a statement of conformity which details the part's lineage, method of fabrication, and compliance with FAA regulations, as was the case when Being scrutinized the titanium alloy used in their aircraft, ensuring the material's provenance and adherence to quality standards despite issues with falsified documentation.
Furthermore, the aerospace supply chain's complexity, highlighted by incidents such as the investigation into titanium supplies from China, underscores the importance of traceability and documentation in PMA parts. Utilizing FAA-approved facilities guarantees this traceability, with non-compliance deemed unlawful by FAA standards.
In the realm of medical devices, which the World Health Organization defines broadly to include everything from simple tools to complex machinery, precision and exacting standards are paramount. CNC machining has revolutionized this sector, transitioning from traditional methods to advanced, computer-controlled systems that deliver products meeting the highest precision and efficiency standards. This technological leap has been transformative, equipping the healthcare industry to generate medical devices with unparalleled accuracy and consistency.
The meticulous calibration of medical components is critical, as underscored by experts in the field. Every phase, from concept to quality control, must conform to stringent criteria to guarantee the medical devices' safety and functionality. The metrology involved in this process is essential for scanning, detecting defects, and ensuring components meet the exact specifications necessary for their intended medical application.
Moreover, the healthcare supply chain management, as shown in the Hospital ISM® Report On Business®, reflects the dynamic nature and the critical role of PMA parts and CNC machining in maintaining product quality and adherence to regulations. The report, a collection of data from hospital supply executives, indicates the ongoing need for efficient, high-quality supply chains in healthcare.
In summary, PMA parts offer a compelling proposition for both aviation and healthcare industries, combining cost savings with reliable quality assurance, as evidenced by the stringent processes and oversight they undergo. The commitment to precision and quality, as seen in the manufacturing of medical devices, is integral to the success and regulatory compliance of these industries.
Ensuring Airworthiness: Standards and Inspections for PMA Parts
In the aviation industry, the term PMA refers to Parts Manufacturer Approval, a combined design and production approval for aircraft and engine parts. It is a critical process for ensuring that replacement parts for aircraft meet rigorous safety, durability, and performance standards. The Federal Aviation Administration (FAA) mandates that all PMA parts be produced following strict guidelines that cover everything from the materials used, the manufacturing processes, assembly, and testing, to the traceability of these components.
For instance, any part produced must have documentation that demonstrates its equivalence to the original part in terms of design and how it conforms to that design, making it legal in the eyes of the FAA.
To clarify, the owner of an aircraft is responsible for the part's 'production,' which means managing the production process including defining the requirements, design, and conformity of the part. This distinction is vital as it expands the scope of owner-produced parts beyond just those with personal manufacturing capabilities. For example, if an aircraft manufacturer is no longer in business or supporting a particular model, aircraft owners can request from the FAA the necessary design data under the Freedom of Information Act to produce a part equivalent to the original.
Regulatory authorities such as the FAA and the UK Civil Aviation Authority emphasize the importance of compliance and regular inspections to uphold these high standards. Recent news highlights the FAA's dedication to safety, as seen in their thorough review process of Boeing's revised instructions for inspections and maintenance following feedback, prioritizing the safety of the flying public.
With over 44,000 compliance actions taken since 2015, the FAA's Compliance Program aims to address regulatory noncompliance collaboratively. Moreover, the aviation sector's safety record continues to improve, with 2023 marking an exceptionally safe year with no fatal accidents or hull losses for jet aircraft, as per the 2023 IATA Annual Safety Report. These improved safety measures underline the industry's commitment to maintaining the highest levels of airworthiness and safety for all aircraft.
Traceability and Documentation of PMA Parts
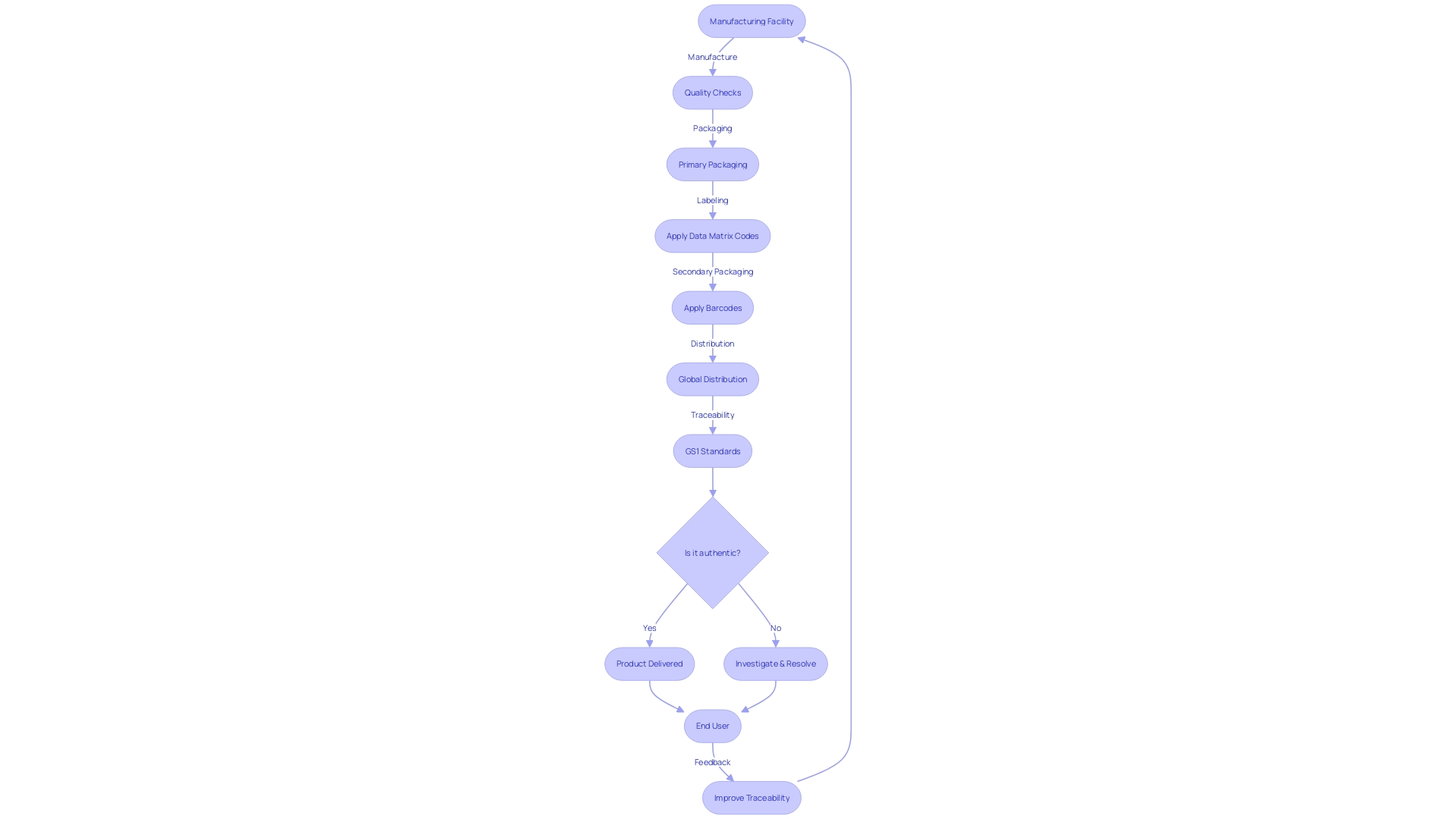
The concept of traceability and documentation in the production and distribution of PMA parts is fundamental, not just in the aviation sector but also in healthcare, particularly in the realm of vaccine distribution. In the pursuit of global health improvements, manufacturers meticulously chronicle the journey of each component, from its origin through manufacturing processes, quality checks, and any alterations. This rigorous record-keeping fosters an environment of transparency and enables precise tracking and identification of parts or products.
One shining example of this practice is the Supply Chain Initiative by the Developing Countries Vaccine Manufacturers Network (DCVMN). This initiative, launched in 2019, underscores the importance of traceability in the vaccine supply chain. By introducing global standards like GS1, complete with barcodes and data matrix codes on vaccine packaging, the DCVMN has enhanced the visibility of product movement, thereby elevating supply chain reliability and mitigating the circulation of counterfeit and expired vaccines.
Pilot studies across various countries have demonstrated that a phased approach to adopting traceability standards not only facilitates learning and lowers initial risks for manufacturers but also allows for a more spread-out investment strategy. These efforts are vital as they underscore the cumulative value of traceability, which hinges on widespread adoption and effective data utilization. The Who's policy paper on medical product traceability further reinforces the critical nature of these global standards, which enable seamless interoperability among supply chain stakeholders and bolster safety monitoring systems.
Moreover, the serial numbers accompanying Global Trade Item Numbers (GTINs) provide a unique identity for each product, offering a granular level of traceability that is particularly useful in the battle against falsified vaccines. The industry's shift towards this level of detail in traceability and documentation is a testament to its commitment to safeguarding public health and ensuring the integrity of global immunization programs.
Industry Impact: How PMA Affects Business and Healthcare
The Pre-Market Approval (PMA) process is a cornerstone of ensuring safety and innovation across various industries, particularly in aviation and healthcare. This rigorous regulatory pathway plays a pivotal role in aviation by fostering a competitive environment that empowers both established and emerging manufacturers of aircraft parts. It opens doors for small and medium-sized enterprises to carve out their niche, contributing to a dynamic market landscape and driving cost efficiencies.
In the healthcare sector, the significance of PMA is further underscored by its stringent safety and efficacy standards for medical devices. Before reaching healthcare professionals and patients, these devices must navigate through a meticulous review process that serves as a bulwark for public health. By vetting medical devices extensively, PMA builds a foundation of trust in the medical device industry, which is critical for patient care.
For instance, Chris, a biomedical engineer with over a decade's experience in the medical device field, has witnessed the evolution of PMA studies and their impact on both pre and post-market scenarios. His role at Greenlight Guru involves guiding solutions that navigate these rigorous regulatory landscapes.
Moreover, companies like Summer Health are leveraging technology to streamline administrative tasks such as medical visit notes, which traditionally consume over half of medical care providers' time. With the aid of generative AI, this initiative not only improves the quality of communication to patients but also allows healthcare providers to dedicate more time to patient care, addressing the issue of burnout.
These advancements resonate with the insights highlighted in the Hospital ISM® Report On Business®, which emphasizes the need for thorough research and data-driven decision-making in service planning and bid submissions by insurers. It is not just consumer demand that shapes today's healthcare landscape but also the strategic insights that inform benefit refinement, ultimately enhancing patient and provider experiences.
Such transformative changes across business and healthcare sectors are a testament to the role of PMA in shaping an environment where safety, innovation, and efficiency coalesce to advance public well-being and market growth.
Conclusion
In conclusion, the Parts Manufacturer Approval (PMA) process is a critical certification issued by the FAA and FDA to ensure safety, quality, and efficacy in aviation and healthcare. PMA allows manufacturers to produce alternative aircraft parts and authorizes medical devices before entering the market.
PMA has a rich history, evolving from the 1950s in aviation to become a robust regulatory framework. In healthcare, PMA plays a vital role in evaluating and authorizing medical devices, focusing on safety and efficacy. Business implications are evident, with companies using data-driven approaches to manage healthcare costs effectively.
Obtaining PMA approval differs between industries. In aviation, compliance with FAA regulations and traceability is crucial. In healthcare, the classification of a medical device determines the approval pathway, with extensive evaluations and safety standard compliance.
Regulatory bodies, like the FAA, ensure airworthiness and safety. The FAA's commitment to safety is unwavering, investing in research and development for advanced technologies. Different types of PMA approvals serve specific purposes in ensuring safety and quality.
PMA parts offer cost savings and quality assurance. They undergo stringent testing, adhering to standards, and are more economical than OEM parts. Traceability and documentation are vital for transparency and compliance.
PMA has a significant impact on business and healthcare, driving market dynamics and cost efficiencies in aviation. It builds trust in medical devices and enables transformative changes in healthcare operations.
In conclusion, PMA is essential for safety, innovation, and quality in aviation and healthcare. It empowers manufacturers, fosters trust, and drives market growth. The rigorous evaluation process, traceability, and documentation ensure the integrity and efficacy of PMA parts.
Ultimately, PMA benefits businesses, healthcare providers, and public well-being.




