Introduction
Premarket Approval (PMA) is the FDA's most stringent regulatory pathway for high-risk medical devices, ensuring their safety and efficacy before they can be marketed. This process involves a thorough evaluation of benefits and risks, including rigorous testing and clinical trials. PMA is crucial in safeguarding public health and ensuring that only safe and effective devices reach the market.
Achieving PMA is a significant milestone for medical device manufacturers, enhancing their brand reputation and facilitating market access. In healthcare, PMA ensures that healthcare providers have access to reliable and proven devices, leading to better patient care. This article explores the PMA process, its requirements, benefits, and its comparison to other approval pathways.
What is PMA?
Premarket Approval (PMA) is the FDA's most stringent regulatory pathway for medical apparatus that pose the highest level of risk, such as life-supporting or implantable apparatus. It involves a rigorous evaluation of safety and efficacy, including benefits and risks, before the product can be marketed. The classification of a device—ranging from low-risk Class I to high-risk Class III—is the first critical step in determining the appropriate registration pathway, which might be a 510(k) clearance, PMA, or De Novo request. High-risk Class III medical instruments, comprising roughly 10% of instruments supervised by the FDA, usually need to go through the PMA procedure because of their substantial contribution to patient care. This procedure is crucial in protecting the well-being of the general public by guaranteeing that only secure and efficient instruments make it to the market, which is essential considering the increasing intricacy of healthcare technology and the critical significance of these instruments in healthcare environments.
PMA in Business
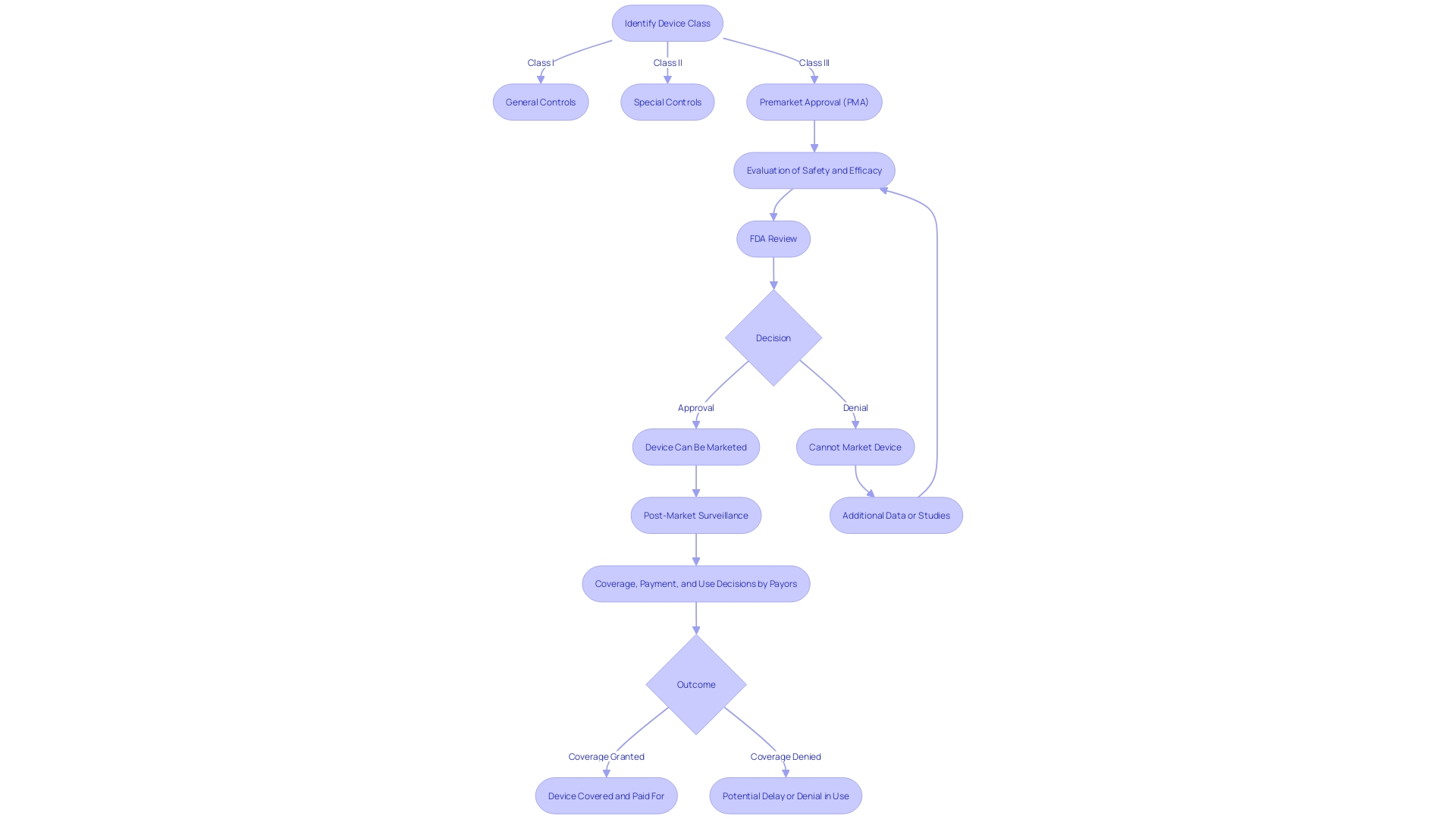
Attaining Pre-Market Approval (PMA) is a crucial milestone for manufacturers of healthcare equipment, indicating that their product has satisfied the Food and Drug Administration's (FDA) rigorous criteria for safety and effectiveness. This authorization is especially crucial for Class III healthcare instruments, which encompass life-supporting or life-sustaining tools, such as implantable pacemakers. These instruments constitute only about 10% of all FDA-regulated medical tools but they undergo the most thorough assessment due to their high-risk nature.
The pathway to PMA is complex, requiring a clear understanding of the classification of the product and the appropriate regulatory method, whether it's a 510(k) notification for lower-risk products, a De Novo request for novel products without a predicate, or the PMA for Class III products. With PMA, manufacturers gain a substantial competitive edge as it indicates that the product has passed exhaustive testing and review. The approval not only instills confidence among healthcare providers, patients, and stakeholders but also facilitates market access and can lead to increased revenue. However, manufacturers should be aware that the data submitted to the FDA might not align with the information needed by payors for coverage and reimbursement decisions, potentially leading to delays.
The regulatory landscape is evolving, with efforts to streamline approval pathways and strengthen collaboration between regulatory bodies and industry participants, especially in the wake of the COVID-19 pandemic. These initiatives are created to expedite the approval process for healthcare tools that address crucial healthcare gaps. Nevertheless, the time needed for a gadget to progress through the regulatory pipeline can differ greatly depending on the intricacy of the apparatus and the particular medical condition it caters to. Companies like PharmaSens have achieved significant milestones such as ISO 13485 certification and the introduction of innovative products like the 'niia essential' insulin pump system, which combines the simplicity of an insulin pen with advanced pump technology. Similarly, Melodi Health has made significant progress with their Melodi Matrix, an implantable tool positioned to support soft tissue and reduce post-surgical infections in breast cancer surgery. These examples emphasize the significance and influence of attaining PMA and other regulatory approvals in introducing advanced healthcare technologies to the market.
PMA in Healthcare
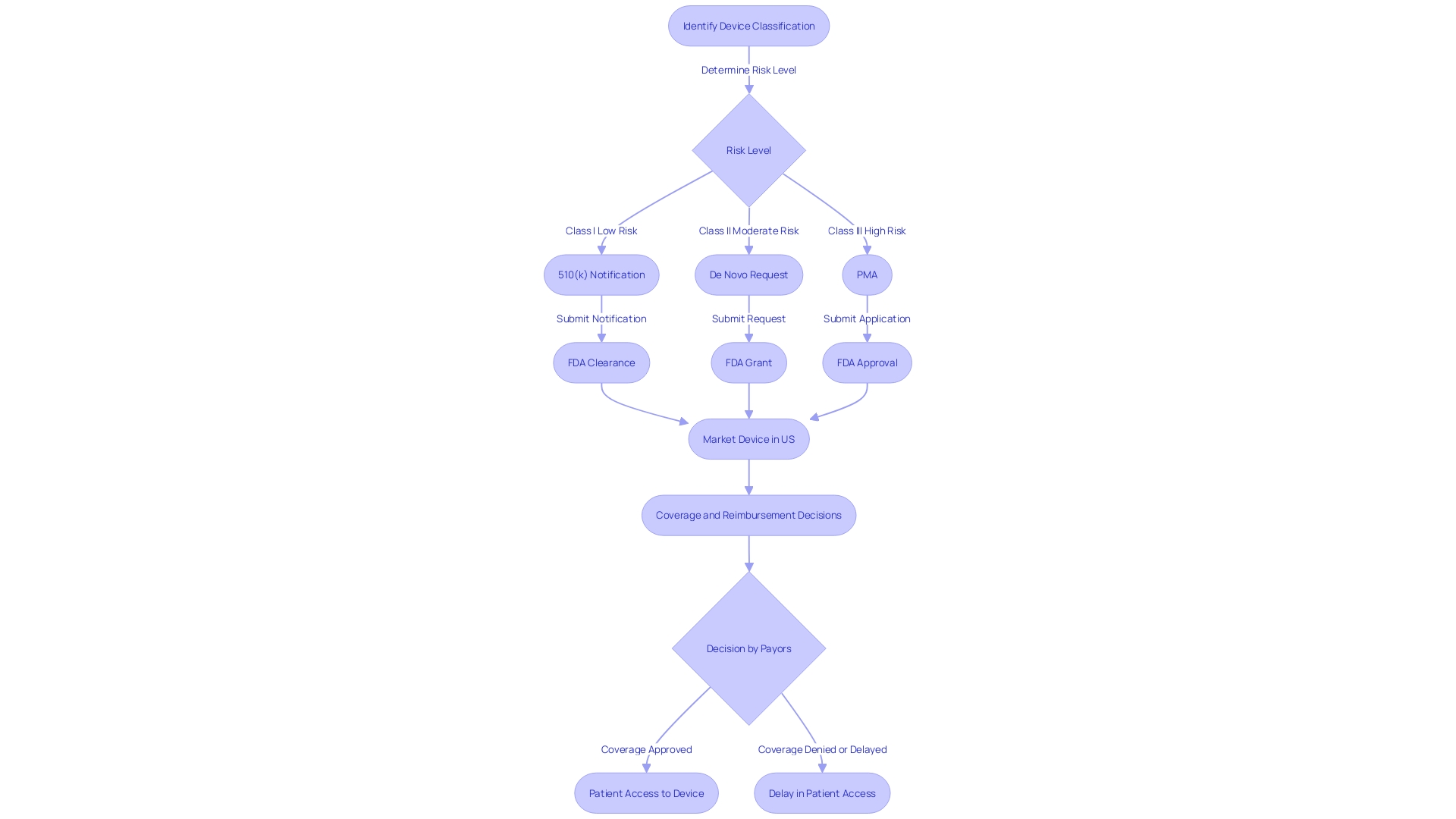
Pre-Market Approval (PMA) is the FDA's rigorous regulatory process to evaluate the safety and effectiveness of Class III medical equipment. These apparatus are regarded as high risk and encompass those that are crucial for life support or sustenance, such as implantable pacemakers. Only approximately 10% of objects belong to this classification, but they endure the most rigorous evaluation because of their crucial characteristics. PMA requires a substantial demonstration of the product's safety and efficacy, typically through significant clinical trials.
In the context of PMA, healthcare practitioners are equipped with tools that have been thoroughly tested and proven to address specific medical conditions effectively. The PMA procedure is vital to guaranteeing that only devices that meet strict standards are introduced into the market, thereby safeguarding patient health. For instance, PharmaSens recently reached a milestone with its insulin pump technology, which, after going through the extensive PMA procedure, has been created to prioritize the needs of patients and be easy to use, demonstrating the equal focus on creativity and patient safety.
Furthermore, predictive analytics plays a pivotal role in enhancing decision-making in healthcare by harnessing vast amounts of data, including records and insurance claims. This approach can lead to more efficient and responsive healthcare services, as demonstrated by the case of Pacific Steel, which used data analysis to move towards reference-based pricing and gain transparency in healthcare costs.
Ultimately, the PMA procedure is not only about compliance but also about enabling healthcare providers to provide the highest quality of care. With the advancement of healthcare technology, the PMA pathway guarantees that only those equipment that can genuinely improve patients' conditions are authorized for utilization, demonstrating a continuous dedication to healthcare innovation and patient well-being.
The PMA Process
The Premarket Approval (PMA) is a thorough assessment conducted by the FDA to assess the safety and effectiveness of Class III healthcare instruments, which are high-risk instruments crucial for maintaining or supporting life. Throughout the PMA procedure, manufacturers must provide significant scientific evidence, which encompasses detailed results from testing, clinical trials, and a comprehensive application that addresses the design, manufacturing processes, and labeling of the product. The evidence must convincingly demonstrate that the equipment is both safe and effective for its intended use. Despite obtaining FDA approval or clearance, manufacturers may encounter challenges with coverage and reimbursement decisions made by other entities like the Centers for Medicare & Medicaid Services (CMS), private health plans, and health technology assessment groups. These decisions are crucial as they impact the actual usage, payment, and availability of the approved medical equipment to patients, potentially leading to delays or denials in access even after FDA clearance. It's also crucial for manufacturers to collaborate with efficient filers who are well-versed in the documentation and importation requirements to prevent delays in the market introduction of the products. Additionally, adherence to voluntary consensus standards and conformity assessments, as outlined by OMB Circular A-119 and ANSI Essential Requirements, is vital in ensuring regulatory quality and successful communication to stakeholders. The transparency of the logic and explainability of the system is a crucial aspect for the FDA's evaluation, influencing risk assessment and patient outcomes.
Requirements for PMA Approval
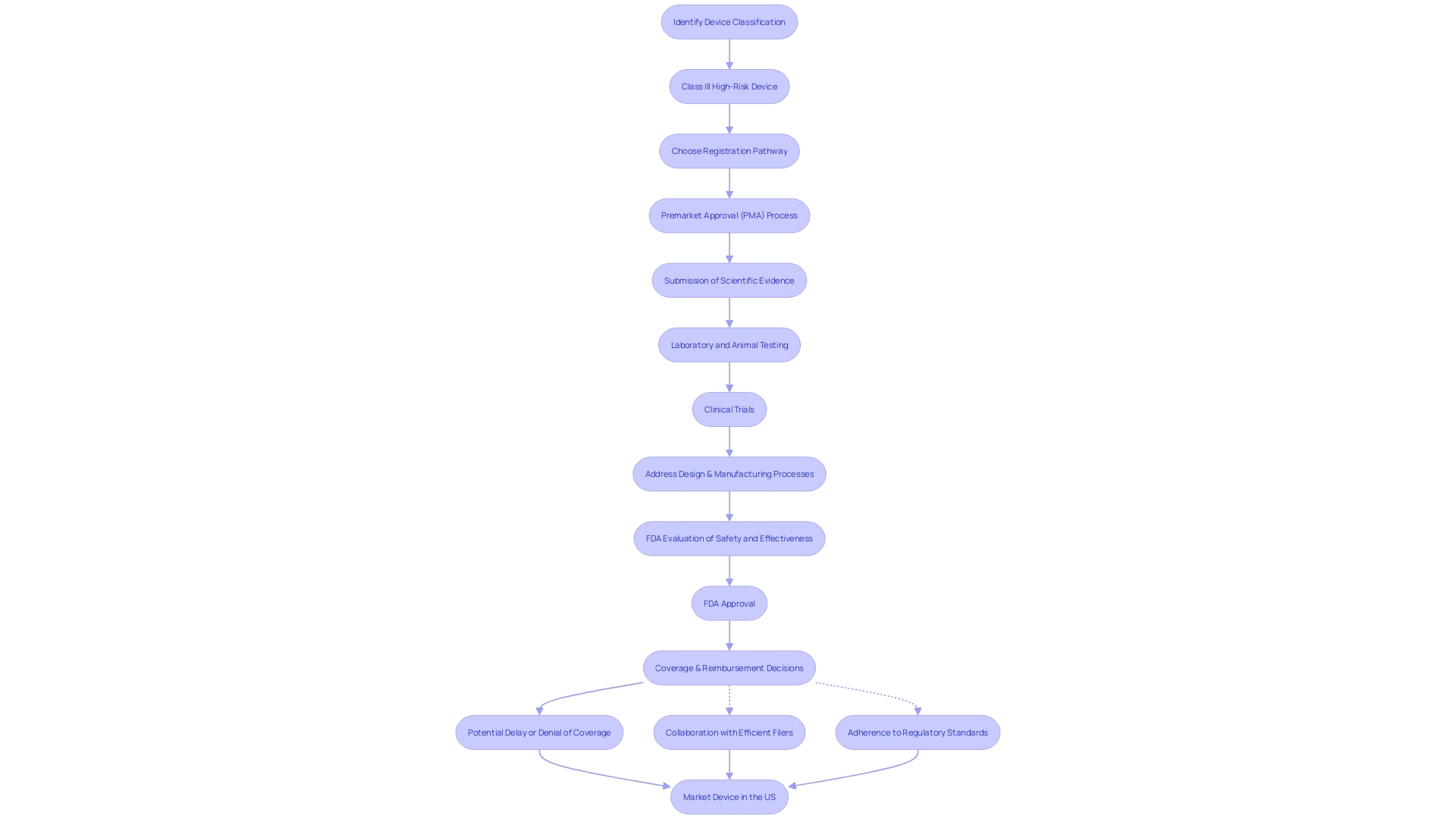
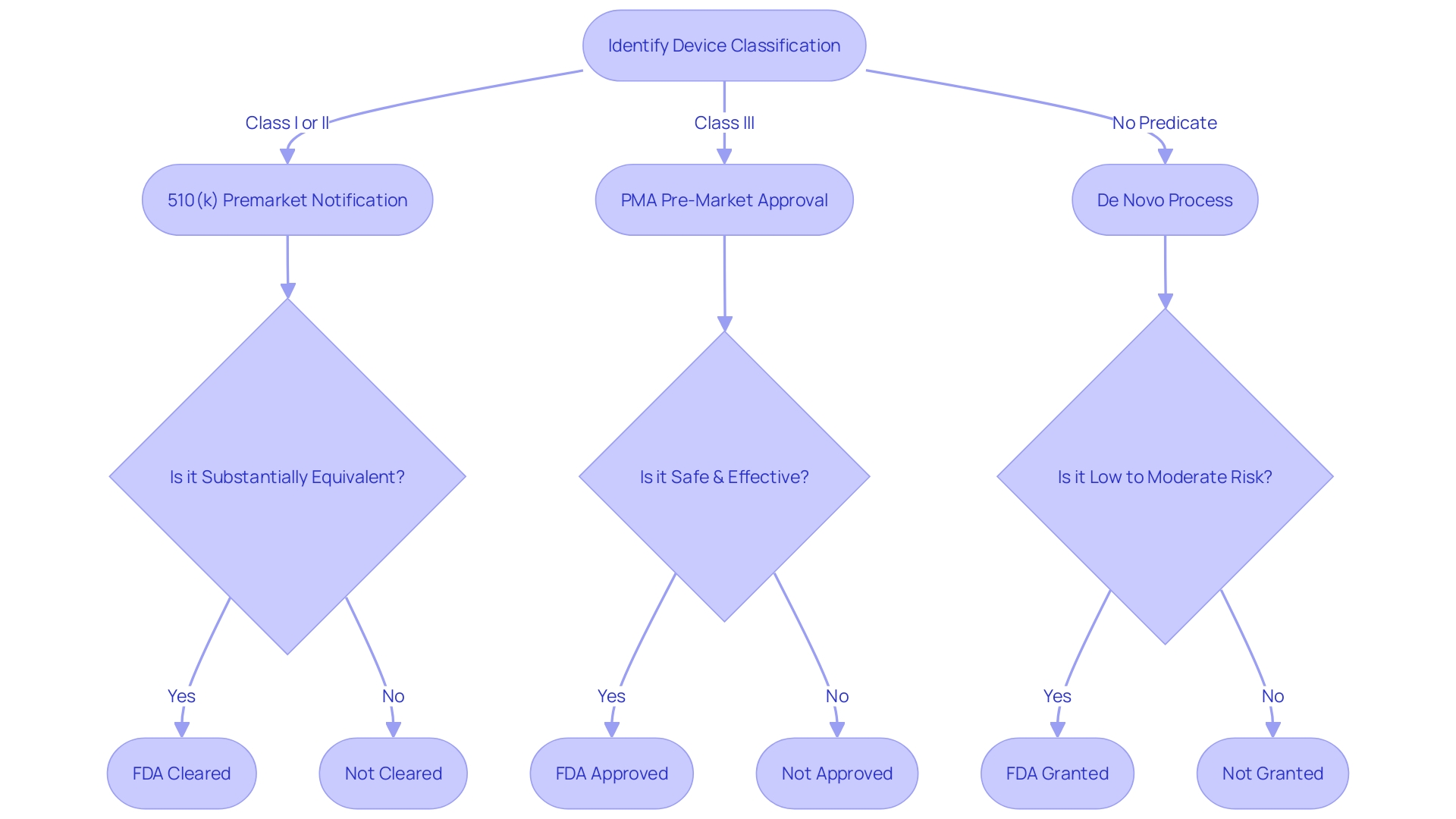
Obtaining Pre-Market Approval (PMA) from the FDA is a demanding procedure that requires manufacturers to extensively showcase the safety and efficacy of their medical products. The process starts by correctly categorizing the equipment based on FDA's three-tier system, taking into account the level of risk it poses to patients. The categorization determines whether a product necessitates a 510(k) Premarket Notification, PMA, or can undergo the De Novo procedure for lower-risk items that do not have a precedent.
Manufacturers seeking PMA must provide a comprehensive dossier of clinical data and evidence supporting the product's safety and efficacy. Adherence to Quality System Regulations (QSR), which encompass the techniques and facilities employed in designing, producing, packaging, labeling, and maintaining medical products, is also obligatory. The CGMP requirements within these regulations are designed to ensure items are safe, effective, and align with the Federal Food, Drug, and Cosmetic Act.
Furthermore, thorough labeling and instructions for use are crucial elements of the application to guarantee that the equipment is utilized accurately and safely, thus protecting patient health. A PMA is granted only after a careful and thorough FDA review, confirming that the equipment is beneficial for its intended purposes and meets all necessary standards for patient safety and care.
It is crucial to understand that FDA approval or clearance does not immediately translate to coverage or payment by healthcare payors. Following the FDA's approval, organizations like CMS and private health plans independently determine coverage and reimbursement, which can impact the availability of new healthcare technology.
The use of voluntary consensus standards is encouraged as they are created with a commitment to transparency and due process, fostering innovation, and facilitating patient access to novel apparatus. The FDA's Division of Standards and Conformity Assessment (DSCA) plays a crucial role in advancing the creation and implementation of such criteria, thereby improving regulatory review effectiveness and the general quality of healthcare instruments accessible in the market.
Benefits of PMA
Premarket Approval, commonly referred to as PMA, plays a pivotal role in the business and healthcare sectors. In the world of business, obtaining PMA for a healthcare product signifies a noteworthy accomplishment for producers, improving their brand's standing and inspiring a strong feeling of confidence among investors, customers, and stakeholders. This approval is often a harbinger of increased market share and growth prospects. In the healthcare field, PMA plays a crucial role in ensuring that healthcare equipment is both secure and efficient for patient use. Devices with PMA have undergone extensive and rigorous analysis, which allows healthcare professionals to rely on them as dependable options for treatment.
In the complex landscape of medical devices, the Food and Drug Administration (FDA) categorizes these devices into three classes based on the level of risk they present, with Class III devices being high-risk. These include life-sustaining products like pacemakers, which require a thorough and stringent PMA procedure. The FDA's classification system is crucial; it dictates the pathway to market, whether it's a 510(k) clearance, PMA, or De Novo process. For instance, a biomedical engineer with 13 years of expertise in the device space, like Chris, would have extensive knowledge managing clinical studies for Class III devices, navigating the intricate requirements of PMA studies and post-market surveillance to ensure compliance and patient safety.
Furthermore, the importance of PMA reaches to the wider healthcare technology market, which is expected to generate significant revenue in the United States. This growth is driven by the growing demand for innovation in healthcare and the development of new healthcare solutions. The market for healthcare equipment, including diagnostics, therapeutics, and patient monitoring, among others, is crucial for enhancing patient care and the overall delivery of healthcare services.
PMA's impact is further illustrated by case studies of companies like Pacific Steel & Recycling, which, through strategic shifts in healthcare administration and cost transparency, managed to significantly reduce their per-person health spending while maintaining benefits. Such examples highlight the significance of transparent and effective healthcare management, of which PMA-approved equipment are a vital component. The push for innovation, combined with strict safety regulations, guarantees that the market for healthcare instruments keeps progressing, providing dependable and cutting-edge treatment choices that are advantageous for both enterprises and patient health results.
Key Components of a PMA Application
The Pre-Market Approval (PMA) process is a pivotal step for medical equipment manufacturers, requiring a thorough compilation of data to demonstrate a product's safety and efficacy. This data encompasses a comprehensive description of the apparatus, including its design and intended use, supported by robust preclinical and clinical studies. Additionally, detailed manufacturing specifications and clear labeling must be included to guide healthcare professionals and patients. This ensures that the FDA can evaluate the equipment comprehensively. Class III items, such as life-sustaining implants like pacemakers, undergo this rigorous scrutiny due to their high-risk nature and represent about 10% of the items reviewed by the FDA. As healthcare tools are becoming more essential in patient care, with advancements in digital health and personalized medicine gaining momentum, regulatory authorities are working to simplify procedures to address pressing healthcare requirements. This includes fostering better collaboration between agencies and industry stakeholders, a movement underscored by the urgency of the COVID-19 pandemic response. The level of information needed in a PMA demonstrates the crucial significance of healthcare equipment in clinical environments and the need for clear communication of their functionality and safety to guarantee the best possible patient results.
PMA vs. 510(k) and De Novo Processes
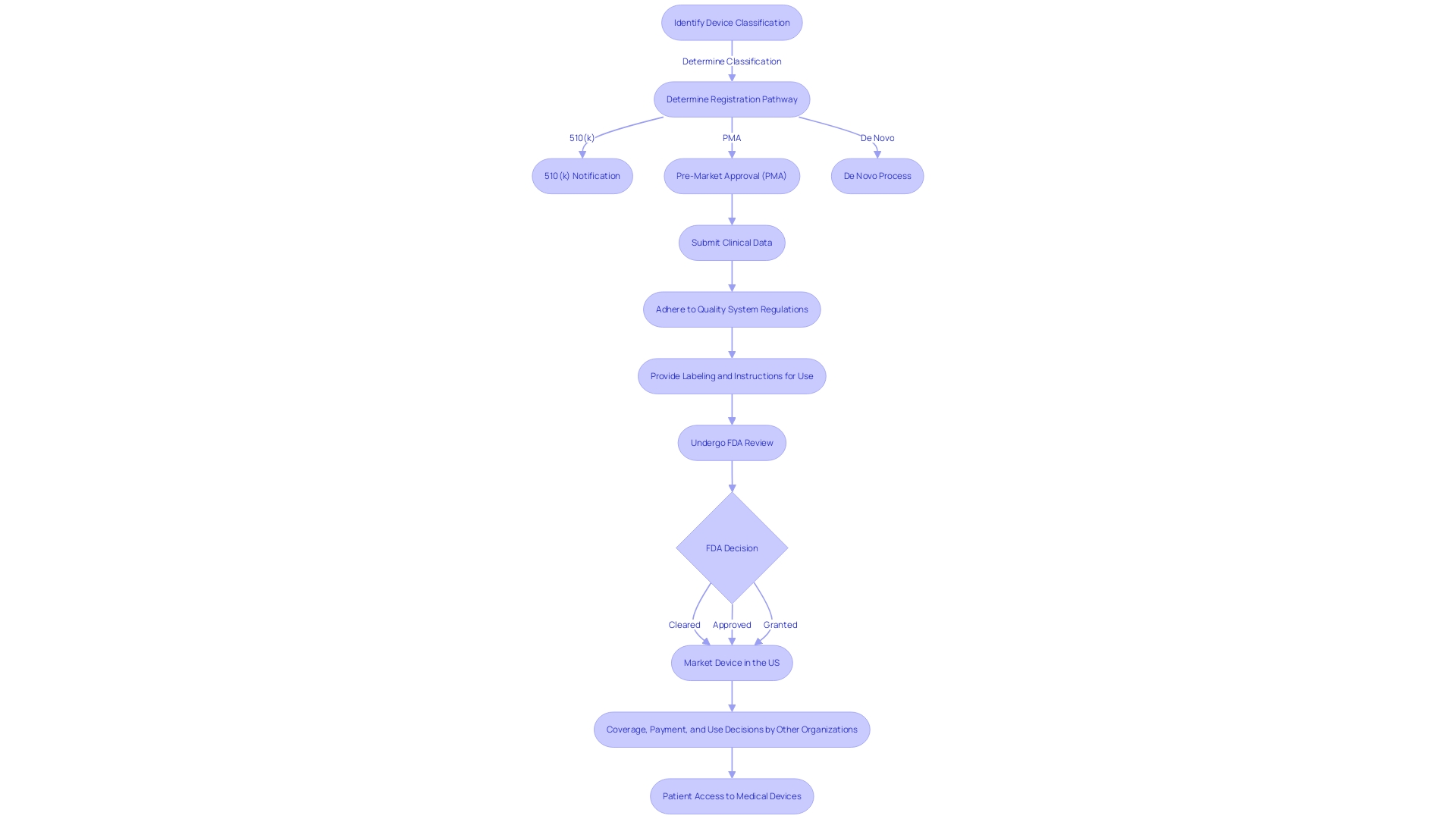
To successfully navigate the intricate process of approving medical devices, it is important to understand the different regulatory pathways and the specific criteria that govern them. The Food and Drug Administration (FDA) outlines several paths, notably the Premarket Notification (510(k)), Pre-Market Approval (PMA), and the De Novo procedure. The 510(k) pathway is created for products in the healthcare field that are considerably similar to those previously accessible on the market, enabling a simplified evaluation process based on comparison to a reference item. In stark contrast, the PMA is required for novel and higher-risk class three items that are critical to patient wellbeing, such as life-sustaining implantables. This pathway requires a more rigorous evaluation because of the increased risks linked to such instruments, which account for approximately 10% of the FDA-regulated healthcare instruments.
However, the De Novo pathway is designed for medical instruments with low to moderate risk that do not have a similar precedent but establish a new classification category, potentially speeding up their journey to the market once they obtain 'Granted' status. As the regulatory landscape changes, grasping these differences is essential for manufacturers to skillfully navigate the approval pathway, guaranteeing prompt entry to inventive healthcare remedies while upholding stringent safety criteria. This knowledge is especially pertinent in light of global efforts, such as the MHRA's policy intent for international recognition of medical devices, which emphasizes the importance of streamlined regulatory processes and international collaboration to facilitate the availability of medical technologies to patients worldwide.
Conclusion
In conclusion, Pre-Market Approval (PMA) is a vital regulatory pathway for high-risk medical devices, ensuring their safety and efficacy before reaching the market. Achieving PMA enhances manufacturers' brand reputation and facilitates market access, instilling confidence among healthcare providers, patients, and stakeholders.
PMA safeguards public health by ensuring only safe and effective devices are available for patient care. It enables healthcare practitioners to access reliable and proven devices, leading to better patient outcomes. The process involves rigorous testing, clinical trials, and a thorough evaluation of benefits and risks.
Manufacturers must provide comprehensive data and evidence demonstrating device safety and effectiveness. Compliance with quality system regulations and detailed labeling is crucial. It's important to note that FDA approval does not guarantee coverage or payment by healthcare payors, necessitating collaboration between regulatory bodies and industry stakeholders.
PMA brings several benefits to the business and healthcare sectors. It enhances manufacturers' brand reputation, market share, and revenue. It also supports the growth of the medical technology market, which plays a vital role in enhancing patient care and healthcare delivery.
Compared to other approval pathways like 510(k) and De Novo processes, PMA is reserved for novel and higher-risk devices, requiring a more stringent review. Understanding these distinctions is crucial for manufacturers to navigate the approval process effectively while maintaining safety standards.
Overall, PMA is a crucial step in ensuring the safety and efficacy of high-risk medical devices. It reflects a commitment to medical innovation and patient safety in an ever-evolving healthcare landscape.




