Introduction
The process of Pre-Market Approval (PMA) by the U.S. Food and Drug Administration (FDA) is a stringent examination of high-risk medical devices, specifically those classified as Class III. These devices, due to their critical functions in sustaining life, preventing significant health impairment, or presenting a potential risk of illness or injury, undergo a comprehensive evaluation of their safety and efficacy prior to entering the market. The FDA categorizes medical devices into three risk-based classes.
Class III devices, which include life-supporting or life-sustaining technologies, must navigate through the PMA route—one of the most rigorous regulatory pathways for device approval. Medical devices play an essential role in healthcare, aiding in diagnosis, treatment, and improving patient quality of life. It's crucial for manufacturers to understand the proper classification and regulatory pathway for their devices to ensure compliance and facilitate patient access to life-enhancing medical technologies.
What is Pre Market Approval?
The procedure of Pre-Market Approval (PMA) by the U.S. Food and Drug Administration (FDA) is a rigorous evaluation of high-risk instruments, particularly those classified as Class III. These instruments, because of their vital roles in sustaining life, preventing significant health impairment, or presenting a potential risk of illness or injury, undergo a thorough evaluation of their safety and efficacy before entering the market. The FDA classifies healthcare tools into three risk-based categories. Class III technologies, which include life-supporting or life-sustaining technologies, must navigate through the PMA route—one of the most rigorous regulatory pathways for approval.
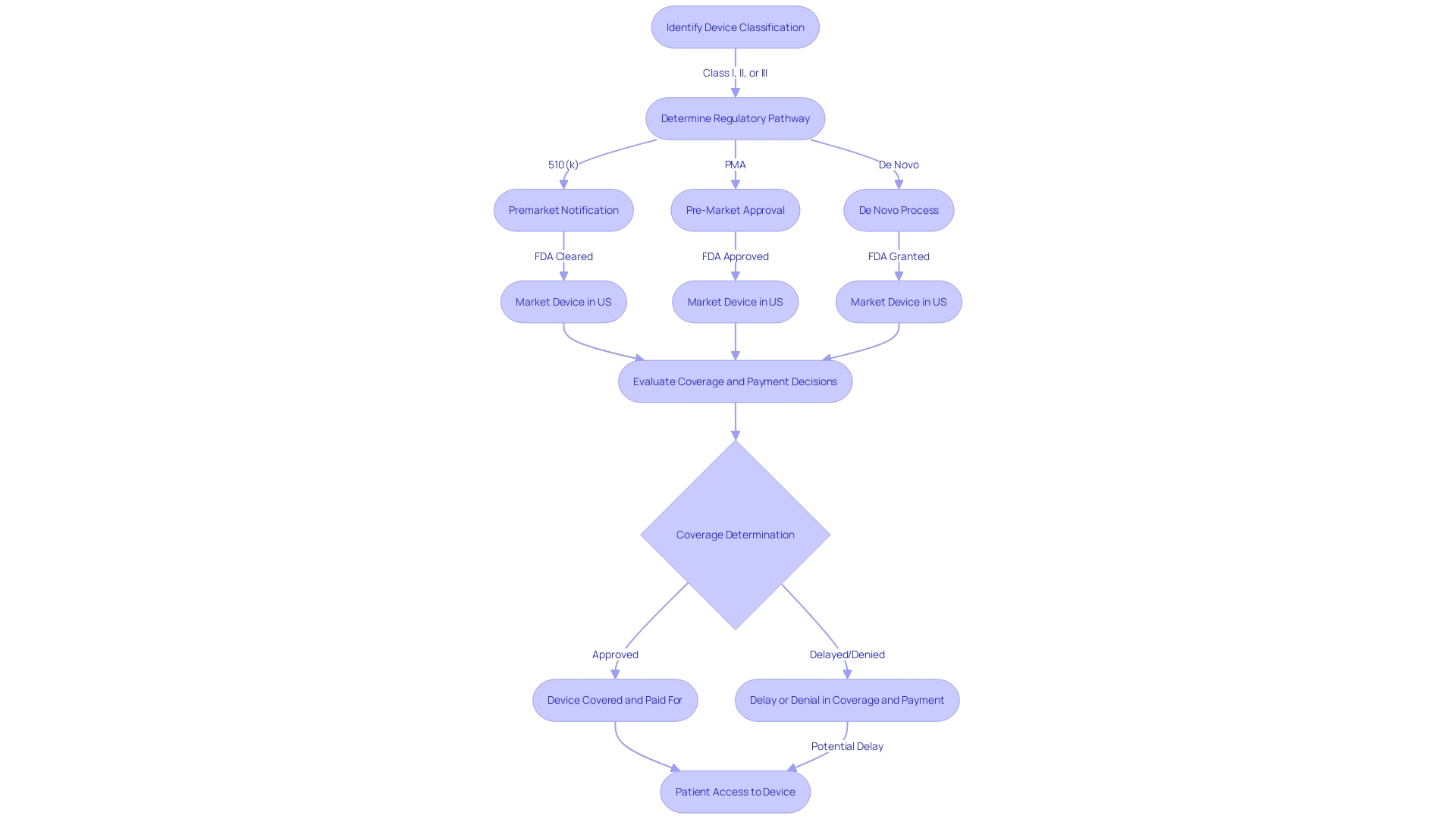
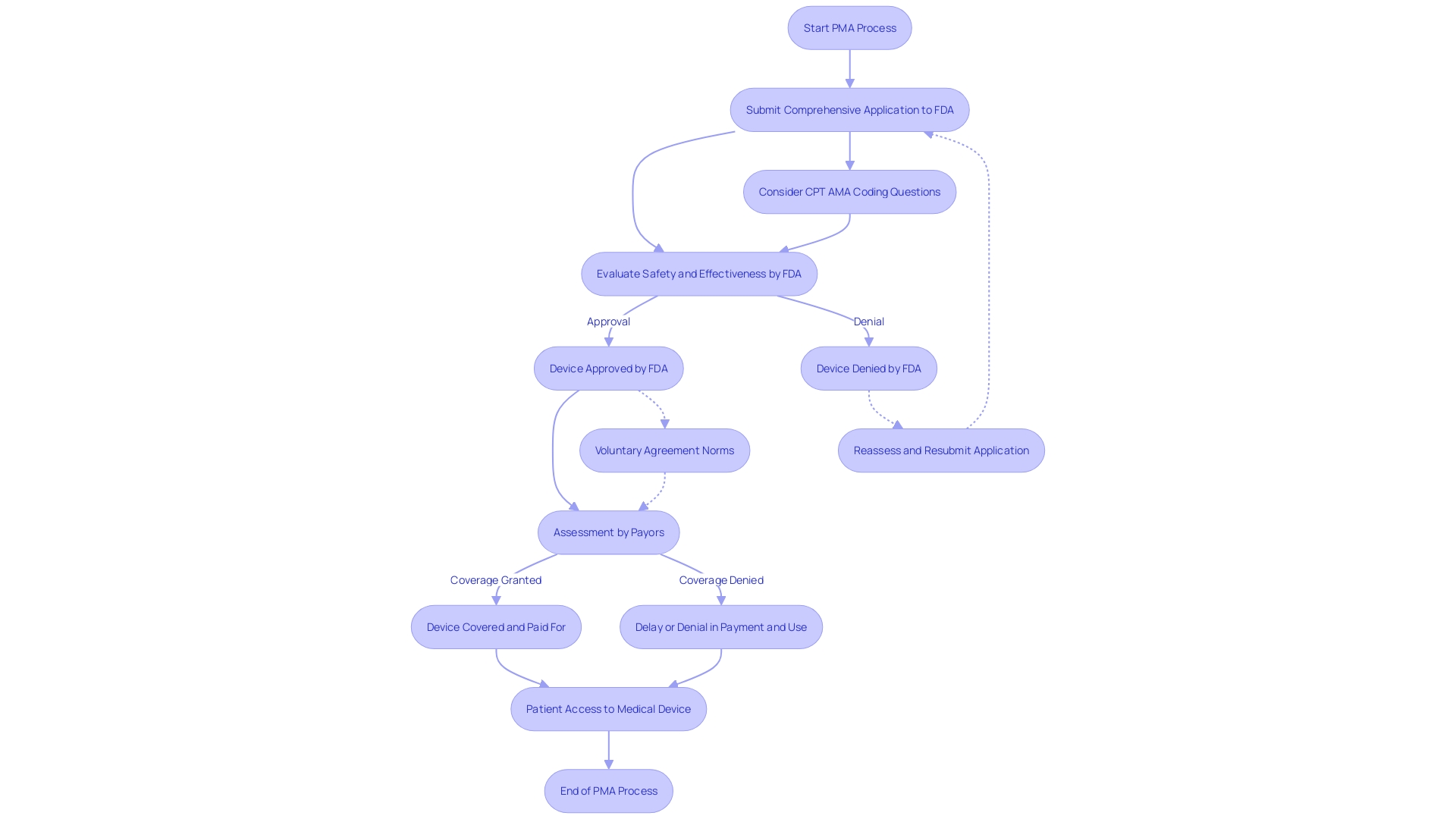
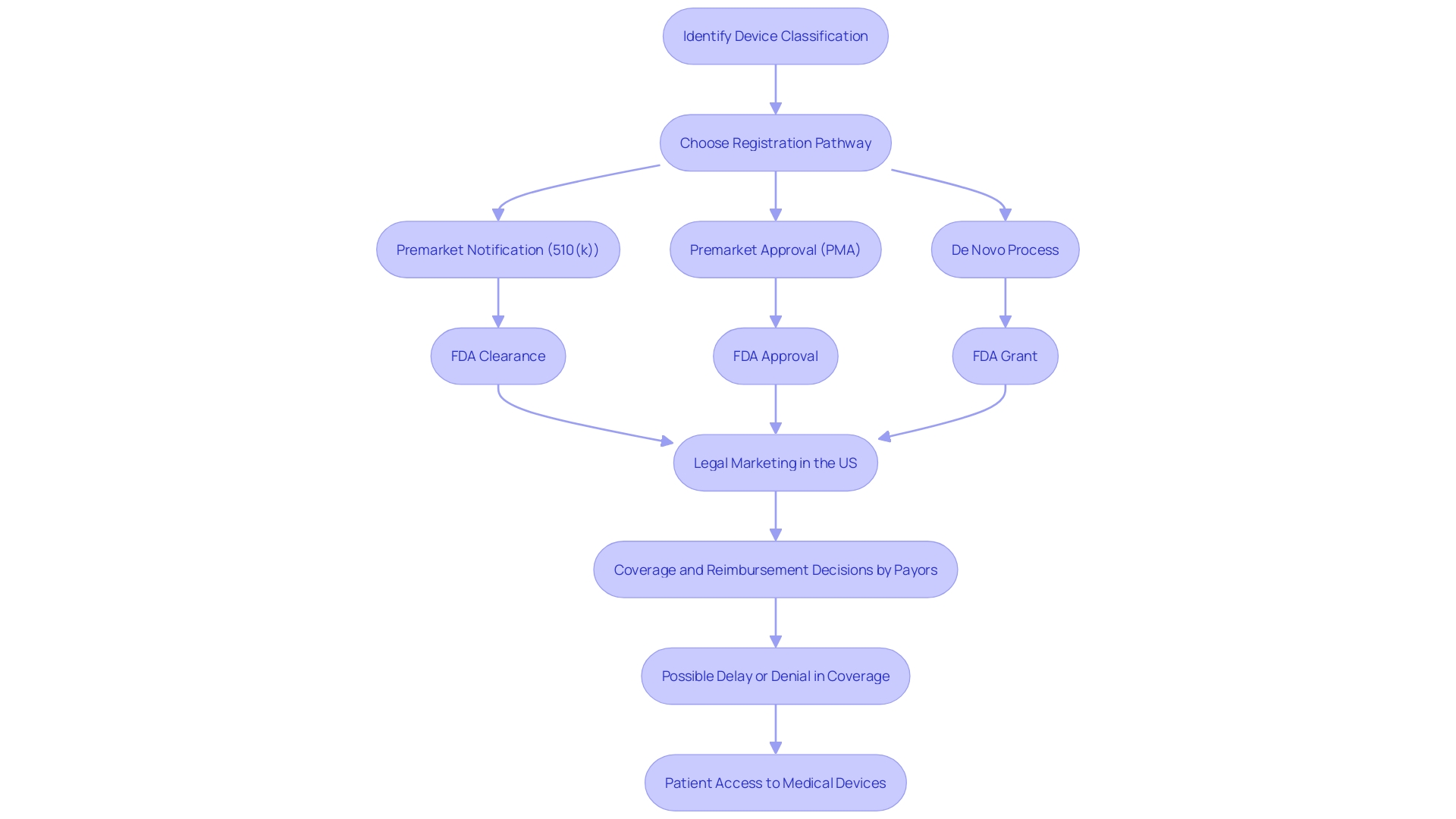
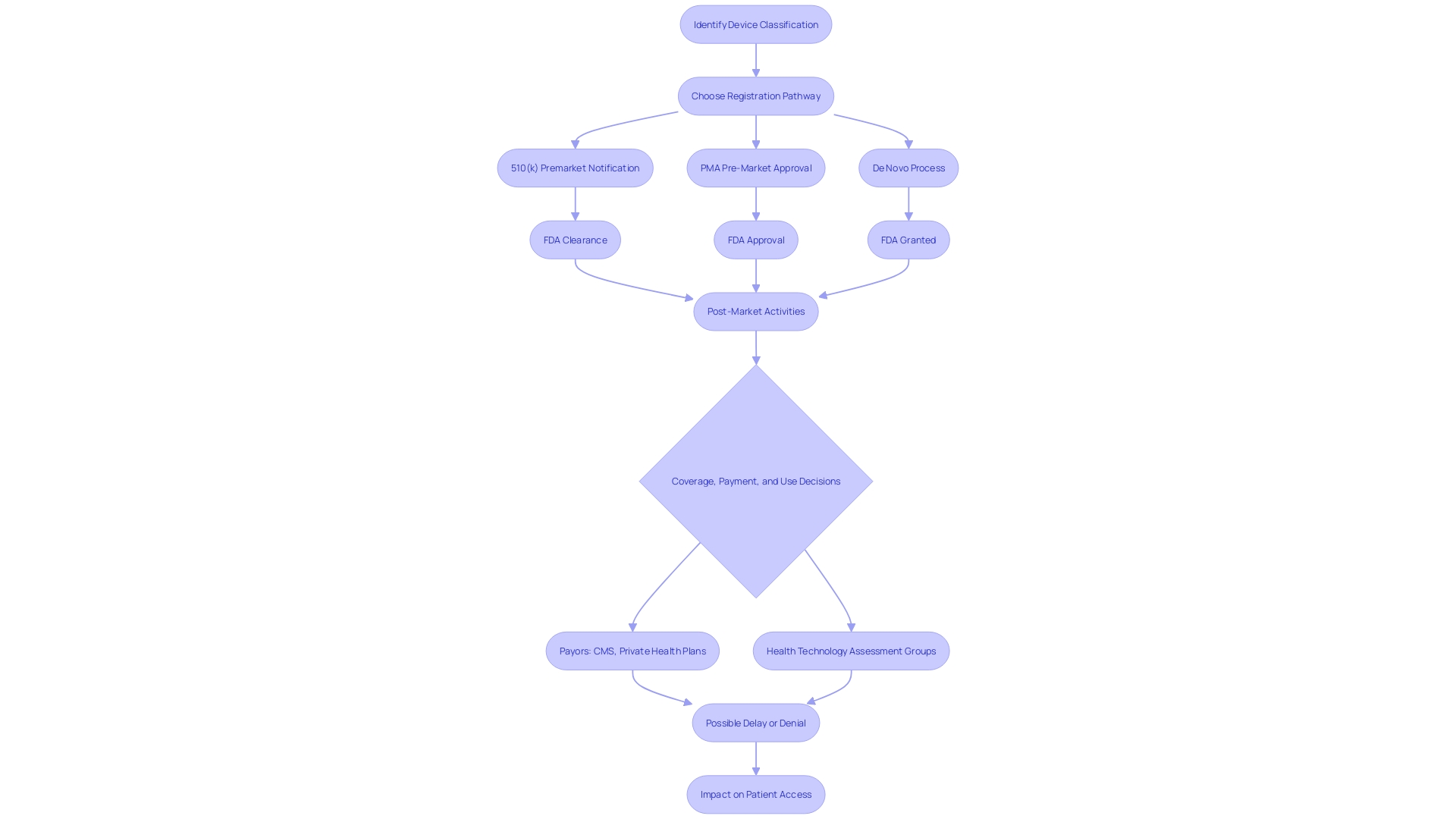
The FDA's responsibility goes further than just registering healthcare equipment; it must also pass, endorse, or authorize them based on their classification and the information provided by manufacturers demonstrating their safety and effectiveness. It's vital for regulatory professionals to understand the nuances and implications of terms like Registered, Cleared, Approved, and Granted. Moreover, the data submitted to the FDA may differ from what payors, such as CMS and private health plans, require for coverage decisions, potentially leading to delays or denials in coverage and impacting patient access to these technologies.
Medical instruments play a crucial part in healthcare, assisting in diagnosis, treatment, and enhancing patient quality of life. They range from simple tools like tongue depressors to complex technologies like prostheses and diagnostic software, each according to their purpose and indications for use. Understanding the proper classification and regulatory pathway for a product is crucial for manufacturers aiming to bring their healthcare innovations to the U.S. market, ensuring compliance and facilitating patient access to life-enhancing medical equipment.
Types of Medical Devices and Their Regulatory Pathways
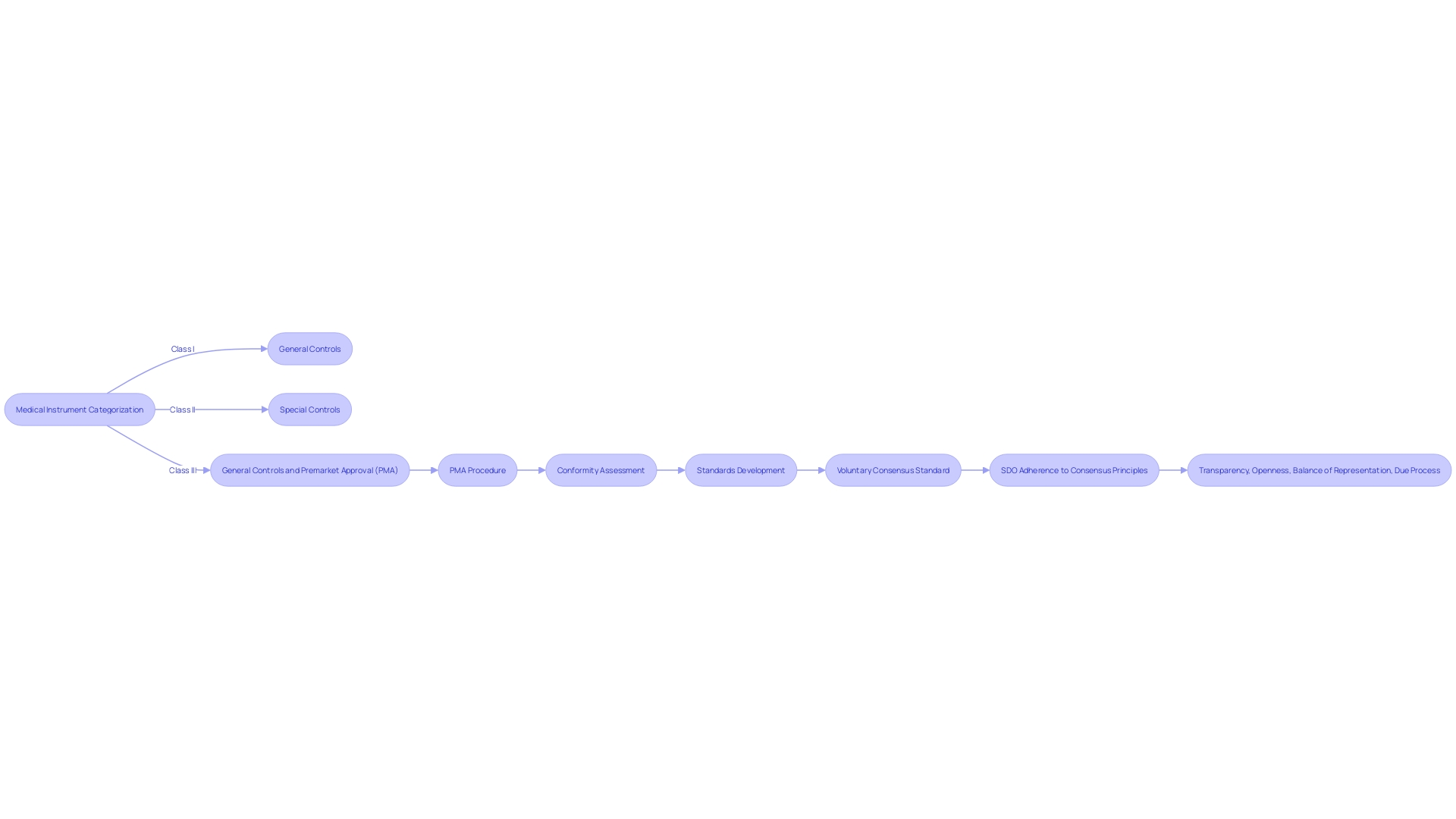
The categorization of medical instruments by the Food and Drug Administration (FDA) is a crucial measure in guaranteeing the well-being and efficiency of these products. Objects are classified into three primary categories, each representing the degree of regulation required to ensure the well-being and efficiency of the object. Class I products are considered low risk and are subject to general controls. Class II instruments, which pose higher risk than Class I, necessitate supplementary regulatory measures, referred to as special controls, to ensure their reliability and effectiveness. Class III products, representing the highest risk category, support or sustain human life, are for a use that is of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury. Therefore, they are required to go through the pre-market approval (PMA) procedure, a thorough scientific and regulatory assessment to guarantee the safety and efficacy of the product.
Around 10% of products in the field of health fall into Class III and include life-critical items such as pacemakers. The rigorous PMA procedure entails a comprehensive analysis of scientific and clinical evidence to evaluate the benefits and risks of the equipment. This procedure is crucial in the administration of healthcare equipment that has a fundamental function in patient care and treatment. As the healthcare equipment industry progresses, it is vital for producers to effectively navigate these regulatory routes, comprehending that the information provided to the FDA may vary from what payors demand for coverage determinations, potentially resulting in delays in patient access to innovative technologies.
Moreover, voluntary consensus standards developed by Standards Development Organizations (SDOs) play a significant role in shaping regulatory quality. These standards are grounded in principles of transparency, openness, balance of representation, and due process. Thorough conformity assessments are essential to a strong regulatory structure, guaranteeing that healthcare instruments satisfy the required criteria for safety and performance. The FDA's dedication to public health is apparent in its continuous efforts to safeguard consumers by regulating not only pharmaceuticals, but also human and veterinary drugs, vaccines, and other products crucial to the well-being and safety of the nation.
Key Characteristics of Class III Medical Devices
Class III tools are crucial to public health, often playing a vital role in sustaining or supporting life. These items, which encompass implantables such as pacemakers, represent a segment of high-risk medical products due to their complexity and critical functions. Acknowledged by governing bodies like the FDA in the US and supervised by the EMA in Europe in collaboration with EU Member States, these instruments are subjected to rigorous regulatory examination. Such instruments must go through a thorough pre-market approval procedure, which evaluates their safety and efficacy in a more rigorous way compared to their Class I and II counterparts.
Roughly 10% of equipment regulated by the FDA fit into this category, demonstrating their specialized nature and the thorough evaluation required to ensure their reliability. Considering their capacity to have a major effect on the health of patients, Class III products go through a comprehensive evaluation, including a review of clinical data, to authenticate their application as life-preserving or life-sustaining remedies. Recent initiatives to streamline regulatory pathways, especially those amplified by the COVID-19 pandemic, highlight the continuous efforts to improve the efficiency of the approval procedures for such crucial healthcare equipment, while upholding the rigorous standards required for patient well-being.
PMA Submission Process
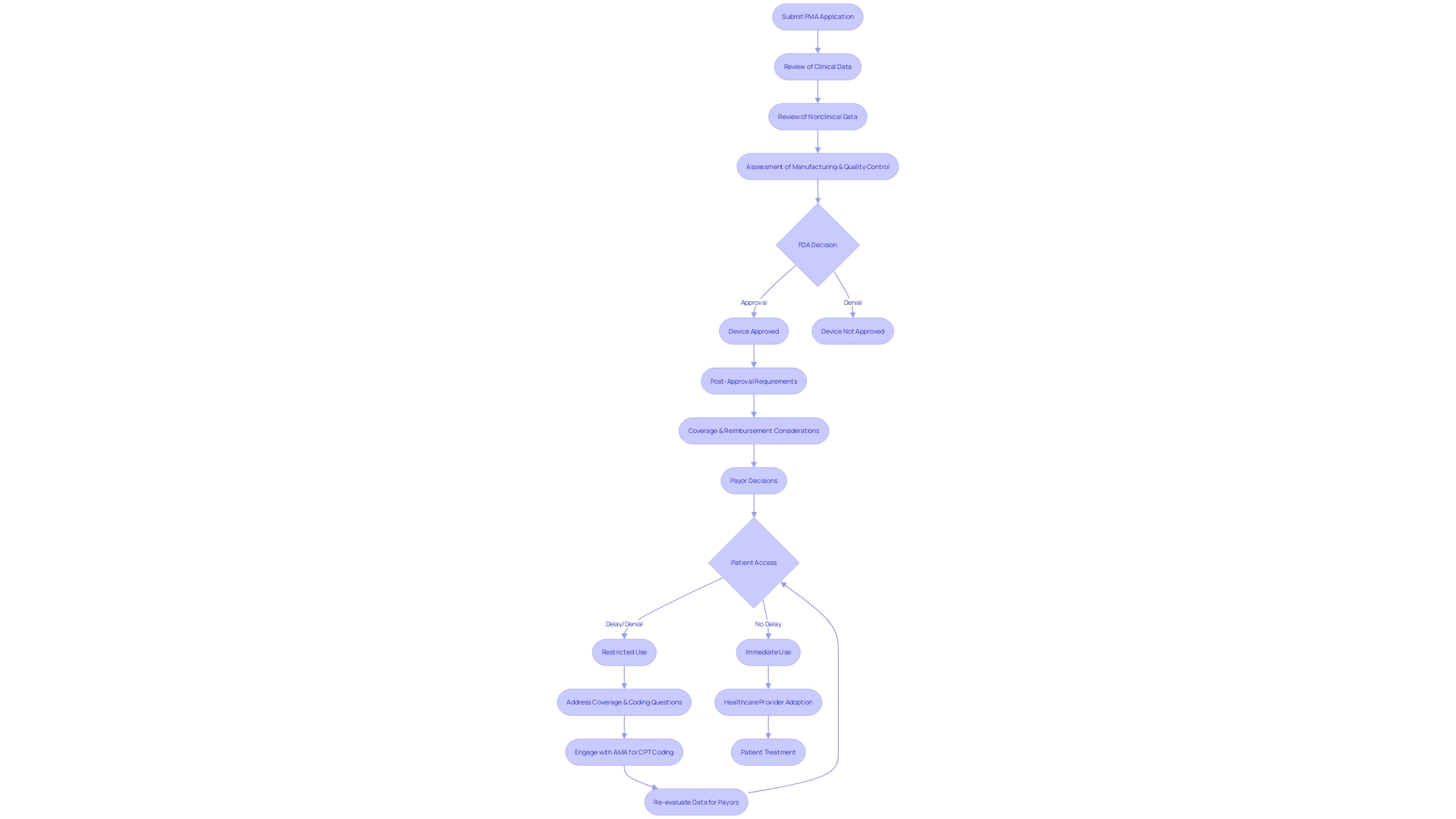
The Premarket Approval (PMA) is a crucial procedure for Class III products, which are regarded as high risk due to their substantial role in sustaining or supporting life. These instruments, such as implantable pacemakers, constitute around 10% of medical items regulated by the FDA and undergo a rigorous evaluation to ensure their safety and effectiveness. The PMA process begins with a comprehensive application that encompasses extensive data from clinical and nonclinical studies, demonstrating the intended use and robustness of the product. Upon submission, the FDA meticulously evaluates the application against stringent regulatory standards. It is important to recognize that the FDA's clearance or approval does not automatically translate to coverage decisions by payors. These organizations, including CMS and private health plans, require their own set of data to assess the product's value, which could lead to delays or denials even after FDA approval. Moreover, the utilization of voluntary agreement norms, as determined by institutions such as SDOs, supports the regulatory structure, guaranteeing openness and engagement of interested parties in the establishment of standards for equipment. These standards are crucial in promoting innovation and ensuring that new healthcare technologies meet the highest quality standards for patient access. Considering the changing healthcare environment, regulatory procedures are being improved to accelerate authorizations for products addressing unfulfilled healthcare requirements, as observed during the COVID-19 outbreak, especially in the emerging areas of digital well-being and individualized therapy.
PMA Application Requirements
To obtain Premarket Approval (PMA) from the FDA, a medical instrument must go through a thorough examination to exhibit its reliability and efficacy. This includes a comprehensive submission of detailed information about the equipment's intended use, clinical performance data, and safety assessments. Moreover, the submission should cover an explanation of the manufacturing procedures, quality assurance measures, and labeling, along with documentation for any expected alterations. The categorization of the equipment, which indicates the linked patient risk, decides the particular course for registration, whether via a 510(k) notice, PMA, or De Novo procedure. Only after receiving FDA clearance, approval, or a grant for De Novo can an item be legally marketed in the United States. This complex procedure is crucial for guaranteeing that healthcare tools meet strict criteria and can safely be employed to identify, prevent, monitor, manage, or alleviate ailments or injuries, ultimately enhancing patient results and quality of life.
Components of a PMA Application
The Premarket Approval (PMA) application is a rigorous process that encompasses an extensive dossier to demonstrate a medical instrument's safety and efficacy. It contains a cover letter and detailed sections such as apparatus description, nonclinical and clinical study data, manufacturing information, labeling, and proposed indications for use. Each aspect is crucial to validate the readiness of the product for market entry. Beyond the premarket phase, Post-Market Surveillance (PMS) becomes a pivotal component, ensuring products continue to perform safely and effectively in real-world conditions through various data collection methods. These may involve passive surveillance like spontaneous reporting and active surveillance via registries, leveraging electronic health records and databases to maintain a product's lifecycle integrity. As revealed by a recent study by Perfuze, which seeks to enroll patients for an interventional study, continuous innovation and monitoring are vital in healthcare technology. Meanwhile, the market for healthcare instruments, such as corneal implants, is projected to see substantial growth, emphasizing the significance of maintaining strong PMA and PMS protocols to support this thriving industry.
The Role of Clinical and Nonclinical Studies in PMA
In the field of medical tool regulation, the Premarket Approval (PMA) pathway is a crucial route that manufacturers must follow to show the effectiveness and soundness of their tools. This rigorous process involves a series of nonclinical and clinical studies. Nonclinical studies provide initial information on the product's well-being through laboratory tests and animal studies. These foundational studies are essential in identifying any potential risks before human trials. Clinical studies, which involve human participants, are crucial for evaluating the effectiveness and reliability of the apparatus within a clinical setting.
The nonclinical phase lays the foundation for clinical studies by confirming the basic safety profile of the equipment. This is a crucial step, as it determines whether the equipment can proceed to human trials. Chris, a biomedical engineer with significant experience in managing clinical studies for Class III products, emphasizes the importance of these initial tests in mitigating risks during subsequent clinical trials. Clinical research, where the medical instrument is tested within a patient population, is where the instrument's true performance and impact on patient health are observed. The data collected from these stages make up the core of the evidence presented in favor of a PMA application, emphasizing the potential of the equipment to enhance patient care.
The importance of these studies is further underscored by the recent findings linking Parkinson's disease with a higher risk of autoimmune disorders. Such insights into disease mechanisms can greatly impact the design and focus of clinical trials, guaranteeing that the technology developed meets the nuanced needs of patients with complex conditions. With the introduction of new healthcare instruments, the PMA evaluation, with its thorough approach to assessing safety and efficacy, continues to be a fundamental aspect in the advancement of healthcare and the provision of novel therapeutic alternatives to patients.
Quality System Regulation and Its Importance in PMA
The Quality System Regulation (QSR) is a cornerstone of the Premarket Approval (PMA) process, setting forth the regulations and requirements that oversee the entire lifecycle of medical devices—from design and manufacturing to distribution. Compliance with QSR is not just a regulatory checkbox but a complete dedication to quality, ensuring that products are consistently manufactured to the highest standards. This is especially important for Class III equipment, which undergoes rigorous scrutiny in clinical studies and Pivotal, PMA, and post-market registries.
Experts such as Chris, a biomedical engineer with 13 years of expertise, recognize the crucial function that QSR has in the triumph of healthcare instruments. Working with Greenlight Guru, Chris leverages his expertise to guide manufacturers through the complexities of QSR compliance. This emphasis on quality is echoed by the FDA-AAMI Nexus, which has been urging the industry to foster a culture of quality—a sentiment reinforced by the FDA's Keisha Thomas, who indicates that the FDA views the QSR overhaul as integral to this quality push, hinting at more stringent manufacturing expectations in the future.
Furthermore, the QSR's recognition of risk management's significance cannot be overstated. By embedding risk management into every stage, from design to post-market surveillance, manufacturers are equipped to proactively address potential risks, leading to safer, higher-quality medical devices. To enhance the measures of precaution, adherence to standards like ISO 14971 is vital, providing a comprehensive risk management framework that complements the QSR.
Patient well-being remains the topmost priority, as emphasized by the recent announcements of the UK Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA's innovative 'roadmap' for regulation of healthcare equipment aims to prioritize patient safety, facilitate access to necessary tools, and foster a conducive environment for technology innovators. The organization's strategy objective for global acknowledgement of healthcare equipment further emphasizes the significance of standardizing rules to enable the introduction of groundbreaking instruments that can improve patient well-being.
Post-Market Surveillance (PMS) is an essential component of this ecosystem, expanding the quality assurance of healthcare equipment beyond pre-market testing. Through various data collection methods—ranging from spontaneous reporting to the use of electronic health records—PMS ensures the continuous evaluation and improvement of equipment performance in real-world scenarios. This continuous watchfulness is crucial for preserving the long-term well-being and efficiency of instruments available in the market.
Medical Device Tracking and Reporting Requirements
The Premarket Approval (PMA) pathway for Class III medical technologies is a meticulous and crucial method to guarantee the effectiveness and reliability of advanced medical innovations. As part of this process, manufacturers are mandated to implement comprehensive tracking systems and post-market surveillance (PMS) strategies. These systems are created to gather information on the real-world performance of their equipment, which is crucial for identifying potential concerns regarding well-being, monitoring the overall functionality of the equipment, and determining the need for product recalls or adjustments. The significance of PMS was emphasized by a 2018 study which revealed that over a 10-year period, healthcare instruments were potentially associated with more than 1.7 million injuries and 83,000 deaths in the United States.
To effectively manage PMS, manufacturers deploy various data collection methods, including both passive and active surveillance systems. Passive systems might involve spontaneous reporting by healthcare professionals and patients, whereas active systems could include patient registries or studies, and the use of electronic health records and administrative databases. This ongoing monitoring in real-world conditions is not only crucial for patient well-being but also for enhancing the performance and reliability of medical devices over time.
Additionally, adherence to these post-market requirements is not only a regulatory obstacle but also an indication of a manufacturer's dedication to patient well-being and product quality. As emphasized by industry experts, integrating quality and regulatory considerations from the earliest stages of product development can streamline the approval process and mitigate risks. Yet, in practice, quality and regulatory concerns are often relegated to later stages, which can lead to retroactive modifications and potential resource constraints.
The FDA plays a crucial part in the supervision of healthcare instruments, guaranteeing that the well-being of the public is safeguarded by verifying the safety and effectiveness of these items. The organization's recent initiatives, like the final rule for direct-to-consumer prescription drug advertisements, emphasize their dedication to clear and understandable communication, a principle that aligns with the transparency required in tracking and reporting.
For manufacturers of healthcare equipment, keeping up-to-date with both local and global regulations is crucial, as the worldwide distribution of their products requires a thorough comprehension of various regulatory environments. Some companies opt for global regulatory compliance to cover all markets. This not only covers the broad range of product technologies and lifecycles but also guarantees the ongoing quality, safety, and effectiveness of items throughout their useful life, as outlined in FDA's guidance differentiating between 'servicing' and 'remanufacturing' activities.
In brief, adherence to PMA requirements, including strong tracking and post-market surveillance, is crucial for ensuring that Class III products remain safe and effective throughout their lifecycle. The integration of quality and regulatory strategies early in product development, coupled with continuous market surveillance, serves as the foundation for maintaining high standards in patient care and innovation.
PMA Review Process and Timeline
The Premarket Approval (PMA) procedure conducted by the FDA is a meticulous and crucial pathway for high-risk medical equipment, guaranteeing that they fulfill the required security and efficacy criteria prior to entering the market. The scrutiny applied to PMA applications is extensive, involving meticulous examination of clinical and nonclinical data. This process is not only about compliance but also about a partnership with developers to align on the shared goal of patient safety and therapeutic advancement.
The timeline for this review varies, influenced by factors such as the equipment's complexity and the comprehensiveness of the submitted data. On average, the PMA review can span from several months to over a year—a reflection of the FDA's commitment to thorough evaluation. It is important to mention that only a portion of instruments used in the field of medicine, roughly 10%, necessitate this degree of evaluation, usually those that aid or maintain life, such as pacemakers.
The FDA's mission extends beyond approval, as it also influences coverage and reimbursement decisions made by payors, such as CMS and private health plans. These organizations consider FDA approval as a critical factor but also evaluate additional data to make informed decisions on coverage, which can lead to delays or denials even after FDA clearance.
In the wider perspective, the FDA's duty is to safeguard public health by ensuring the safety and security of other products and equipment. In doing so, the agency adapts to emerging health needs and technological advancements, as seen through the push for more streamlined regulatory pathways, especially during critical times like the COVID-19 pandemic. These efforts aim to expedite the delivery of innovative healthcare solutions to patients, particularly in areas like digital health and personalized medicine.
The FDA's Center for Drug Evaluation and Research (CDER) plays a similar role in drug regulation, providing guidance for drug developers to support comprehensive assessments of new drugs and biological products. The center leverages scientific understanding to evaluate new therapies, which ultimately leads to a diverse array of approved treatments each year, expanding the horizons of healthcare.
Alternative PMA Submission Pathways (Modular PMA, PDP)
For Class III products that are crucial and necessary for maintaining or assisting life—such as implantable pacemakers—manufacturers may choose alternative routes to the conventional Premarket Approval (PMA) procedure. The modular PMA option allows for submission in segments, facilitating a more efficient review by the FDA and potentially hastening the availability of crucial components in the market. Delve’s Senior Director of Interaction Design, Ken Soliva, underscores the importance of such innovations in offering better interactions with technology. Furthermore, the Product Development Protocol (PDP) serves as an alternative pathway, especially for products still in progress that offer to address unfulfilled healthcare requirements. The PDP enables a collaborative review, providing manufacturers with timely feedback during various development stages.
Archetype, a MedTech innovation consultancy, highlights the intricacy of acquiring market approval for healthcare instruments. With the National Library of Medicine reporting that approximately three-quarters of MedTech innovations do not reach the market, pathways like modular PMA and PDP become critical for innovators. Dr. Stuart Grant, Archetype's Principal Consultant, with his vast experience in orthopedic technology, emphasizes the diverse and ever-changing nature of the MedTech product approval process. These alternative pathways align with the project management principles in the healthcare equipment industry, where patient safety and product quality are prioritized to improve patient outcomes. As medical devices are subject to rigorous regulatory controls and classifications by the FDA and involvement by the EMA in Europe, these pathways offer a structured yet flexible approach to navigate the intricate regulatory landscape.
Conclusion
In conclusion, the PMA process by the FDA is a stringent examination for Class III medical devices. These devices undergo comprehensive evaluations to ensure their safety and efficacy before entering the market. Medical devices play a vital role in healthcare, improving patient quality of life and aiding in diagnosis and treatment.
Manufacturers must understand device classification and regulatory pathways to ensure compliance and patient access to life-enhancing technologies.
Device classification into risk-based classes is crucial for safety and effectiveness. Class III devices, such as life-supporting technologies, undergo extensive scientific and regulatory reviews. Efforts to streamline regulatory pathways have been made to expedite approvals for devices addressing unmet medical needs, especially during the COVID-19 pandemic.
The PMA submission process involves detailed applications with data from clinical and nonclinical studies. The FDA evaluates applications against rigorous standards. It's important to note that FDA approval doesn't guarantee coverage decisions by payors, who may have their own data requirements.
Voluntary consensus standards developed by SDOs ensure devices meet safety and performance standards.
The PMA review process duration varies based on device complexity. The FDA's mission extends beyond approval, influencing coverage and reimbursement decisions. The agency adapts to emerging health needs and technological advancements, expediting innovative medical solutions to patients.
Alternative pathways, like modular PMA and PDP, offer efficient review processes and timely feedback during development stages for Class III devices. Adherence to PMA requirements, including device tracking and post-market surveillance, is crucial for device safety throughout their lifecycle. Integrating quality and regulatory strategies early in product development, along with continuous market surveillance, maintains high standards in patient care and medical innovation.




