Introduction
In the complex landscape of medical device regulation, understanding the role of predicate devices is paramount for manufacturers navigating the FDA's 510(k) submission process. Predicate devices, which have already received FDA clearance, serve as essential benchmarks for establishing substantial equivalence of new devices. This foundational concept not only influences regulatory compliance but also underscores the need for rigorous safety and efficacy standards.
As the medical technology sector continues to evolve, the implications of predicate devices become increasingly significant, particularly in light of recent analyses highlighting their impact on device recalls and patient safety.
This article delves into the intricacies of predicate devices, outlining best practices for their identification, documentation, and utilization within the 510(k) framework, while also addressing common challenges faced by manufacturers in the submission process.
Defining Predicate Devices: The Foundation of FDA 510(k) Submissions
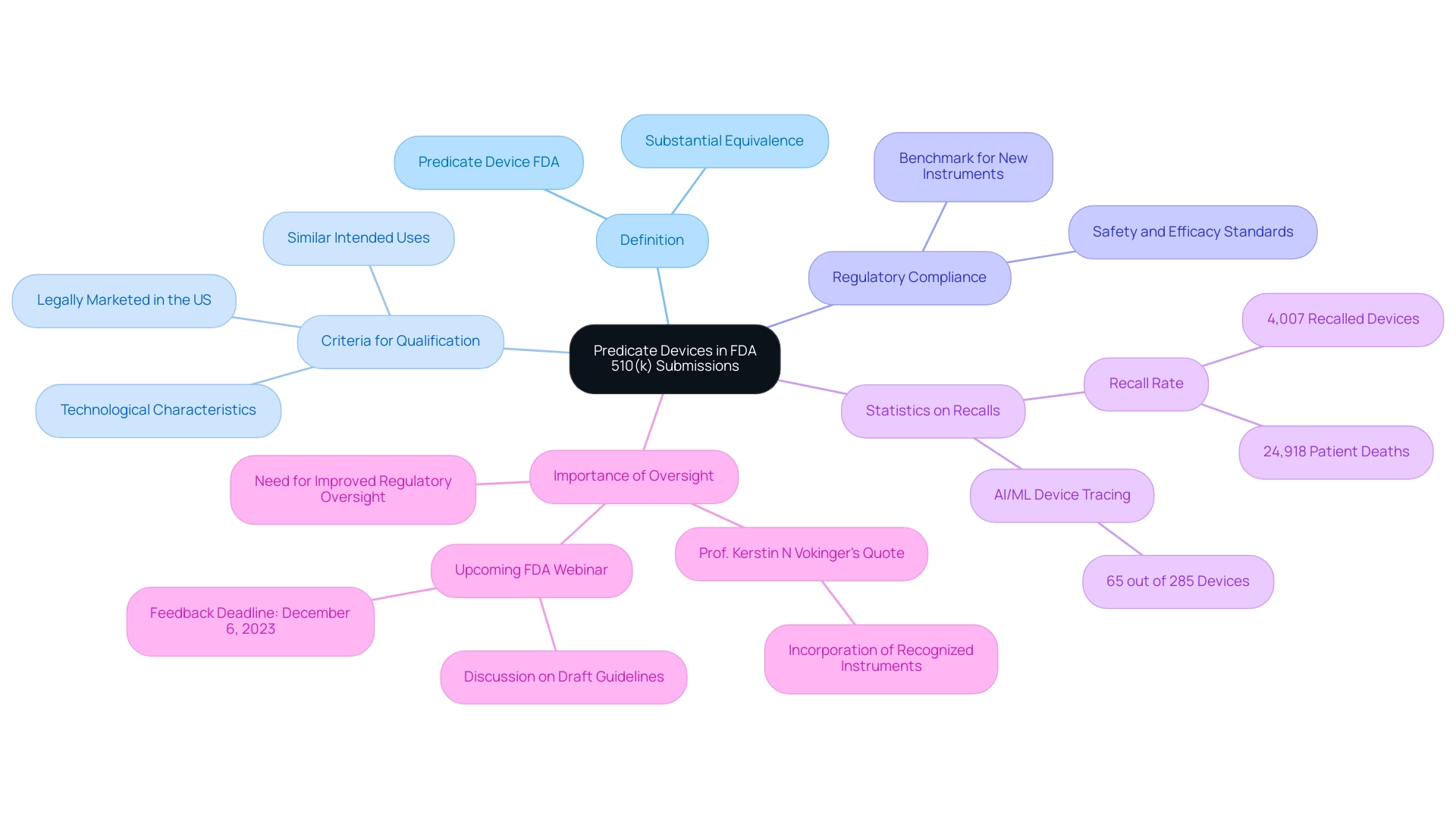
A type of medical equipment that has obtained clearance from the FDA via a 510(k) application is referred to as a predicate device FDA, serving as an essential benchmark for new instruments aiming to show substantial equivalence. For a product to qualify as a predicate device FDA, it must be legally marketed in the United States and share similar intended uses and technological characteristics with the new product. This definition is foundational, as it directly influences the FDA 510(k) submission process for the predicate device FDA.
Predicate devices FDA not only provide a benchmark for regulatory compliance but also ensure that the new products are founded on established safety and efficacy standards. Recent analyses uncover the significant implications of classification tools in regulatory oversight, particularly as 65 out of 285 AI/ML-based medical instruments were traced back to de novo cleared products, illustrating their critical role in the evolution of medical technology. Furthermore, a study on recalls and patient deaths associated with 510(k) products found that among 35,176 items analyzed, 4,007 (11.4%) were recalled, linked to 24,918 patient fatalities from 2003 to 2020.
This underscores the need for improved regulatory oversight. As Prof. Kerstin N Vokinger observed, 'The incorporation of recognized instruments is essential for guaranteeing patient safety and efficient regulatory practices.' Moreover, the FDA will conduct a webinar on October 26 to discuss draft guidelines related to reference products, accepting feedback until December 6, 2023, emphasizing the ongoing importance of this subject in medical product regulation.
Establishing Substantial Equivalence: Key Criteria and Evaluation Process
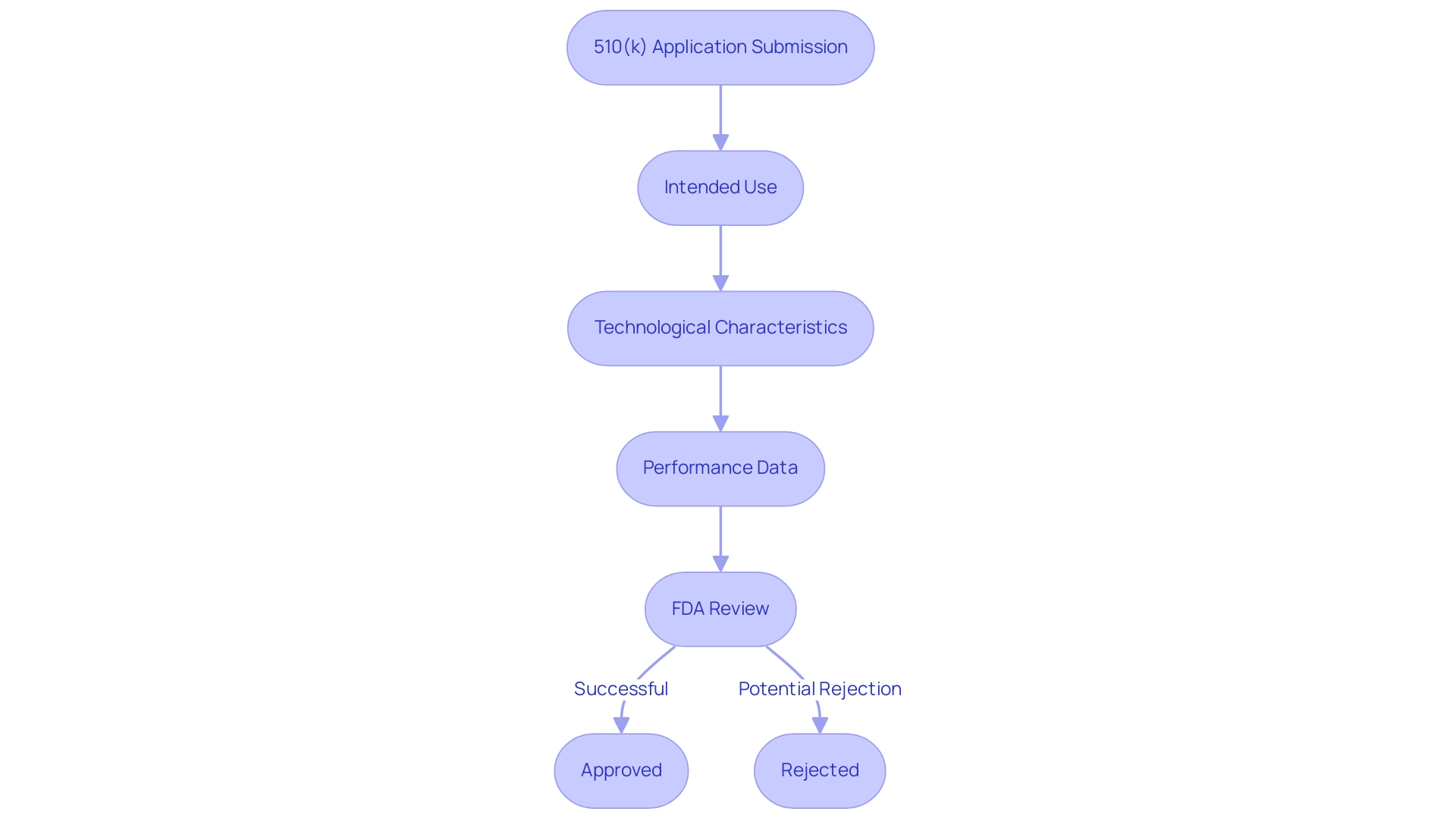
Establishing substantial equivalence is a crucial step in the 510(k) application process, requiring manufacturers to convincingly demonstrate that their new product is as safe and effective as an existing reference product. This evaluation encompasses a comprehensive comparison across several key aspects, including intended use, design, materials, and performance specifications. The FDA conducts a meticulous review, considering both clinical and non-clinical data to ensure compliance with Regulatory standards.
Experts like Ana Criado, with her extensive background in Regulatory Affairs, biomedical engineering, and health economics, underscore the importance of this process, particularly in the context of Colombian regulations. Ana has directly contributed to numerous successful regulatory submissions, providing invaluable insights into the nuances of the 510(k) process. The main criteria for determining substantial equivalence, as defined by the predicate device FDA, include:
- Intended Use: The item's purpose must align with that of the reference.
- Technological Characteristics: Similarities in design and materials are necessary to affirm equivalence.
- Performance Data: Manufacturers must provide robust evidence demonstrating that the new product performs comparably to the predicate device FDA.
It's important to note that a 510(k) filing may be rejected not solely due to inaccuracies but also due to the absence of compelling arguments supporting substantial equivalence; indeed, a filing can be rejected simply for lacking convincing arguments.
A comprehensive strategy, such as clearly separating intended use from technological characteristics and confirming the device classification, can significantly enhance the likelihood of a successful application while minimizing the risk of rejection. As a cautionary note, if you see a lot of Red, maybe this is not a good predicate or if there is no other predicate, then it could become a 'de novo'. Additionally, the 510(k) Statement must be left blank for new entries until the 510(k) number is received in the acknowledgment letter.
As an example, historical data shows that the first 510(k) filing, K760001, took place in 1976, signifying the start of an essential Regulatory route for medical equipment. Katherine Ruiz's expertise in Regulatory Affairs for medical equipment and in vitro diagnostics further highlights the significance of complying with these standards in the changing environment of medical technology. Katherine has successfully navigated complex regulatory challenges, offering guidance that can assist clinical researchers in aligning their studies with regulatory expectations, ultimately facilitating easier applications and approvals.
Navigating the Selection Process: Steps to Identify and Document a Predicate Device
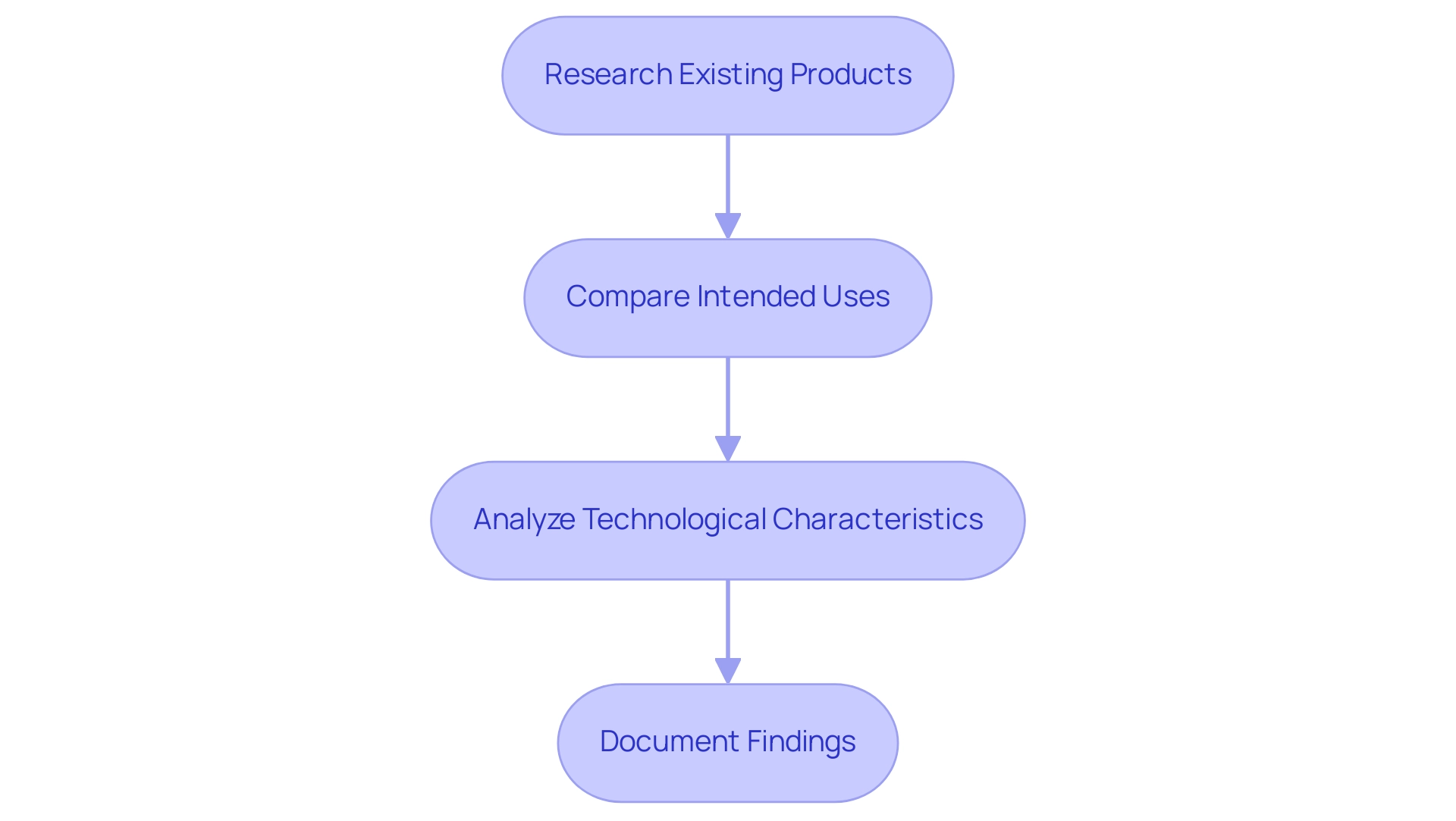
To successfully identify and document a comparable product for a 510(k) submission, adhere to the following steps:
-
Research Existing Products: Begin by utilizing the FDA's 510(k) database, which has seen an uptick in usage, to identify products that have previously received clearance, such as the Bipolar Forceps cleared on 6/24/1991. This database is essential for ensuring that the referenced item is relevant and suitably categorized.
-
Compare Intended Uses: Verify that the intended use of the reference item aligns with that of your new item.
Discrepancies in intended use are a common reason for receiving a Not Substantially Equivalent (NSE) letter from the FDA, as outlined in the case study regarding NSE letters, which highlights the critical nature of this comparison.
-
Analyze Technological Characteristics: Conduct a thorough evaluation of the design, materials, and performance features of potential comparable items to confirm similarities. This analysis is vital in establishing substantial equivalence.
-
Document Findings: Compile a comprehensive report detailing the comparison between your apparatus and the selected predicate. This documentation should include references to relevant FDA guidance, product codes, and clear explanations of how your predicate device meets the criteria for substantial equivalence. Such thorough documentation is essential for the filing process and must be precise to facilitate FDA review.
By following these steps, you can enhance the likelihood of a successful 510(k) application. Furthermore, the heightened reference to the FDA's 510(k) database, highlighted in the RDC statistic, emphasizes its importance in the application process.
Common Challenges in 510(k) Submissions: Avoiding Pitfalls with Predicate Devices
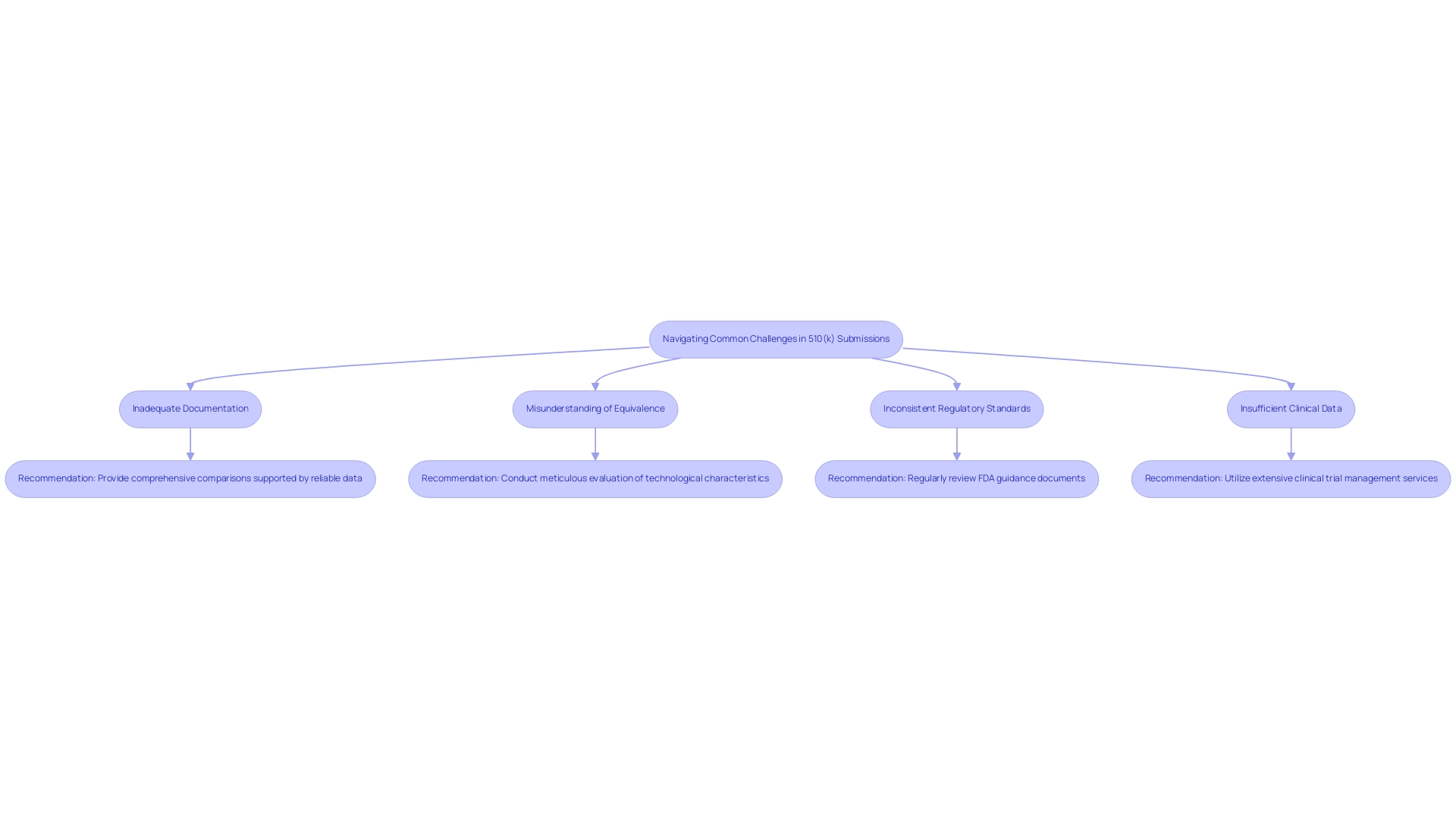
The 510(k) application process presents several common challenges that manufacturers must navigate effectively to enhance their chances of approval. Recently, the FDA launched a program for lowered medical equipment user fees for small businesses, which can assist producers in handling application costs. Key issues include:
-
Inadequate Documentation: Insufficient documentation concerning the reference item can significantly postpone the filing process.
It is crucial to provide comprehensive comparisons supported by reliable data and references.
-
Misunderstanding of Equivalence: Manufacturers frequently encounter challenges in precisely evaluating the equivalence of their products to current standards, which can lead to submission rejections.
A meticulous evaluation of technological characteristics is essential to demonstrate substantial equivalence.
As Mike Drues, President of Vascular Sciences, aptly notes,
The whole premise of a 510(k) is that you are demonstrating substantial equivalents to a predicate device FDA.
-
Inconsistent Regulatory Standards: The evolving nature of FDA regulations can complicate compliance.
Regularly reviewing FDA guidance documents and updates is imperative to stay informed.
-
Insufficient Clinical Data: When clinical data is mandatory, it must be robust and effectively convey the safety and effectiveness of the product.
A recent study titled 'Implications for Device Safety and Regulatory Practices' highlighted that while six specialties showed a decreased risk of recall compared to orthopedic surgery, the importance of strong clinical evidence remains paramount.
To effectively tackle these challenges, utilizing extensive clinical trial management services—such as feasibility studies, site selection, compliance evaluations, trial setup, project oversight, and reporting—guided by specialists like Katherine Ruiz can greatly enhance applications and boost the chances of regulatory approval.
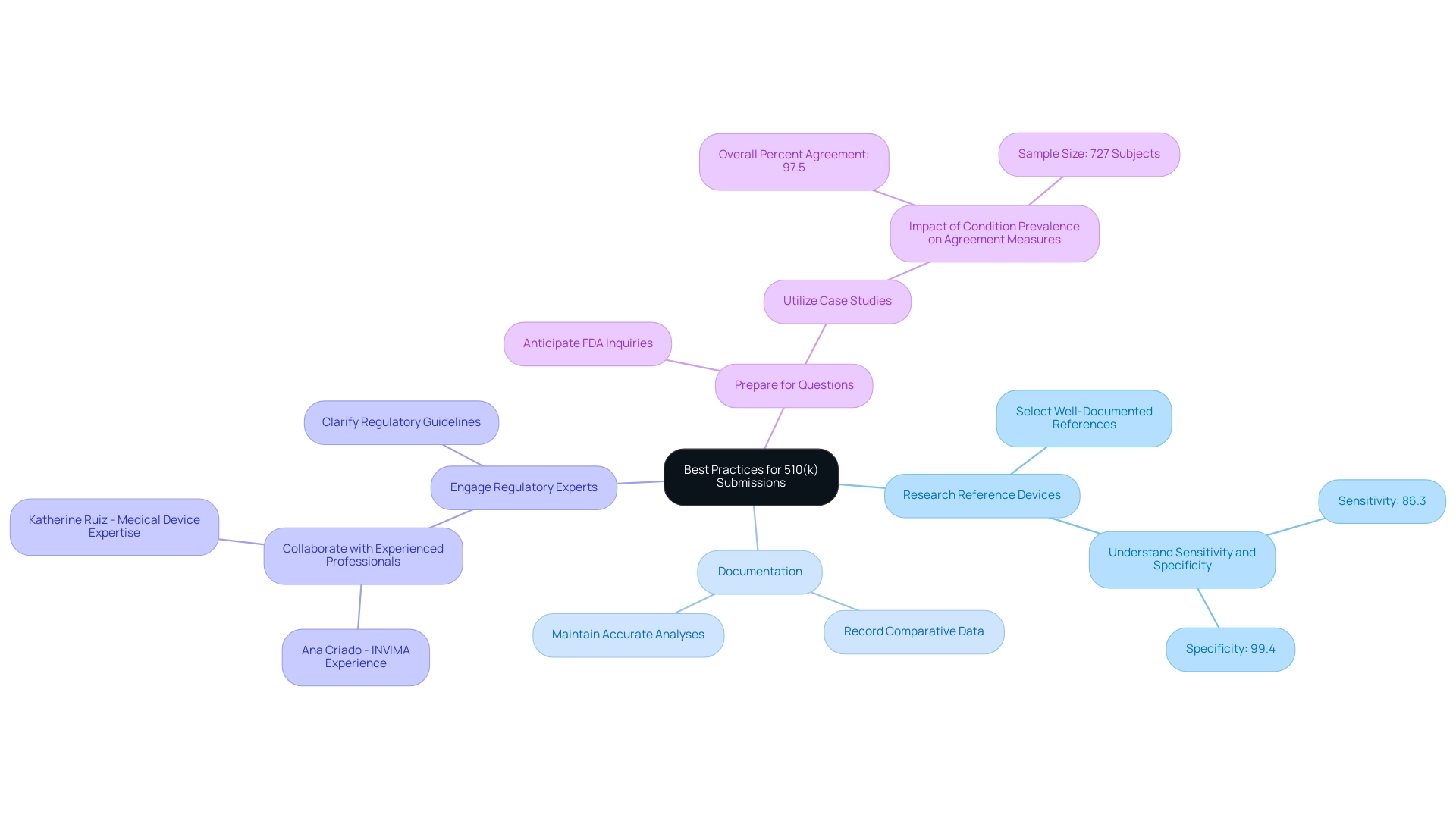
Best Practices for Utilizing Predicate Devices in 510(k) Submissions
To improve the chances of a successful 510(k) filing involving reference devices, it is essential to adopt the following best practices regarding the predicate device FDA:
-
Thoroughly Research Reference Devices:
Select references that demonstrate substantial similarity and are well-documented. This strategic choice strengthens your submission by providing a solid foundation for equivalence. For instance, understanding the sensitivity of 86.3% and specificity of 99.4% from relevant studies can reinforce your rationale for selected predicates.
-
Maintain Clear and Accurate Documentation:
Keep meticulous records of all comparative data and analyses that support your claims of equivalence. This level of detail facilitates the FDA's review process regarding the predicate device FDA and mitigates potential concerns.
-
Engage with Regulatory Experts:
Collaborating with Regulatory Affairs professionals, such as Ana Criado, who has over five years of experience at INVIMA and serves as a professor at Universidad Javeriana and Universidad de los Andes, or Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, is crucial.
Their extensive backgrounds ensure compliance with the latest guidelines and assist in clarifying any ambiguities that may arise during the application process. For example, Ana's experience in regulatory oversight and her academic role in training future professionals provide valuable insights into navigating complex regulatory landscapes.
-
Prepare for Potential Questions:
Anticipate inquiries from the FDA regarding your predicate device FDA application and be ready with comprehensive responses. This proactive approach can significantly enhance the review experience.
The notable case study, 'Impact of Condition Prevalence on Agreement Measures,' illustrates how understanding population characteristics can influence agreement measures between tests. It highlights the significance of context in your contributions, especially how the variability in agreement measures according to population traits pertains to the choice of suitable predicate instruments.
With an overall percent agreement of 97.5% derived from a sample of 727 subjects, this study underscores the need for precise documentation and testing strategies in your 510(k) submissions.
Lastly, for any inquiries related to the predicate device FDA, you can contact the FDA at their phone number or email, ensuring you have direct access to regulatory guidance.
Conclusion
Navigating the complexities of the FDA's 510(k) submission process necessitates a thorough understanding of predicate devices and their critical role in ensuring the safety and efficacy of new medical technologies. By defining predicate devices as benchmarks for establishing substantial equivalence, manufacturers can better align their submissions with regulatory expectations. The importance of accurately identifying and documenting predicate devices cannot be overstated, as these practices directly influence the likelihood of successful regulatory approval.
In addition to understanding the criteria for substantial equivalence, manufacturers must be aware of common challenges that can arise during the submission process. Notable pitfalls that can hinder progress include:
- Inadequate documentation
- Misinterpretation of equivalence
- Insufficient clinical data
By adopting best practices such as:
- Engaging with regulatory experts
- Maintaining meticulous records
- Preparing for potential inquiries
manufacturers can significantly enhance their chances of navigating the 510(k) landscape successfully.
As the medical device industry continues to evolve, the implications of predicate devices remain increasingly significant. The ongoing discussions and updates from the FDA, including upcoming webinars and guidance documents, highlight the need for manufacturers to stay informed and proactive. Ultimately, a commitment to rigorous evaluation and documentation not only fosters compliance but also supports the overarching goal of patient safety in the ever-advancing field of medical technology.
Frequently Asked Questions
What is a predicate device FDA?
A predicate device FDA is a type of medical equipment that has obtained clearance from the FDA via a 510(k) application. It serves as a benchmark for new instruments that aim to demonstrate substantial equivalence.
What criteria must a product meet to qualify as a predicate device FDA?
To qualify as a predicate device FDA, a product must be legally marketed in the United States and share similar intended uses and technological characteristics with the new product.
Why are predicate devices important in the FDA 510(k) submission process?
Predicate devices provide a benchmark for regulatory compliance and ensure that new products are based on established safety and efficacy standards, influencing the FDA 510(k) submission process.
What recent findings highlight the implications of predicate devices in medical technology?
Recent analyses found that 65 out of 285 AI/ML-based medical instruments were traced back to de novo cleared products, illustrating the critical role of predicate devices in the evolution of medical technology.
What concerns have been raised regarding the safety of 510(k) products?
A study analyzing 35,176 510(k) products found that 4,007 (11.4%) were recalled, linked to 24,918 patient fatalities from 2003 to 2020, underscoring the need for improved regulatory oversight.
What is the significance of the FDA's upcoming webinar related to predicate devices?
The FDA will conduct a webinar on October 26 to discuss draft guidelines related to reference products, accepting feedback until December 6, 2023, emphasizing the ongoing importance of this subject in medical product regulation.
What is required to establish substantial equivalence during the 510(k) application process?
Manufacturers must convincingly demonstrate that their new product is as safe and effective as an existing reference product, comparing intended use, design, materials, and performance specifications.
What key criteria are considered for determining substantial equivalence?
The main criteria include: 1. Intended Use: The purpose must align with that of the reference. 2. Technological Characteristics: Similarities in design and materials are necessary. 3. Performance Data: Robust evidence must demonstrate comparable performance to the predicate device FDA.
What can lead to the rejection of a 510(k) filing?
A 510(k) filing may be rejected not only due to inaccuracies but also for lacking compelling arguments supporting substantial equivalence.
What historical significance does the 510(k) process hold?
The first 510(k) filing, K760001, took place in 1976, marking the beginning of an essential regulatory route for medical equipment.




