Overview
Real-world evidence (RWE) plays a pivotal role in clinical trials in Mexico, offering essential insights into the effectiveness and safety of medical products within everyday healthcare contexts. This evidence significantly influences regulatory decisions and enhances patient care.
The article emphasizes how RWE, bolstered by a variety of methodologies and technological advancements, deepens the understanding of treatment outcomes. It also addresses the unique healthcare dynamics present in Mexico, ultimately leading to improved quality in clinical research and better patient outcomes.
Introduction
In the evolving landscape of healthcare, Real-World Evidence (RWE) has emerged as a pivotal element, reshaping how clinical research is conducted and interpreted. Unlike traditional clinical trials, RWE draws from data collected outside controlled environments, providing insights into the effectiveness and safety of medical products in everyday clinical practice.
This article delves into the significance of RWE, particularly in regions like Latin America, where unique healthcare dynamics necessitate a deeper understanding of treatment outcomes across diverse populations. With advancements in technology and regulatory frameworks, the integration of RWE into clinical trials is not just a trend but a crucial step towards enhancing patient care and optimizing healthcare decisions.
Through a series of case studies and expert analyses, the following sections will explore the methodologies, challenges, and future directions of RWE, highlighting its transformative potential in the medical field.
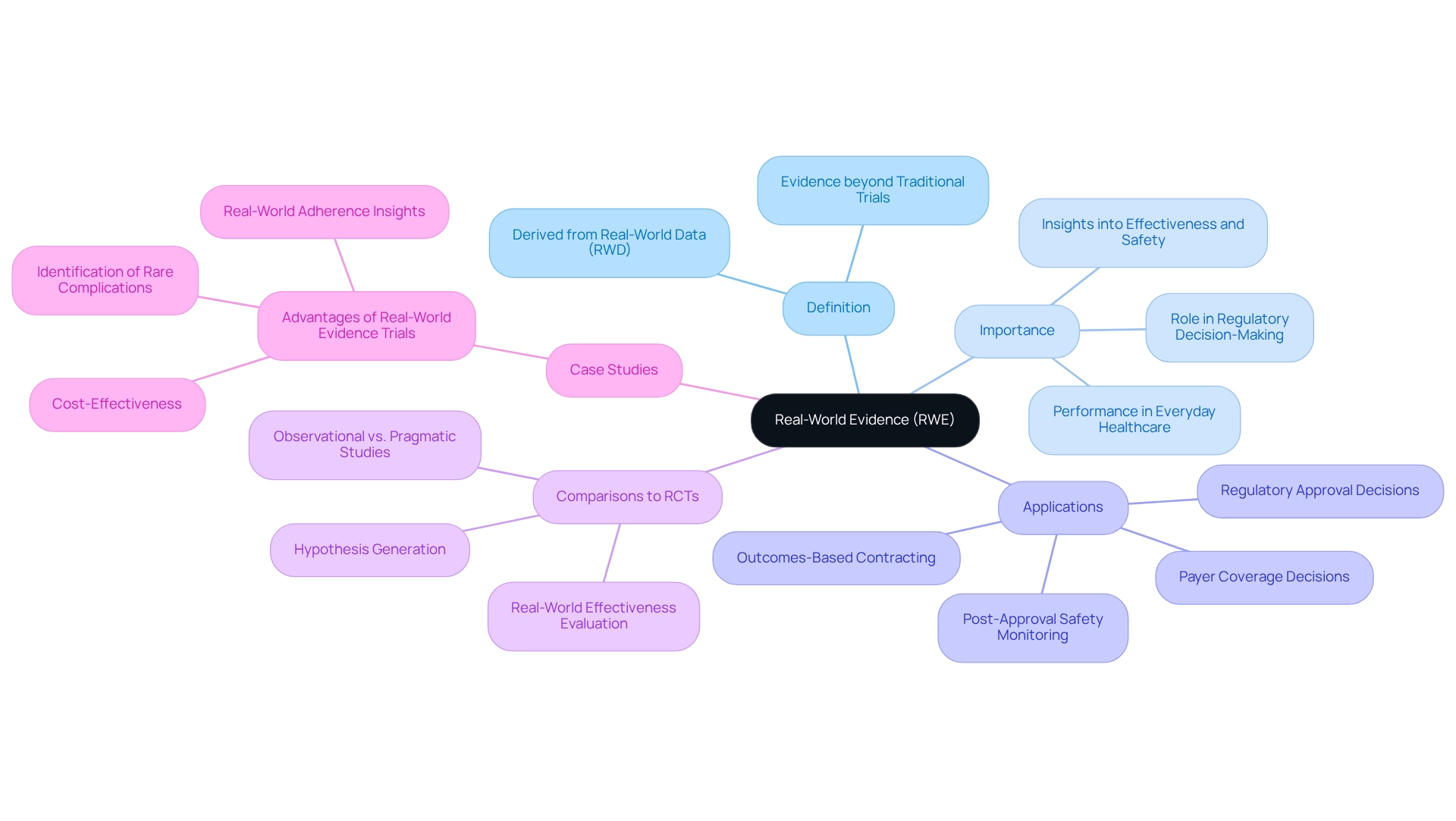
What is Real-World Evidence and Why is it Important?
Real-World Evidence (RWE) is defined as the evidence derived from the analysis of Real-World Data (RWD), which includes information collected beyond the confines of traditional trials. The significance of RWE lies in its ability to illustrate the performance of medical products within everyday healthcare settings, offering crucial insights into their effectiveness, safety, and potential risks. By 2025, RWE is set to play an increasingly pivotal role in regulatory decision-making, practice guidance, and health technology assessments (HTAs).
Integrating RWE into the clinical research framework empowers stakeholders to acquire a nuanced understanding of treatment outcomes across diverse patient populations. This is particularly vital in Latin America, where the real-world evidence for trials in Mexico may be shaped by distinct demographic and healthcare dynamics that influence the efficacy of medical devices. For instance, RWE studies, whether observational or pragmatic, provide a different perspective compared to randomized controlled trials (RCTs).
These studies are especially valuable for generating hypotheses and evaluating the effectiveness of interventions in real-world conditions.
Recent developments underscore the growing dependence on RWE for regulatory approval decisions, post-approval safety monitoring, payer coverage decisions, and outcomes-based contracting. A notable case study titled "Advantages of Real-World Evidence Trials" demonstrates that RWE trials are not only more cost-effective but also proficient at identifying rare complications and shedding light on real-world adherence to treatments. This positions RWE as an essential tool for regulatory decisions and healthcare planning, ultimately enhancing the quality of patient care.
Particularly noteworthy is the presentation of one-year first-in-human data for the VenoValve® by Dr. Jorge Hernando Ulloa at the Charing Cross International Symposium, which highlights advancements in vascular medicine and the critical role of RWE in assessing new medical technologies. bioaccess® is instrumental in managing research studies across Latin America, leveraging real-world evidence for trials in Mexico, and specializing in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. This expertise is vital for navigating the complexities of regulatory frameworks, such as those governed by INVIMA, Colombia's National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO.
INVIMA regulates medical devices, ensuring their safety and efficacy within the Colombian market. As multiple FDA centers increasingly incorporate RWD and RWE into their daily operations, this trend highlights the growing acknowledgment of RWE's role in shaping healthcare decisions. By harnessing the power of RWE, stakeholders are positioned to make informed decisions that enhance patient outcomes and optimize the deployment of medical technologies.
Fang Liu, who conducted the literature review and authored the manuscript, emphasizes the importance of these insights in advancing research within the medical field. The information presented here is timely, reflecting developments as of 05 November 2022.
Navigating the Regulatory Landscape for RWE in Mexico
In Mexico, the regulatory framework governing Real-World Evidence (RWE) is primarily shaped by the Federal Commission for Protection against Sanitary Risks (COFEPRIS). Researchers must adhere to stringent guidelines that dictate the collection and utilization of Real-World Data (RWD), ensuring compliance with ethical standards and data protection laws. A critical component of this framework is the mandatory Health Technology Assessments (HTAs), which necessitate the submission of evidence derived from both randomized trials and RWE.
This dual requirement underscores the importance of integrating RWE into medical studies, enhancing the credibility and acceptance of findings among regulatory bodies and stakeholders within the healthcare system. Furthermore, compliance rates with COFEPRIS regulations have shown significant improvement, reflecting a growing commitment to maintaining high research standards. Notably, Class I Low Risk devices enjoy expedited review and approval times compared to higher classes, illustrating the efficiency of the regulatory process. As of 2025, the regulatory environment continues to evolve, with revised guidelines that further clarify the procedures for gathering RWD in Mexican studies.
Understanding these regulations is paramount for researchers seeking to effectively leverage RWE, as it not only streamlines regulatory approvals but also fosters trust and transparency in the research process. Moreover, the ethical responsibilities of sponsors and CROs are emphasized in the case study titled "Participation and Participant Rights," which highlights the necessity of ensuring that all therapies and procedures associated with research studies are promptly and complimentary offered to participants. This approach promotes trust and ethical conduct in medical research. Furthermore, as noted by Qserve, "With expertise across the entire process, we simplify compliance, providing a seamless path to success in the Mexican market."
By navigating this complex landscape with a comprehensive understanding of the current guidelines, researchers can ensure that their studies contribute valuable insights to the medical community and ultimately enhance patient care. The mention of import permits and USMCA preferential treatment further emphasizes the importance of adhering to regulatory requirements, enhancing the section's comprehensiveness.
At bioaccess®, we specialize in extensive research study management services, which include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
With over 20 years of experience in Medtech, our expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies positions us as a trusted partner in navigating the regulatory landscape in Latin America. Our tailored approach ensures that your medical studies are conducted effectively and ethically, with experts such as Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, leading the process.
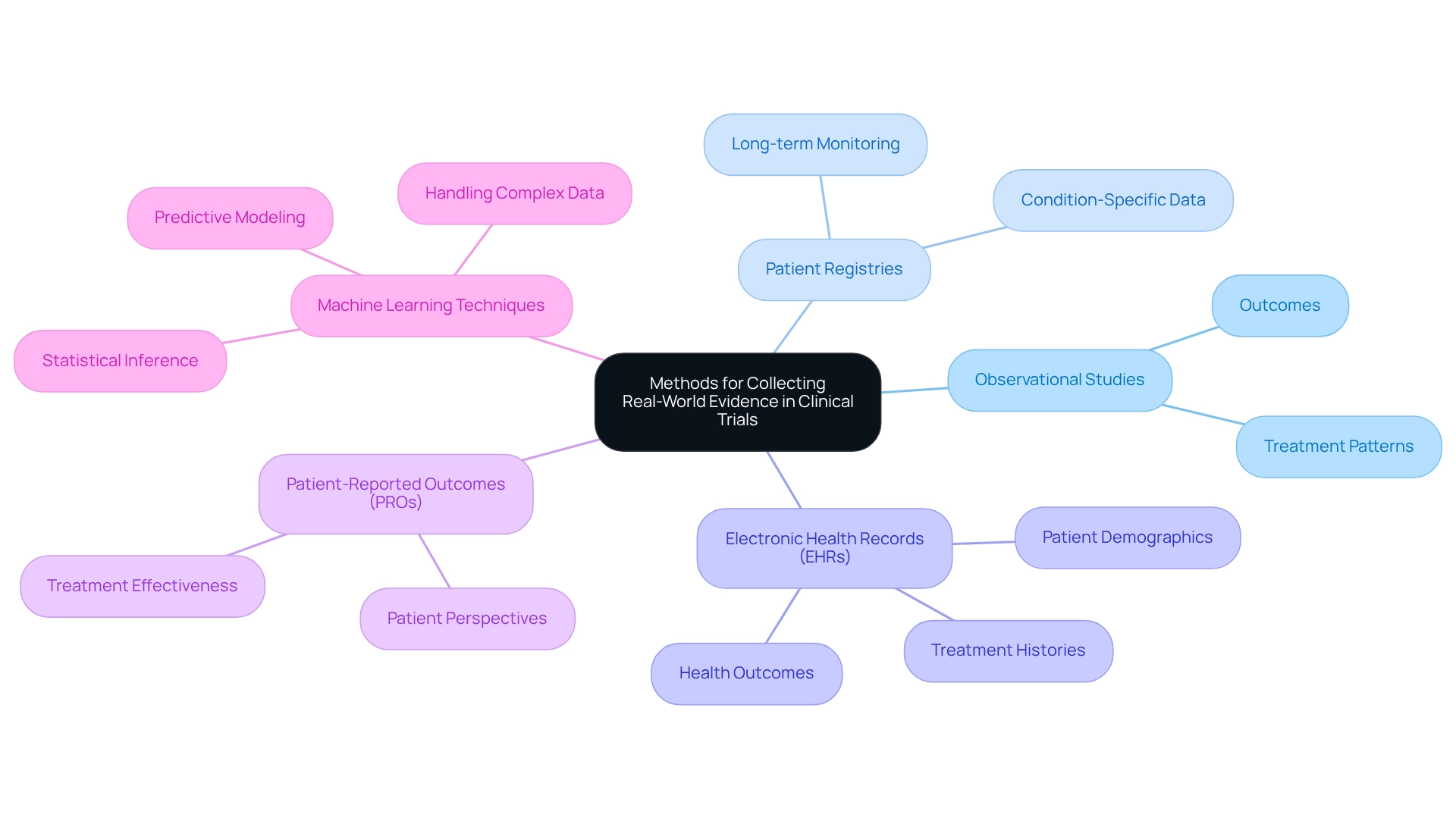
Methods for Collecting Real-World Evidence in Clinical Trials
Collecting real-world evidence for trials in Mexico employs a diverse array of methodologies, prominently featuring observational studies, patient registries, and electronic health records (EHRs). Observational studies are crucial for uncovering treatment patterns and outcomes within real-world contexts, providing valuable insights that often contrast with regulated trial environments. Conversely, patient registries systematically gather information on specific conditions or treatments over extended periods, enabling researchers to monitor long-term outcomes and variations in patient responses.
EHRs serve as a vital resource, offering comprehensive information on patient demographics, treatment histories, and health outcomes. This extensive data enables efficient analysis of large datasets, facilitating the identification of trends and correlations that may otherwise remain obscured. Moreover, integrating patient-reported outcomes (PROs) into these methodologies enhances the understanding of treatment effectiveness from the patient's perspective, ensuring that their experiences and preferences are integral to clinical evaluations.
The combination of these methods not only enriches the information landscape but also aligns with current trends in observational studies and patient registries, increasingly recognized for their role in generating real-world evidence for trials in Mexico. For instance, recent advancements in privacy-preserving record linkage (PPRL) techniques have been successfully applied in large-scale RWE studies, ensuring patient confidentiality while maximizing information utility.
Employing a multifaceted approach to collecting real-world evidence for trials in Mexico is essential for informing clinical practice and regulatory decisions. Optimal methods involve guaranteeing information quality, preserving patient confidentiality, and adhering to legal and regulatory requirements. As Zhan, T. emphasizes, "It is crucial to ensure that information is used in a way that honors patient autonomy and adheres to legal and regulatory requirements."
As methodologies evolve, expert opinions stress the necessity for adaptive model selection to mitigate bias and variance, particularly in high-dimensional scenarios. Targeted learning proves beneficial for managing such information, facilitating more effective model selection.
Furthermore, the application of machine learning techniques in analyzing real-world data (RWD) has shown considerable promise, as illustrated in the case study titled "Machine Learning Techniques in Real-World Data Analysis." This case study underscores the increasing use of ML techniques to handle complex and unstructured information, producing real-world evidence and enhancing predictive modeling. By leveraging these methodologies effectively, researchers can generate meaningful evidence that supports the advancement of medical technologies and improves patient outcomes.
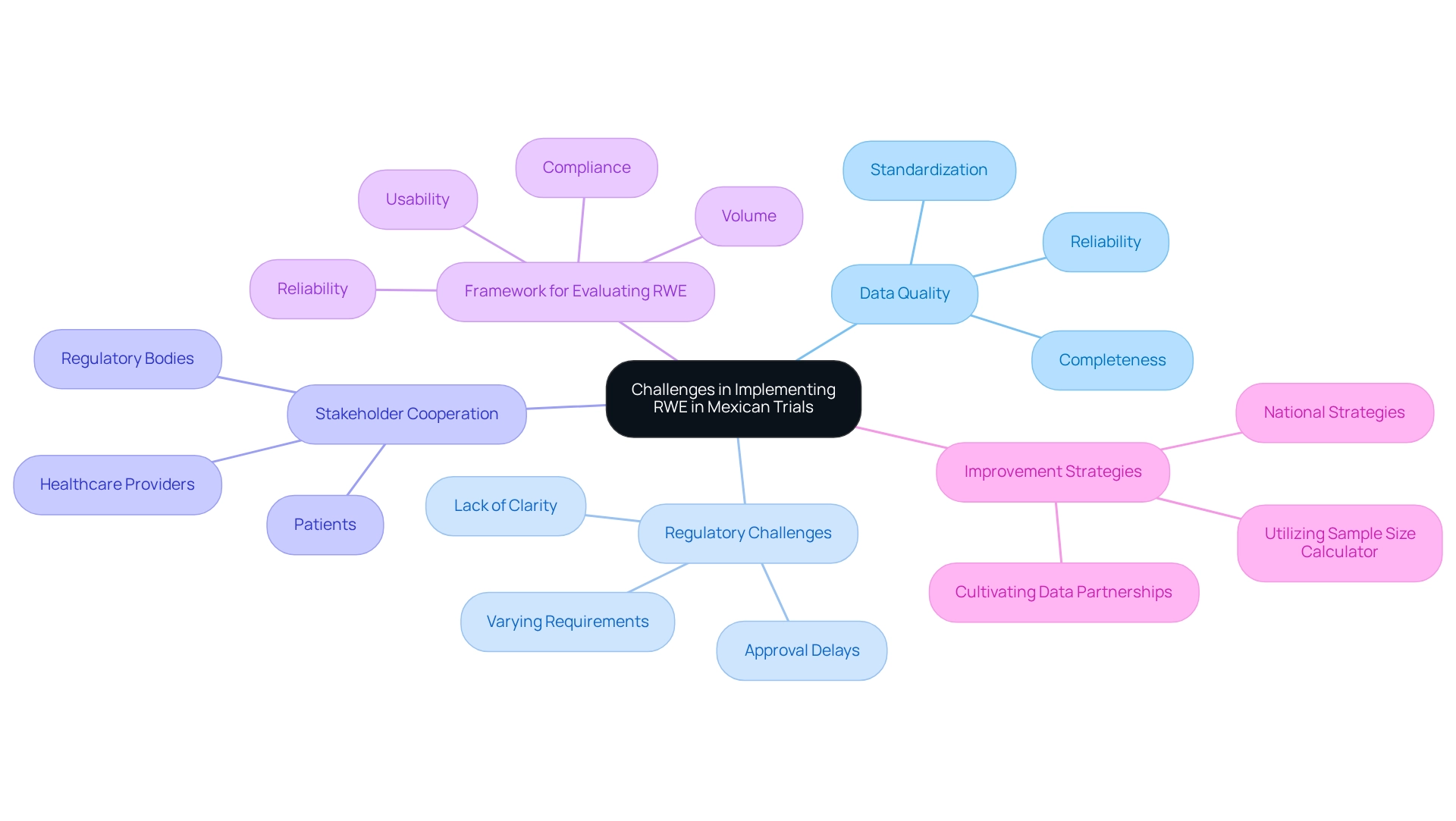
Challenges in Implementing Real-World Evidence in Mexican Trials
The challenges of implementing real-world evidence (RWE) for trials in Mexico can significantly impact research outcomes. A primary concern is the quality of information; researchers often struggle to ensure the reliability and completeness of data sourced from various channels. This challenge is compounded by the lack of standardized practices for information collection and management, leading to inconsistencies and gaps in the data.
A recent framework for evaluating RWE quality highlights four critical dimensions: volume, reliability, usability, and compliance. By utilizing this framework, organizations such as bioaccess can better assess the suitability of their information for specific purposes, ultimately enhancing the robustness of their analyses.
Regulatory hurdles also pose significant challenges for researchers in Mexico. The landscape is characterized by varying requirements across different health authorities, making it essential for researchers to navigate a complex regulatory environment. Existing policies often lack clarity, which can delay the approval process for clinical trials and obstruct timely access to essential information.
As emphasized in recent discussions, promoting an information-driven culture through national and organizational strategies is crucial for addressing these regulatory challenges. Cooperation among stakeholders—including healthcare providers, patients, and regulatory bodies—is vital for enhancing information-sharing practices. Strengthened collaborations can lead to more comprehensive information-gathering initiatives and a more unified strategy for RWE execution. Fostering relationships with information partners can significantly improve data quality and access to suitable resources, which is essential for successful health research.
In 2025, addressing these challenges is more critical than ever, as the demand for high-quality RWE continues to rise. By focusing on improving information quality and managing regulatory challenges, researchers can unlock the full potential of RWE, ultimately leading to better research outcomes and advancements in medical technology. As Marshall H. Chin noted, moving towards transparency and explicit measures of fairness can optimize traditional objectives such as predictive accuracy, underscoring the ethical considerations in RWE.
Additionally, utilizing tools like the sample size calculator developed by Grayling MJ et al. can assist researchers in designing robust studies that effectively address data quality issues. By leveraging insights from extensive research management services provided by bioaccess—including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and detailed reporting on study status, inventory, and adverse events—organizations can enhance their strategies for real-world evidence in trials in Mexico and contribute to the advancement of medical technology.
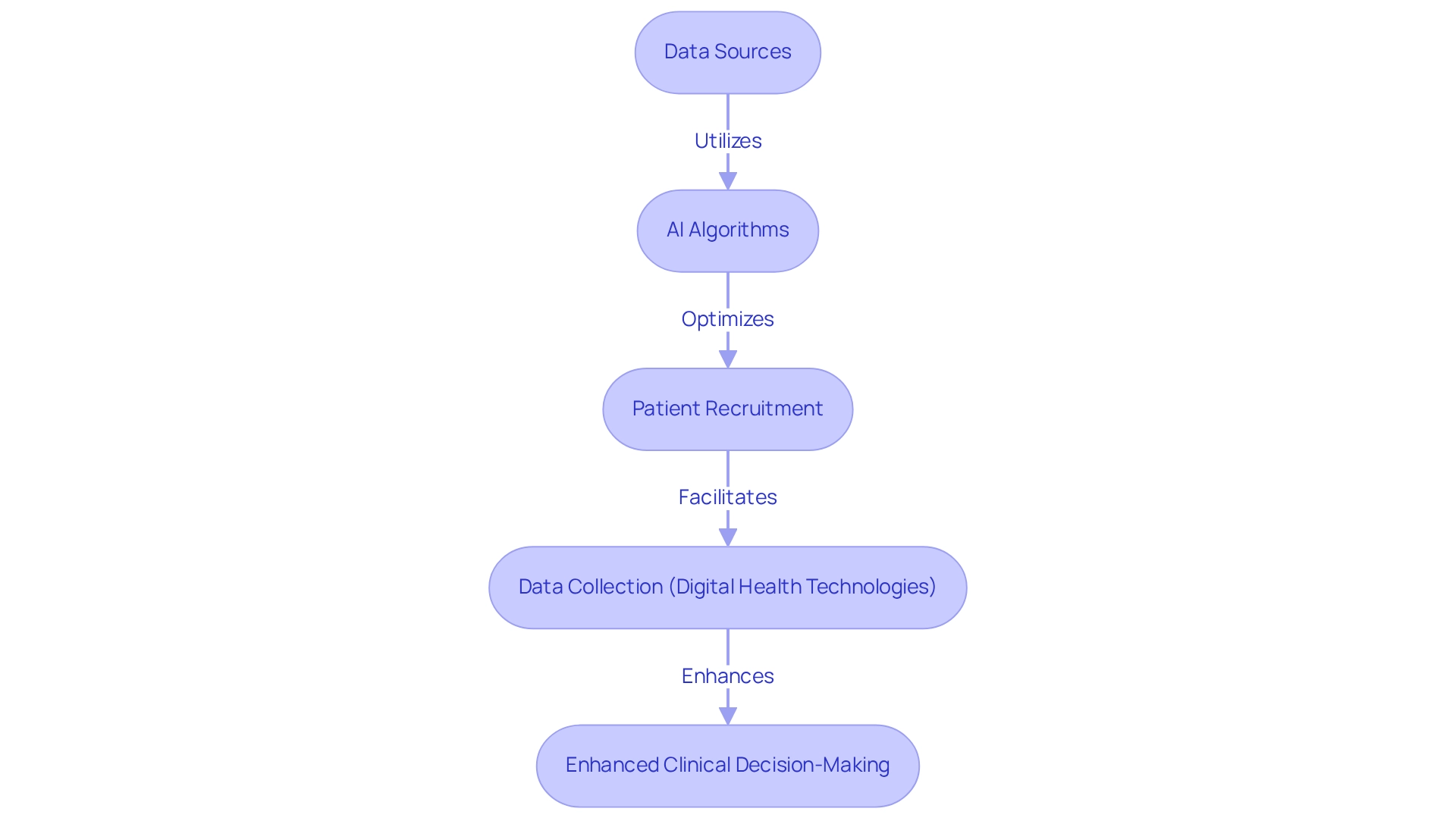
The Role of Technology and AI in Enhancing RWE Collection
Technology and artificial intelligence are revolutionizing the gathering and examination of real-world evidence (RWE) for trials in Mexico, particularly within research studies across Latin America, where bioaccess® is leading the charge. Advanced analytics tools are adept at processing extensive amounts of real-world data from various sources, enabling researchers to identify trends and patterns that significantly enhance clinical decision-making. For instance, AI algorithms streamline patient recruitment by more effectively pinpointing suitable candidates, optimizing study designs, and predicting outcomes based on historical data.
Furthermore, the integration of digital health technologies, including mobile health applications and wearable devices, facilitates real-time data collection from patients, yielding richer datasets for analysis. This approach not only simplifies the research process but also elevates the quality of evidence gathered, aligning seamlessly with bioaccess®'s comprehensive trial management services, which cover feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
As we look ahead to 2025, the significance of technology and AI in RWE collection is more pronounced than ever, with current statistics indicating that over 60% of research professionals are employing AI tools to enhance study outcomes. Bernard Marr underscores that scaling AI systems necessitates careful consideration of deployment modalities, model updates, regulatory frameworks, and ongoing risk monitoring. This insight underscores the necessity of addressing challenges in AI adoption, such as data quality and ethical considerations, as illustrated in the case study titled "Challenges in AI Adoption."
Tackling these challenges can unlock the transformative potential of AI, paving the way for personalized and equitable healthcare solutions.
With over 20 years of experience in the Medtech sector, bioaccess® leverages its expertise and tailored strategies to expedite medical devices for firms in the Medtech field. This is particularly evident through partnerships like that with Caribbean Health Group, which positions Barranquilla as a premier research hub in Latin America, supported by Colombia's Minister of Health. By harnessing these advancements, researchers can significantly enhance the efficiency and effectiveness of studies, especially those focused on real-world evidence for trials in Mexico, ultimately leading to improved patient outcomes. bioaccess® specializes in various types of research, such as Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), ensuring a comprehensive approach to study management.
Case Studies: Successful Applications of RWE in Mexican Clinical Trials
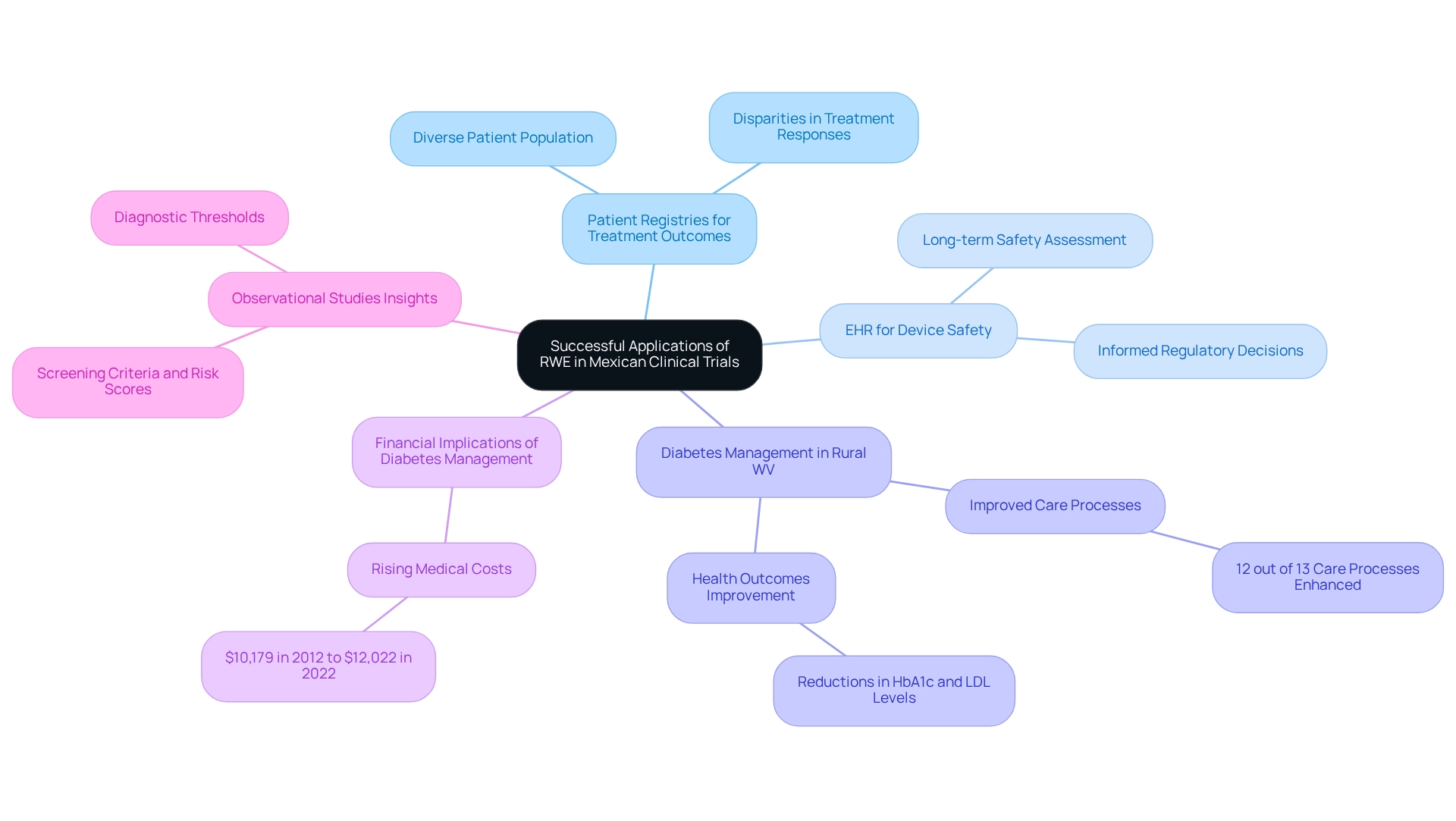
Numerous case studies illustrate the effective application of real-world evidence (RWE) in trials conducted in Mexico, particularly within the realm of diabetes management. A prominent study utilized patient registries to monitor treatment outcomes across a diverse patient population, effectively showcasing the success of a novel therapeutic method in real-world contexts. This approach not only facilitated improved tracking of patient progress but also revealed disparities in treatment responses among various demographic groups.
In another significant case, researchers harnessed electronic health record (EHR) information to assess the long-term safety of a medical device. This analysis yielded essential insights that informed regulatory decisions, underscoring the pivotal role of RWE in safeguarding patient safety and ensuring device efficacy. The findings from these studies underscore how RWE can enhance conventional trial data, offering a more nuanced understanding of treatment effectiveness and safety in routine medical practice.
Moreover, a recent study focusing on diabetes management in rural West Virginia demonstrated that increased utilization of electronic patient registries significantly improved care processes and health outcomes. Specifically, enhancements were noted in 12 out of 13 care processes and three out of six health outcomes, including reductions in HbA1c and LDL levels. This evidence indicates that even basic registry usage can markedly enhance diabetes care, especially in resource-limited settings, serving as a compelling example of how RWE can lead to better health outcomes.
The financial implications of diabetes management are substantial, with excess medical costs per person associated with diabetes rising from $10,179 in 2012 to $12,022 in 2022. This trend emphasizes the urgent necessity for effective management strategies. As A.T.M. from the Office of Physician-Scientist Development observes, "RCTs demonstrate necessary causal evidence of efficacy; however, they are typically costly, take a long time to be conducted, expose participants to experimentation, and are usually designed to answer a narrow hypothesis in highly selected populations." This perspective highlights the critical importance of RWE for trials in Mexico in enhancing the effectiveness of traditional trials.
Furthermore, observational studies have provided valuable insights into screening criteria, risk scores, and diagnostic thresholds, further emphasizing the significance of RWE in research. Collectively, these case studies illustrate the effectiveness of RWE in diabetes management and medical device safety, reinforcing the necessity of integrating real-world evidence into research to enhance patient care and inform healthcare policies.
At bioaccess®, we leverage over 20 years of expertise in managing research studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Follow-Up Studies (PMCF), to empower our clients in effectively utilizing RWE in their research. Our comprehensive research study management services encompass feasibility assessments, site selection, compliance evaluations, study preparation, import permits, project oversight, and reporting, all tailored to optimize the success of your research initiatives in Latin America. Our customized approach and flexibility enable us to adapt to the unique needs of each study, ensuring optimal outcomes for our clients.
Future Trends in Real-World Evidence for Clinical Trials in Mexico
The future of research studies is poised for significant expansion in Mexico, driven by advancements in technology and an increasing recognition of the value of real-world evidence in healthcare decision-making. Emerging trends highlight the integration of artificial intelligence and machine learning to refine data analysis and streamline patient recruitment processes. Moreover, there is a growing focus on patient-centered approaches that prioritize the collection of patient-reported outcomes, enabling a deeper understanding of treatment effectiveness from the patient's perspective.
As regulatory bodies continue to endorse real-world evidence for trials in Mexico, researchers will find enhanced opportunities to leverage this evidence in their studies, ultimately improving patient care and outcomes within the Mexican healthcare system.
To facilitate this growth, bioaccess provides comprehensive study management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These services employ methodologies such as rigorous site assessments, stakeholder engagement, and continuous monitoring to ensure high-quality outcomes. Under the leadership of Dr. Sergio Alvarado, a Clinical Study Manager specializing in innovative medical research and artificial intelligence in Latin America, bioaccess is well-equipped to navigate the complexities of studies and enhance the integration of real-world evidence for trials in Mexico into research practices.
This holistic approach not only streamlines the clinical study process but also guarantees compliance with regulatory standards, fostering a more efficient and effective research environment. The anticipated outcomes of these services include improved trial efficiency, enhanced data quality, and ultimately, superior patient outcomes.
Conclusion
Real-World Evidence (RWE) is revolutionizing the field of clinical research, particularly in regions like Latin America where healthcare dynamics are distinct. The insights derived from RWE, which reflects the performance of medical products in everyday settings, empower stakeholders to gain a deeper understanding of treatment outcomes across diverse populations. By integrating RWE into clinical trials, researchers can produce valuable evidence that enhances regulatory decision-making, informs clinical practice, and ultimately leads to improved patient care.
Despite challenges related to data quality, regulatory complexities, and the necessity for standardized practices, the potential of RWE is indisputable. Technological advancements, particularly in artificial intelligence and machine learning, are further refining the collection and analysis of real-world data, facilitating more efficient and effective clinical trials. As demonstrated by various case studies, RWE not only complements traditional clinical trial data but also offers critical insights into treatment effectiveness and safety, especially in managing chronic conditions such as diabetes.
Looking forward, the future of RWE in clinical trials in Mexico appears promising, with an increasing focus on patient-centered approaches and the ongoing evolution of regulatory frameworks. By leveraging comprehensive clinical trial management services and adopting innovative methodologies, researchers can adeptly navigate the complexities of the healthcare landscape and unlock the full potential of RWE. This dedication to advancing clinical research through real-world insights is set to drive better health outcomes and optimize healthcare decisions, ultimately benefiting patients and healthcare systems alike.
Frequently Asked Questions
What is Real-World Evidence (RWE)?
Real-World Evidence (RWE) is defined as the evidence derived from the analysis of Real-World Data (RWD), which includes information collected outside traditional clinical trials. It illustrates the performance of medical products in everyday healthcare settings, providing insights into their effectiveness, safety, and potential risks.
Why is RWE important in healthcare?
RWE is significant because it offers a nuanced understanding of treatment outcomes across diverse patient populations. It is particularly useful for regulatory decision-making, practice guidance, and health technology assessments (HTAs), thereby enhancing the quality of patient care.
How does RWE compare to randomized controlled trials (RCTs)?
RWE studies, whether observational or pragmatic, provide different perspectives compared to randomized controlled trials (RCTs). They are valuable for generating hypotheses and evaluating the effectiveness of interventions in real-world conditions.
How is RWE being utilized in regulatory processes?
Recent developments indicate a growing reliance on RWE for regulatory approval decisions, post-approval safety monitoring, payer coverage decisions, and outcomes-based contracting. RWE trials are recognized for being cost-effective and for identifying rare complications.
What role does bioaccess® play in RWE research in Latin America?
bioaccess® manages research studies across Latin America, specializing in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, and leverages RWE for trials in Mexico, which is essential for navigating complex regulatory frameworks.
What is the regulatory framework for RWE in Mexico?
In Mexico, the regulatory framework is primarily shaped by the Federal Commission for Protection against Sanitary Risks (COFEPRIS), which mandates adherence to guidelines for RWD collection and requires Health Technology Assessments (HTAs) that include evidence from both randomized trials and RWE.
What improvements have been observed in compliance with COFEPRIS regulations?
Compliance rates with COFEPRIS regulations have significantly improved, indicating a stronger commitment to maintaining high research standards within the regulatory process.
What are the ethical responsibilities highlighted in RWE research?
Ethical responsibilities include ensuring that all therapies and procedures associated with research studies are offered promptly and at no cost to participants, thereby promoting trust and ethical conduct in medical research.
How does bioaccess® assist researchers in Mexico?
bioaccess® offers extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, with a focus on navigating the regulatory landscape effectively.
What is the significance of the case study titled "Participation and Participant Rights"?
This case study emphasizes the necessity of ensuring ethical conduct in research by guaranteeing that participants receive all therapies and procedures related to the study promptly and at no cost, fostering trust in the research process.




