Introduction
Navigating the complex landscape of medical device regulation is crucial for ensuring the safety and efficacy of products within the European Union. The EU Medical Device Regulation (MDR) categorizes devices based on their risk, with Class 2b devices requiring stringent regulatory scrutiny due to their moderate to high-risk profile. This article delves into the intricacies of the EU MDR classification system, focusing on the key regulatory requirements, conformity assessment procedures, and the importance of post-market surveillance for Class 2b medical devices.
It also highlights the critical role of a robust Quality Management System (QMS) in maintaining compliance and explores best practices for achieving regulatory success. As the regulatory environment continues to evolve, staying informed and proactive is essential for manufacturers to ensure their devices meet the highest standards of safety and performance.
EU MDR Classification System Overview
'The EU Regulation for Healthcare Instruments (MDR) establishes a classification system for healthcare products based on their risk to patients and users.'. Class 2b items, identified as moderate to high-risk, undergo a more rigorous assessment process compared to lower-class items. This classification is crucial to guaranteeing the protection and effectiveness of medical instruments prior to their sale in the European Union. Key criteria for classification include intended use, duration of contact, and invasiveness, which dictate the regulatory pathway manufacturers must follow.
Recent data indicates a significant number of in vitro diagnostic instruments (IVDs), particularly high-risk Class D instruments, have not yet transitioned to the new rules. This includes critical tests for infections in blood transfusions and organ donations. To address this, the European Commission has proposed extending the transition periods to give manufacturers and notified bodies more time to complete the necessary conformity assessments. This extension aims to safeguard the high standards of safety and public health set by the MDR. Furthermore, actions to expedite the deployment of EUDAMED, a comprehensive database of all healthcare products and IVDs in the EU market, are suggested to improve transparency and assist in the execution of the regulatory framework.
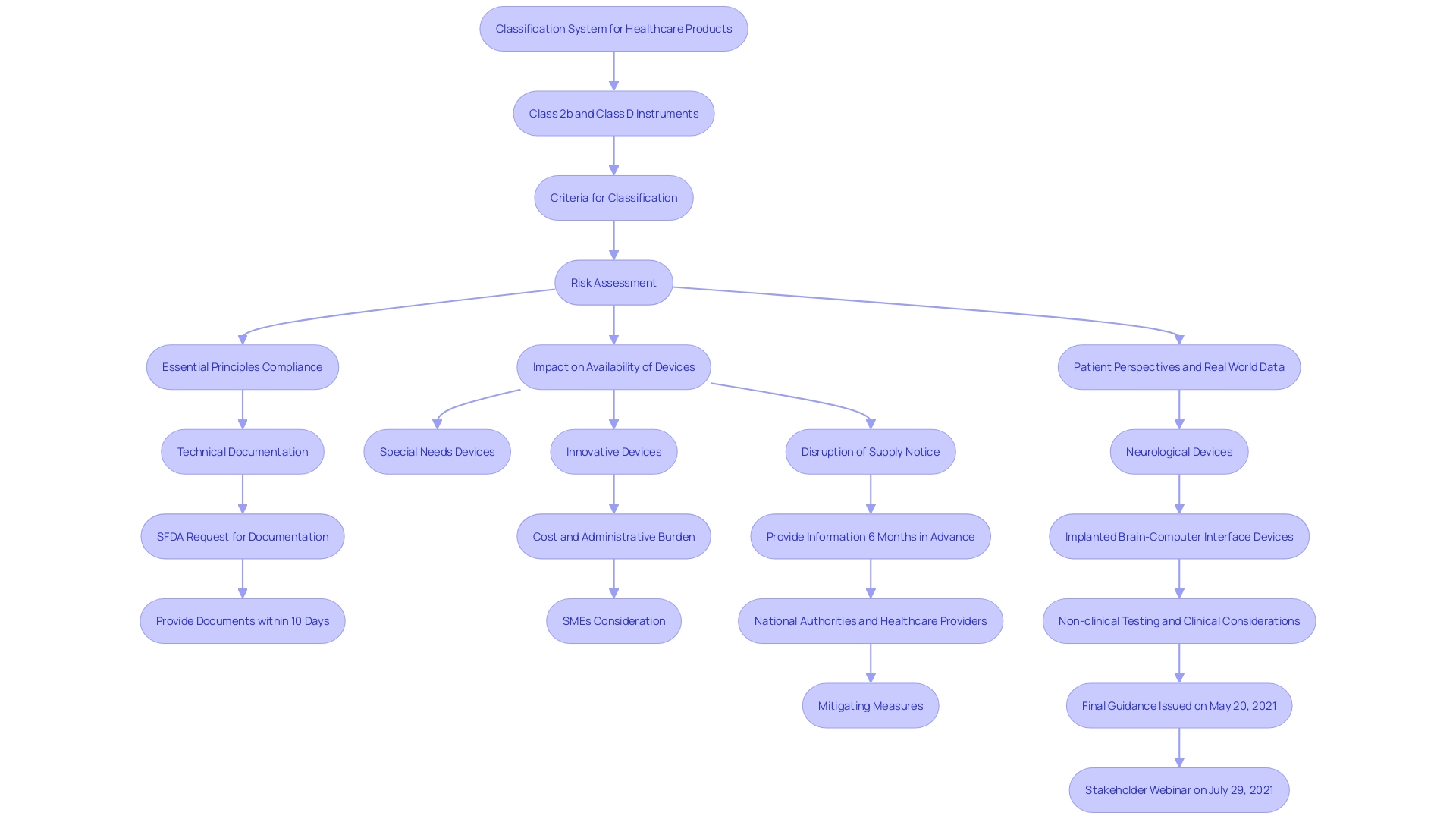
Key Regulatory Requirements for Class 2b Medical Devices
Producers of Class 2b medical equipment must comply with strict regulatory standards as detailed in the EU Medical Equipment Regulation (MDR). Foremost among these is the implementation of a comprehensive Quality Management System (QMS), essential for maintaining high standards in security and performance. A meticulous risk assessment process is imperative, identifying potential hazards and mitigating risks effectively. Clinical information plays an essential part, as it must clearly show the product’s reliability and effectiveness, aligning with the strict standards outlined in the EU MDR.
Additionally, manufacturers are required to compile extensive technical documentation. This documentation must provide robust evidence of compliance with applicable regulations, including detailed evaluation reports (CERs). The CER is especially important, acting as a thorough evaluation of the safety and performance based on clinical data gathered from multiple sources. This is an essential element for acquiring the CE marking, which is required for promoting health products within the European Union.
Staying updated with regulatory requirements is paramount. The EUDAMED database improves clarity, offering a thorough summary of all healthcare products accessible in the European market. This initiative intends to enhance the traceability and supervision of medical instruments, ensuring that they meet the highest standards of security and effectiveness. 'Recent proposals by the European Commission seek to expedite the mandatory launch of EUDAMED components and evaluate the impact of current legislation on availability, particularly for specialized equipment like those for pediatric or orphan diseases.'.
In this evolving regulatory landscape, post-market surveillance (PMS) is essential. It entails ongoing observation of equipment in practical environments, utilizing techniques such as unplanned reporting, registries, and electronic health records to collect information on long-term reliability and efficacy. This ongoing vigilance helps identify and mitigate potential risks, thereby safeguarding patient health.
Ensuring compliance requires a proactive approach. As regulations and guidelines are subject to change, manufacturers must be prepared to adapt their processes and documentation accordingly. This dynamic environment necessitates vigilance and flexibility, underscoring the importance of staying informed and compliant to maintain market access and uphold patient well-being.
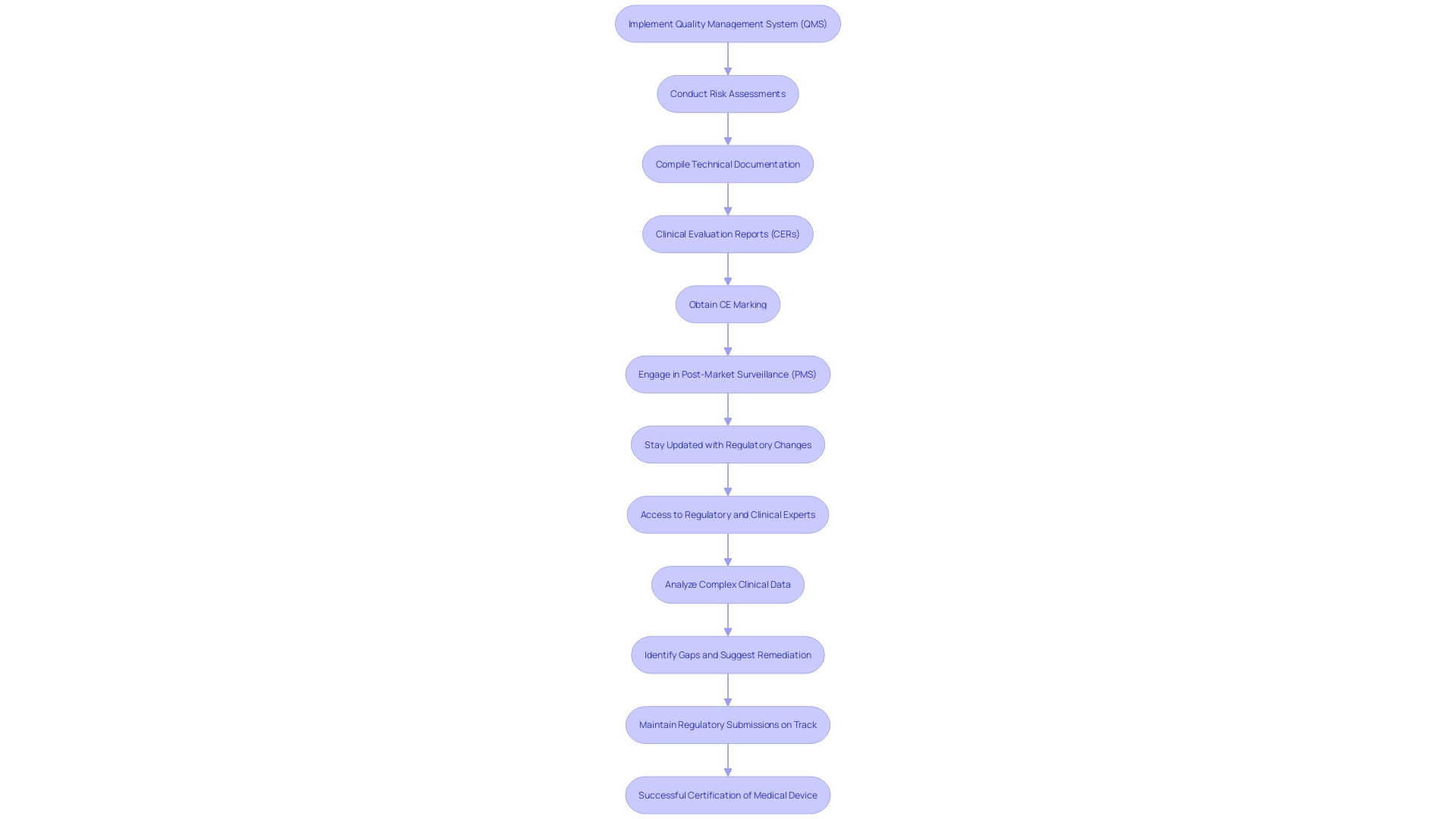
Clinical Evidence and Performance Standards
For Class 2b instruments, medical proof is essential to show safety and effectiveness. This evidence generally includes medical investigations, literature reviews, and post-market data. Producers must create a clear assessment plan aligned with the device's intended use and risk profile. Following set performance standards and benchmarks improves the credibility of the medical evidence. Moreover, interacting with regulatory agencies early in the development process can offer insights into the essential data requirements.
Essential elements of medical evidence consist of information from research carried out for the product being assessed and from research for previously sold comparable products. A state-of-the-art report, which includes a literature review of medical texts, guidelines, and peer-reviewed literature, is essential to demonstrate what is currently accepted as good practice. This aids in demonstrating that an apparatus is comparable to similar products available and poses minimal risk.
A thorough risk-benefit evaluation is essential, including negative occurrences, equipment failures, and possible concerns from the medical assessment. A summary of clinical evidence, including a meta-summary of overall clinical evidence supporting the reliability and performance of the instrument, is necessary to conclude its ability to meet the intended clinical purpose. Once these reports are assembled, manufacturers must submit a Declaration of Conformity to indicate the product complies with MDR stipulations. This declaration must be kept up to date and available upon request to any competent authority.
Post-market reports mandated by the FDA provide information on a product and enable manufacturers to address concerns raised through passive and active monitoring systems. These encompass 522 Studies, which assess specific features of or overall performance of the product once it is accessible in the marketplace, and Post-Approval Studies (PAS), which collect further information on the product's long-term reliability, performance, and effectiveness, providing interim results to the FDA as research is conducted. Recalls must also be reported, detailing any action by manufacturers to recall, withdraw, or correct a product.
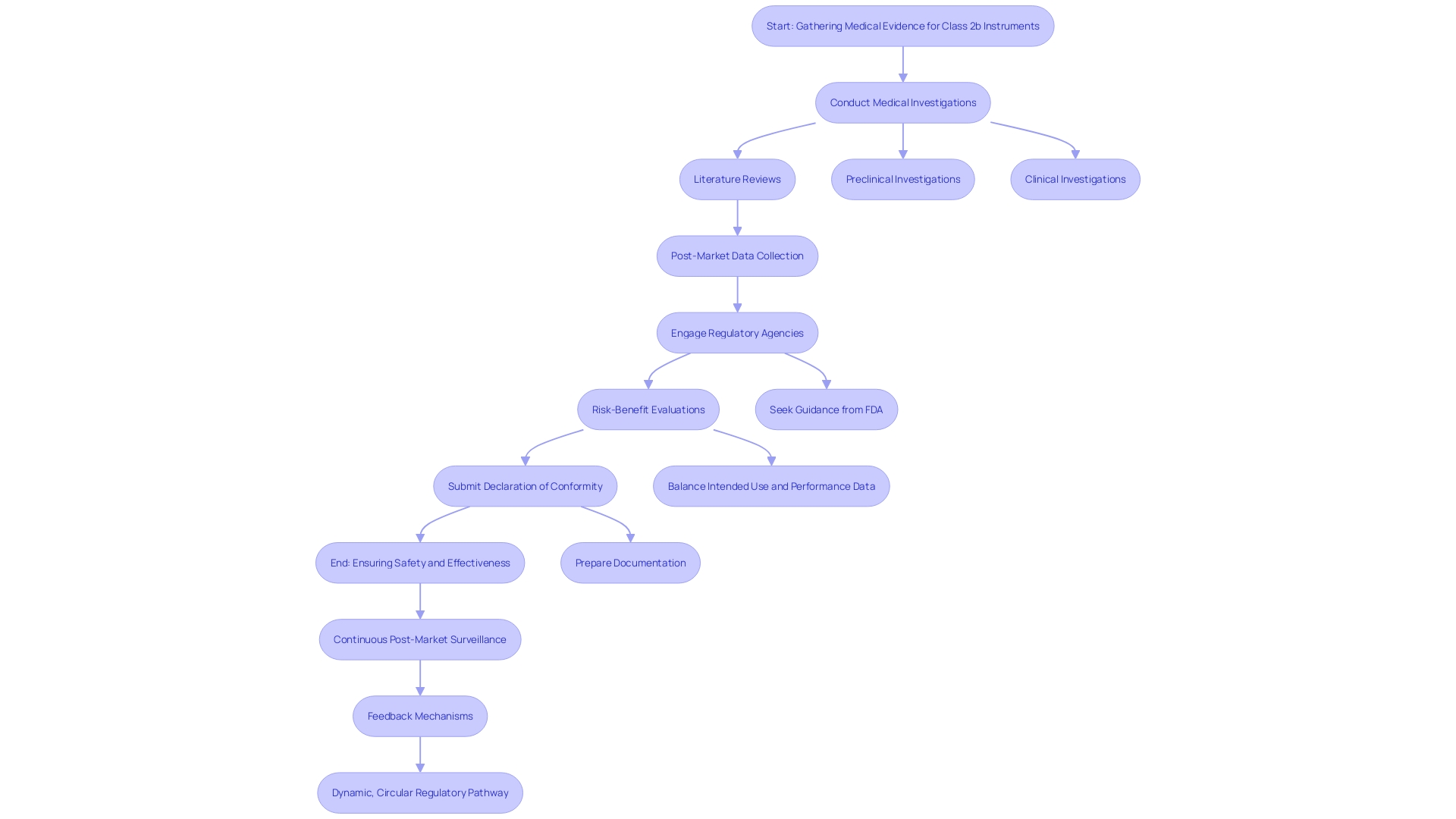
Conformity Assessment Procedures for Class 2b Devices
The conformity assessment procedure for Class 2b medical instruments involves a comprehensive evaluation to ensure adherence to protection and performance criteria. 'Producers must involve a Notified Body to carry out this assessment, which encompasses audits of the Quality Management System (QMS), review of technical documentation, and evaluation of medical data.'. The clinical evaluation is especially vital, as it entails a thorough appraisal of the equipment’s safety and performance based on gathered clinical data. The Clinical Evaluation Report (CER), a key component of the technical documentation, plays a pivotal role in this process by demonstrating compliance with EU regulations.
The result of the Notified Body's evaluation decides if the product can carry the CE mark, indicating compliance with EU regulations. Maintaining open communication with the Notified Body throughout the process is essential to address any concerns or additional requirements.
The European Commission's recent proposal to extend the application period for the In Vitro Diagnostic Medical Devices Regulation (IVDR) underlines the importance of ensuring patient care while improving the availability of essential healthcare products. This action seeks to improve clarity and accelerate the introduction of components in the European Database on Medical Devices (EUDAMED), thus offering a complete summary of all items accessible in the European market.
In summary, the conformity evaluation for Class 2b healthcare instruments is an essential procedure that guarantees the security and effectiveness of these instruments through thorough assessment and compliance with regulatory criteria.
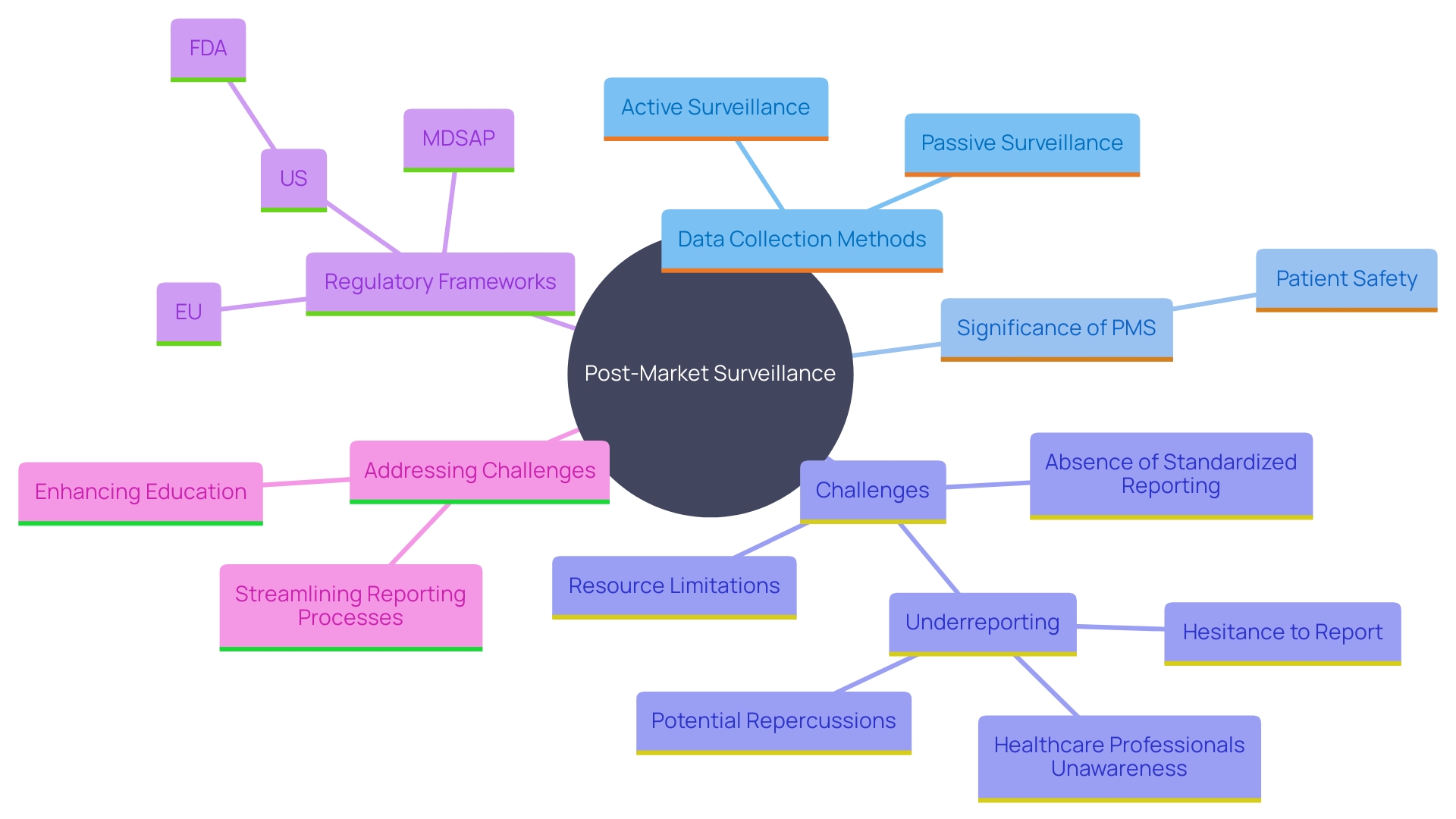
Post-Market Surveillance and Reporting Obligations
Post-market surveillance (PMS) is a critical component of the lifecycle management of Class 2b medical products. This stage is essential for recognizing and tackling possible concerns and enhancing equipment performance over time. Manufacturers are required to monitor the performance of their products after they are on the market, collecting data on any adverse events, incidents, or trends that may arise. Various methods are employed to collect this crucial data, including passive surveillance systems like spontaneous reporting by healthcare professionals and patients, active surveillance through registries or studies, and the utilization of electronic health records and administrative databases. These methods allow for the ongoing observation of equipment in practical environments, offering important information about their long-term reliability and efficacy.
The significance of PMS cannot be overstated. It serves a crucial function in patient protection, assisting in identifying and reducing possible hazards related to healthcare instruments. For instance, more than 1.7 million injuries and 83,000 deaths over a recent 10-year period in the U.S. have been potentially connected to defective medical equipment. Swift action based on PMS findings can prevent harm and contribute to the long-term well-being of patients. The FDA has started developing a monitoring system to search for possible concerns regarding these products, beginning with a small number and growing gradually, despite difficulties in financing and patient recognition.
Reporting duties to regulatory bodies, including incident documentation and periodic update reports (PSURs), must be followed, ensuring transparency and adherence. New regulatory structures are being created to improve patient protection and guarantee prompt access to essential equipment. For instance, the UK's new regulations aim to provide greater international harmonization and patient-centered requirements, reflecting the rapid advancements in healthcare technology. Dr. Laura Squire, Med Tech Regulatory Reform Lead, emphasized that these regulations will strengthen the MHRA’s ability to keep patients safe while fostering an environment that encourages the launch of innovative healthcare products.
Despite its importance, effective PMS faces challenges such as underreporting of adverse events, limited resources for monitoring, and the absence of standardized reporting processes. Tackling these issues is essential to guaranteeing the ongoing security and efficacy of healthcare tools in practical environments. Manufacturers must remain vigilant and proactive in their PMS efforts to safeguard patient health and comply with regulatory standards.
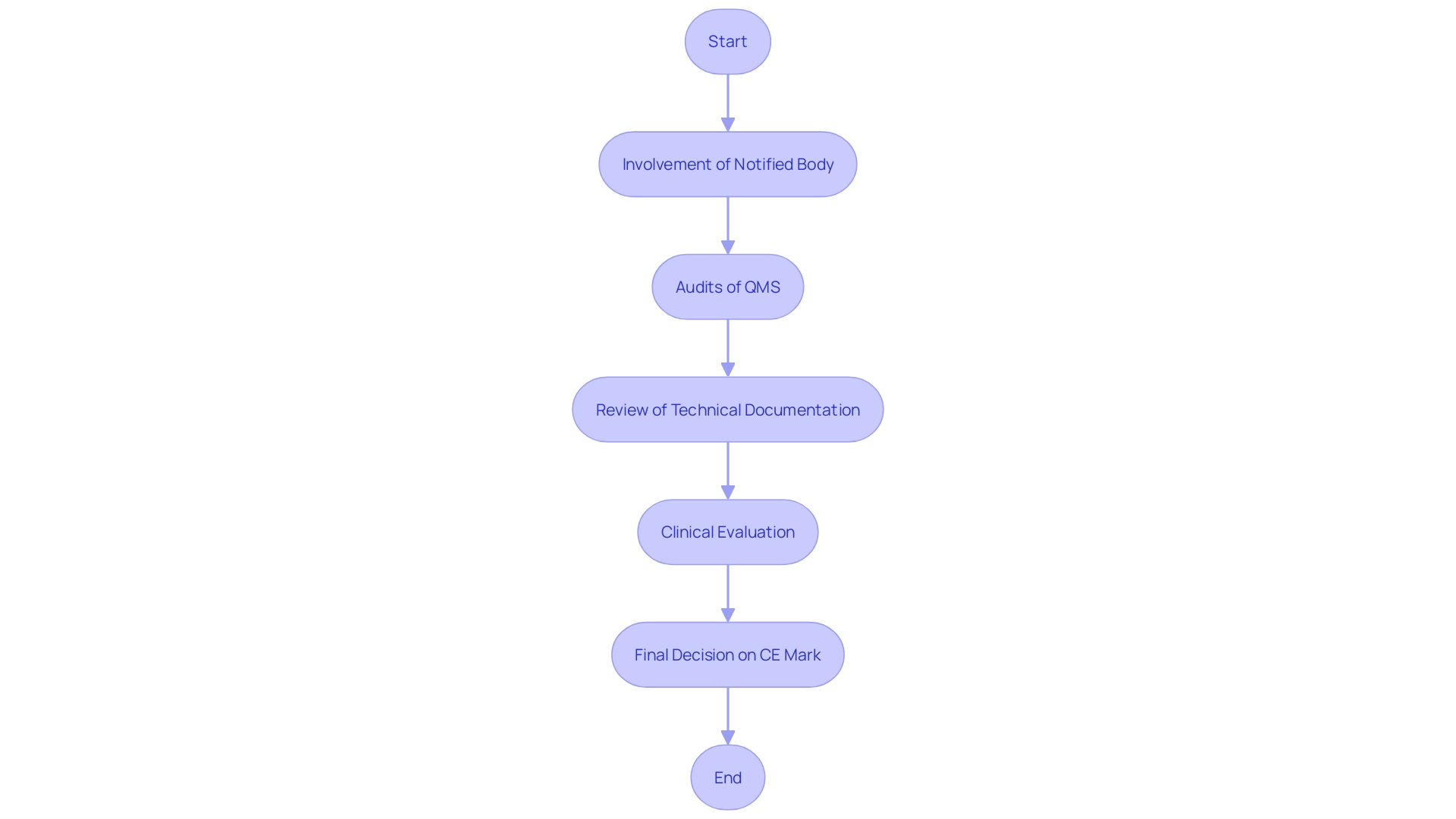
Quality Management System (QMS) Requirements
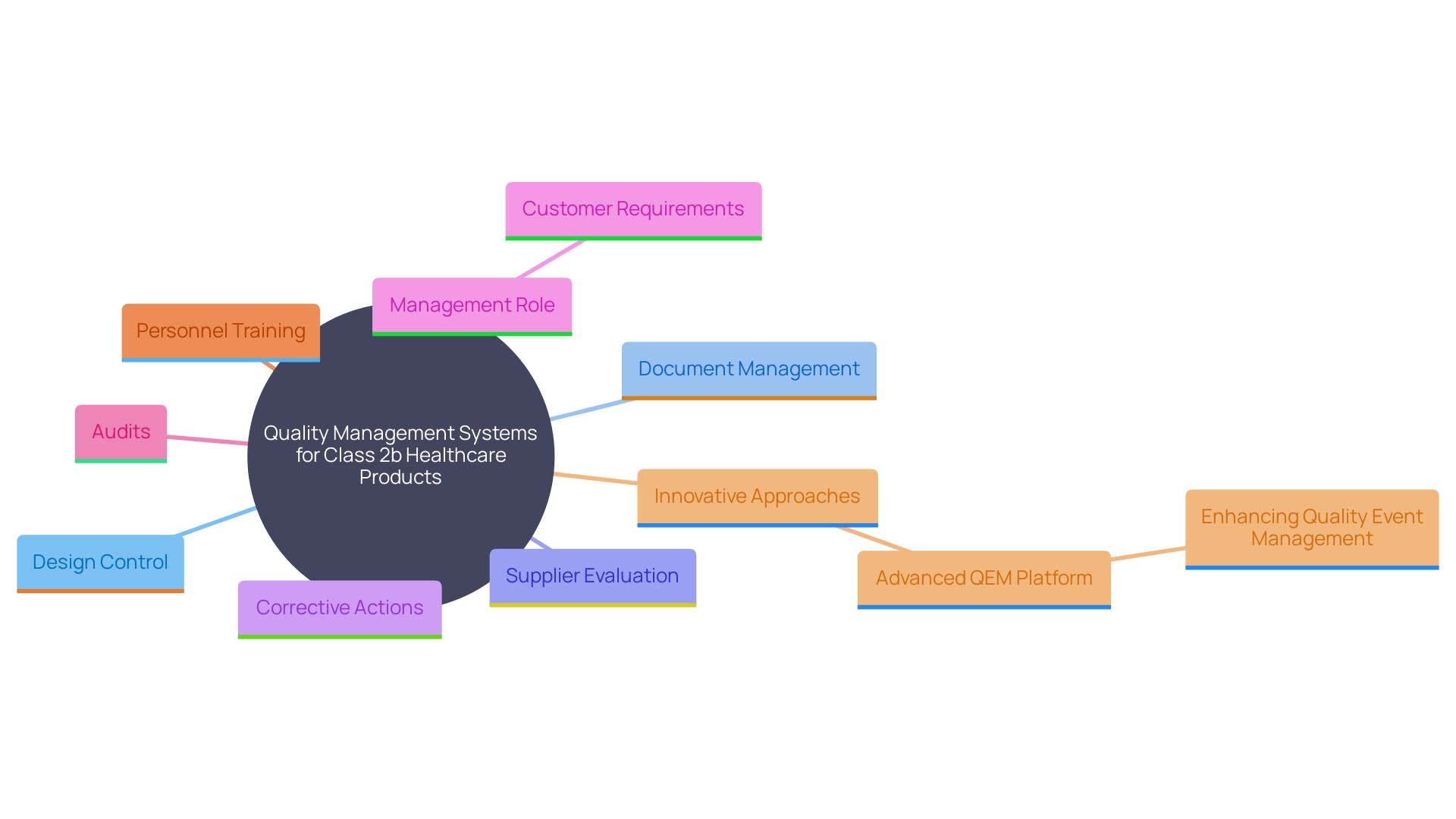
A strong Quality Management System (QMS) is essential for the compliance of Class 2b healthcare products. Manufacturers must establish and maintain a QMS that adheres to ISO 13485 standards, which provide a comprehensive framework for quality management. This standard is specifically designed to help manufacturers develop strong systems from the ground up, ensuring that they meet regulations, assess and improve supply bases, and maintain “best-in-class” management standards. Key components of the QMS include processes for design control, document management, supplier evaluation, and corrective actions.
The ISO 13485 standard specifies that management must ensure customer requirements are met and maintain the integrity of the QMS when changes are implemented. Regular audits and reviews of the QMS ensure its effectiveness and compliance with regulatory requirements. Additionally, the competence, awareness, and training of personnel are crucial, particularly in roles that impact product quality. By following this quality system, manufacturers gain a competitive edge in quality, reliability, delivery, and service, fostering enhanced trust with customers.
Furthermore, the QMS must include dynamic forms for quality event management, as highlighted by the innovative Advanced QEM platform, which allows for more efficient and adaptable quality event management processes. This advanced approach has been recognized as one of the most innovative products in the industry, significantly improving the approach to quality event management.
By adhering to these standards and continuously enhancing risk management processes, manufacturers can proactively identify and mitigate potential risks. This results in the creation of high-quality instruments that meet regulatory standards and offer optimal patient results, aiding in the overall progress of the healthcare equipment sector.
Best Practices for Compliance and Regulatory Success
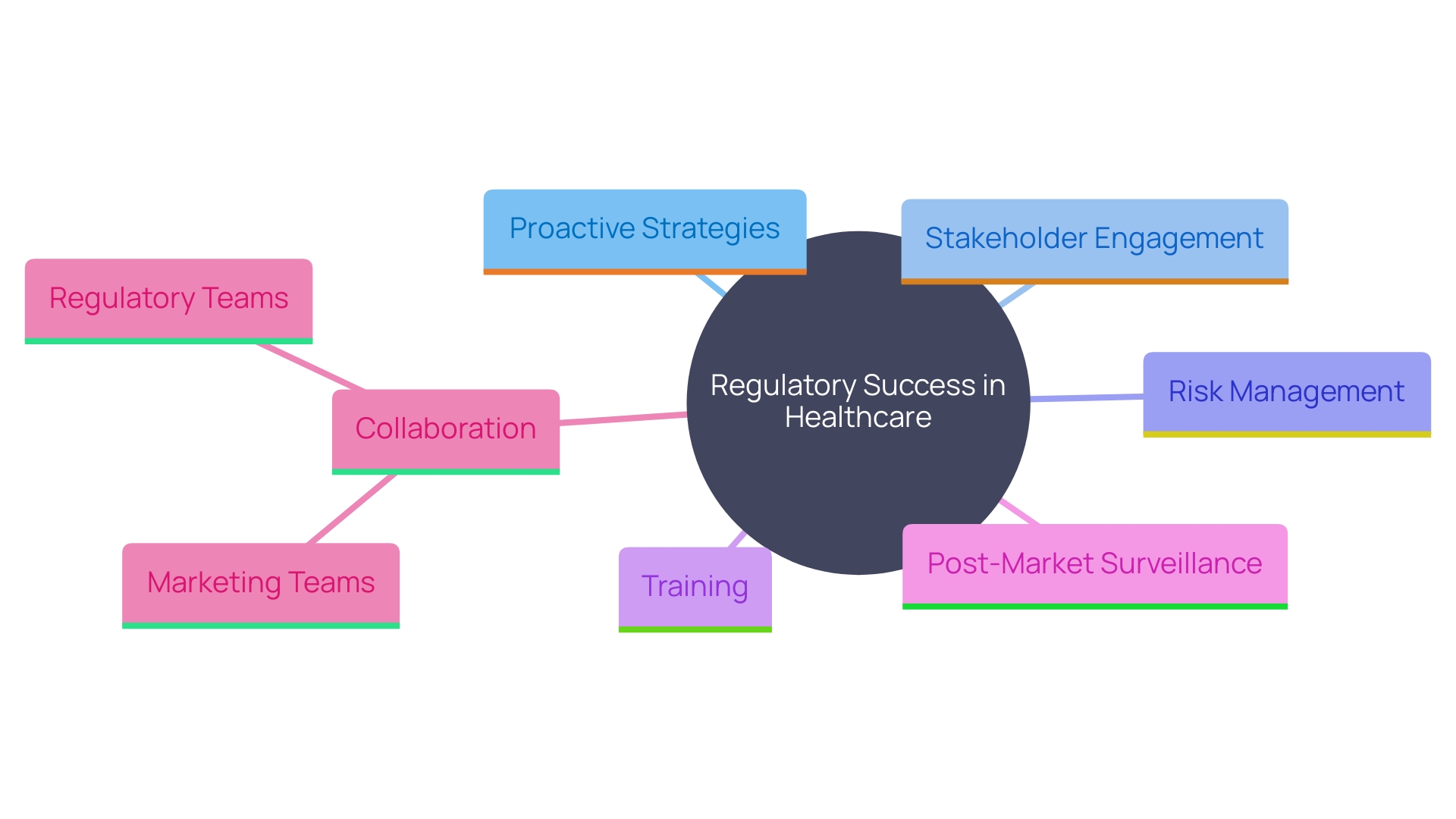
To attain adherence and regulatory success, producers of Class 2b healthcare instruments should embrace a proactive strategy. This involves staying informed about changes in regulations and engaging with stakeholders early in the development process. Utilizing a risk-based approach to quality management is essential. Establishing strong relationships with Notified Bodies and regulatory authorities can facilitate smoother interactions and enhance understanding of compliance requirements.
Putting resources into training for personnel engaged in regulatory matters guarantees that the group is adequately prepared to handle the intricacies of health product regulations. 'The importance of Post-Market Surveillance (PMS) cannot be emphasized enough, as it plays a crucial role in patient well-being by identifying and reducing potential hazards linked to healthcare tools.'. Different approaches, such as passive and active monitoring systems, are used to gather important information concerning the reliability and performance of medical devices.
Furthermore, distinguishing and accurately representing health benefits and claims is critical. The EU MDR and MDCG emphasize that claims about a product's intended purpose, safety, and performance must be supported by factual evidence and data. This necessitates close collaboration between regulatory teams and marketing departments to align the product's market expectations with its actual clinical benefits.
A certificate of competence in ISO 14971 requirements can further enhance a professional's ability to establish and maintain a compliant risk management system for healthcare products. This is a very adaptable skill throughout the healthcare equipment sector and is in great demand. Understanding how software is regulated as a medical device is also essential, as the Same market is poised for significant growth. Navigating this complex terrain requires a firm grasp of regional nuances, documentation requirements, and compliance processes.
Conclusion
Navigating the complexities of the EU Medical Device Regulation (MDR) is essential for ensuring the safety and efficacy of Class 2b medical devices. The classification system, which categorizes devices based on their risk profile, underscores the importance of adhering to stringent regulatory requirements. Manufacturers must implement a robust Quality Management System (QMS) and engage in thorough conformity assessment procedures to demonstrate compliance with safety standards.
The significance of clinical evidence and a comprehensive risk assessment cannot be overstated, as they are pivotal in establishing a device's safety and performance.
Post-market surveillance (PMS) plays a critical role in the lifecycle management of Class 2b devices. Continuous monitoring allows manufacturers to identify and address potential safety issues, thereby safeguarding patient health. The obligation to maintain transparent reporting practices further reinforces the commitment to quality and compliance.
As the regulatory landscape evolves, manufacturers must remain vigilant, adapting to changes and ensuring that their devices not only meet current standards but also anticipate future requirements.
In summary, achieving regulatory success in the medical device sector requires a proactive approach, characterized by strong stakeholder engagement, ongoing education, and a commitment to quality. By fostering a culture of compliance and innovation, manufacturers can enhance patient safety while navigating the challenges posed by the evolving regulatory environment. The importance of aligning clinical claims with factual evidence and maintaining effective communication with regulatory bodies will be critical for sustained market access and the overall advancement of the medical device industry.




