Introduction
Serious Adverse Events (SAEs) are critical medical occurrences that arise unexpectedly during clinical trials, warranting immediate investigation. These events encompass situations that can potentially threaten life, lead to hospital admissions, result in significant disability, or even cause death. The identification and categorization of SAEs follow a structured process guided by well-defined criteria.
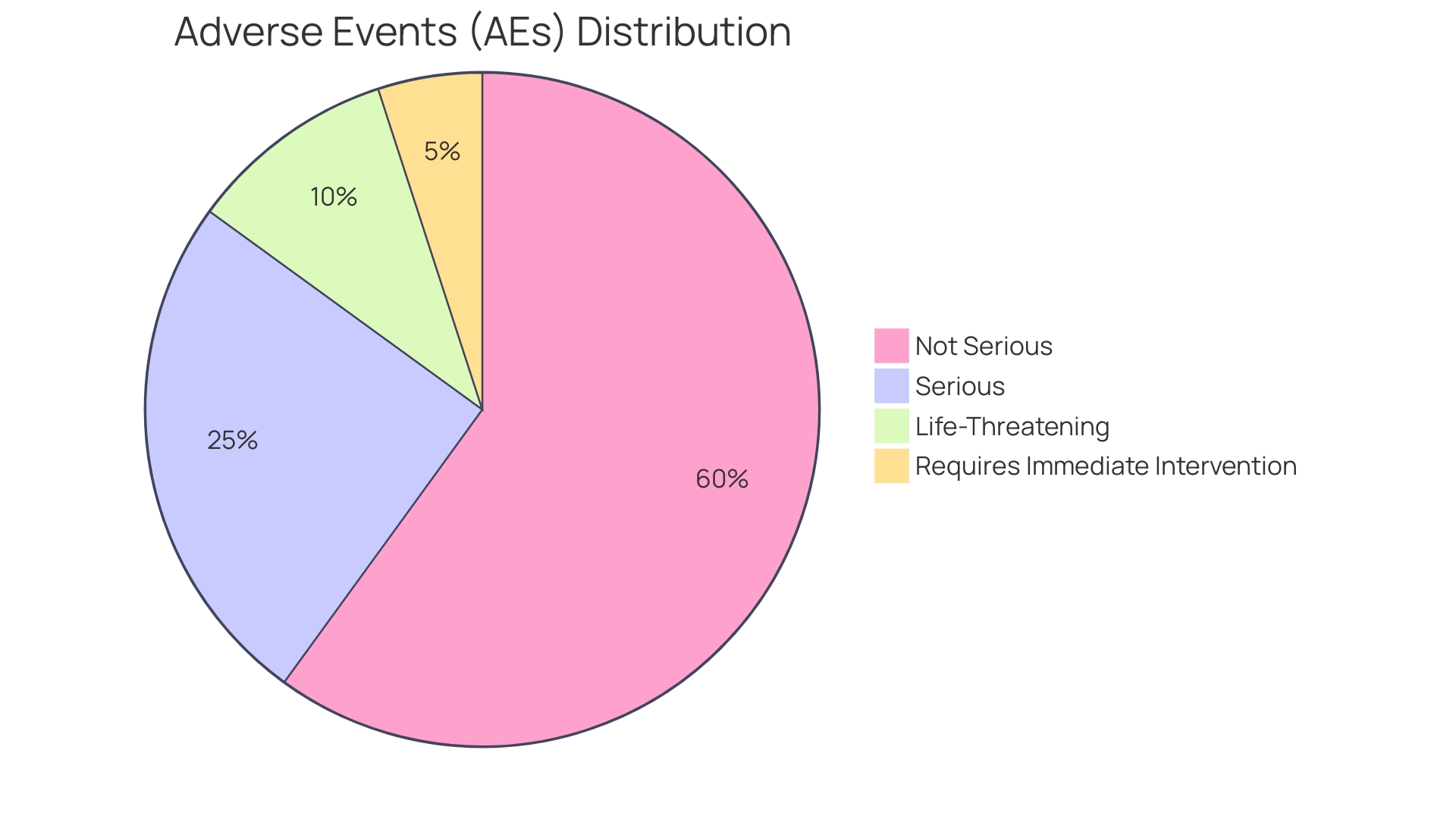
Distinguishing between Adverse Events (AEs) and SAEs is crucial for patient safety and regulatory compliance. While AEs encompass any unfavorable and unintended sign, symptom, or disease associated with the use of a medicinal product, SAEs represent a narrower category that requires immediate medical intervention or poses life-threatening conditions. The meticulous reporting of SAEs is a shared responsibility of investigators and sponsors, ensuring transparency and the comprehensive assessment of patient safety.
Additionally, Suspected Unexpected Serious Adverse Reactions (SUSARs) play a significant role in safety monitoring during clinical trials, uncovering new safety concerns that may not have been previously recognized. The reporting of SAEs and SUSARs must adhere to specific requirements and timelines to maintain trial integrity and patient safety. Annual Safety Reports (ASRs) and continuous safety monitoring are crucial components of clinical trials, providing a snapshot of the investigational product's safety profile and facilitating early identification of potential safety issues.
Through these processes, the medical community strives to ensure the highest standards of patient safety, transparency, and informed decision-making.
Definition of Serious Adverse Events (SAEs)
Critical medical occurrences that are serious and arise unexpectedly during clinical trials require immediate investigation. Distinguished from more common adverse reactions, these events encompass occurrences that can potentially threaten life, necessitate hospital admissions, result in persistent or significant disability or incapacity, or even lead to death. The happening of severe adverse events might also initiate medical or surgical interventions to prevent any of these serious outcomes. Such events require a strict, standardized approach to guarantee the well-being of participants, as specified in detailed reports including the Development Safety Update Report (DSUR) which contains crucial sections such as Introduction, Worldwide Marketing Authorization Status, Clinical Study Status, and a comprehensive Safety Data Summary.
Reflecting on the importance of effectively communicating risks, the FDA's recent final rule on direct-to-consumer prescription drug advertisements underscores the criticality of presenting major side effects and contraindications - akin to SAEs in clinical trials - in a manner that is clear, conspicuous, and neutral. This aligns with the broader objective of enhancing patient care through informed decision-making, echoing the standards set by the Vaccine Adverse Event Reporting System (VAERS), praised for its role in identifying vaccine-related concerns. These regulations and systems exemplify the ongoing efforts to improve transparency and understanding of the potential risks associated with medical interventions, thereby fostering trust and safety in public health initiatives.
Criteria for Identifying SAEs
Accurate identification and categorization of Serious Adverse Events (SAEs) in clinical trials is a structured process guided by well-defined criteria. These criteria are crucial in differentiating serious adverse events from other adverse events that are less critical in nature. The severity of the event, the necessity for medical intervention, the length of hospital stay, and the extent to which the event disrupts the patient's routine activities are key factors considered in this assessment. By following these standards, researchers guarantee that serious adverse events are accurately categorized, facilitating a targeted response and precise reporting that is crucial to patient well-being and regulatory adherence. The significance of this distinction is emphasized by the FDA's role in ensuring the effectiveness and well-being of medical products, as they need dependable information from trials to make informed judgments about the advantages and hazards of a product. This careful approach to adverse events contributes to the overall goal of safeguarding public health and promoting the advancement of medical treatments.
Differences Between Adverse Events (AEs) and SAEs
In clinical research, distinguishing between Adverse Events (AEs) and Serious Untoward Incidents (SUIs) is crucial for patient safety and regulatory compliance. AEs encompass any unfavorable and unintended sign, symptom, or disease temporally associated with the use of a medicinal product, whether or not related to that product. In contrast, another group of adverse events represents a narrower category of AEs that lead to life-threatening conditions, hospitalization, disability or permanent damage, congenital anomalies, or birth defects, and are critical enough to require immediate medical intervention or even death.
The FDA emphasizes the importance of clear communication regarding the risks associated with medical products. Their recent final rule on "Direct-to-Consumer Prescription Drug Advertisements" underscores the need for presenting significant side effects and contraindications (the 'major statement') in a manner that is clear, conspicuous, and neutral. This rule reflects the commitment to using consumer-friendly language and ensuring that the information is easily understandable, which is particularly relevant when conveying the distinctions between adverse events and significant adverse events.
Moreover, the FDA's Postmarketing Requirements and Commitments Searchable Database is evidence of the agency’s commitment to monitoring the well-being of medical interventions post-approval. The database contains important information on PMRs and 506B PMCs for efficacy and the ongoing duty to monitor and examine AEs and serious adverse events.
As healthcare professionals and investigators, comprehending the consequences of adverse events is not only a regulatory necessity but also a ethical obligation to guarantee patient well-being and maintain the credibility of medical research. Through vigilant monitoring and reporting, healthcare providers can contribute to the broader goals of improving patient care and advancing medical knowledge.
Reporting SAEs: Responsibilities and Deadlines
The thorough reporting of significant negative occurrences is a fundamental aspect of upholding the honesty and dependability of clinical trials. It is the shared obligation of investigators and sponsors to report SAEs accurately and promptly, adhering to the timelines stipulated in the study protocol. These reports not only guarantee transparency but are also crucial for a thorough evaluation of patient well-being. They play a significant role in evaluating the potential risks and benefits of new treatments, ultimately influencing patient care and outcomes. ClinicalTrials. Gov entries, which summarize study protocols and results, have evolved over the past 25 years, reflecting changes in trial reporting laws and policies. This evolution underscores the importance of precise SAE reporting, which contributes to the body of information that ensures treatments are safe and effective for patient use. Recent updates from the FDA further emphasize the necessity for clarity and accessibility in communicating risks associated with new treatments, ensuring that information about the well-being is presented to the public in a manner that is both understandable and transparent.
Understanding Suspected Unexpected Serious Adverse Reactions (SUSARs)
The research landscape in the medical field is constantly changing, and with it, the understanding of Serious Adverse Events (SAEs). In this range, Suspected Unexpected Serious Adverse Reactions (SUSARs) are significant for the monitoring of well-being in experiments. These events are not previously recognized from the pharmacological properties of a drug or medical device. SUSARs can emerge from various factors such as unforeseen drug interactions, extending the drug to new patient populations, altering dosage levels, or modifying the frequency of drug administration.
The importance of SUSARs lies in their ability to reveal new concerns regarding the well-being of individuals that may not have been detected during earlier stages of a drug or device development. Therefore, identifying and handling these occurrences is an essential aspect of overseeing medical examinations. Recent updates by the FDA, such as the final rule on advertising prescription drugs, emphasize the importance of presenting drug side effects clearly and prominently to consumers, reflecting a broader commitment to transparency and safety in healthcare communications.
Considering these advancements, publications in the realm of medical research are progressively concentrated on inventive and unique investigations to improve the structure and decision-making in trials. Peer-reviewed journals are instrumental in disseminating in-depth articles that catalyze the transformation of healthcare delivery, while digital platforms offer concise summaries and expert commentary necessary for busy clinicians. These resources serve as educational tools for practitioners to learn and enhance their practice, particularly in the realm of reasoning skills, which are essential when dealing with complex issues such as SUSARs.
A strong comprehension of SUSARs not only contributes to the safe conduct of current trials but also informs post-marketing surveillance and regulatory decisions. This knowledge is crucial for the medical community to prepare for and manage the risks associated with new therapeutic interventions, thus ensuring the well-being and efficacy of treatments offered to patients.
Reporting SUSARs: Specific Requirements and Timelines
The process of notifying authorities about Serious Adverse Events (SAEs) is a critical and regulated task in clinical research. Not only are there strict guidelines to adhere to, but the reporting must be completed within specified timelines to uphold the integrity of the evaluation and ensure patient well-being. Regulatory bodies stipulate the specifics of these reports, including their content and format, which often necessitates a thorough understanding of the regulations.
For example, if an event occurs during manufacturing, including any phase from testing to distribution, that deviates from the norm or is unexpected and could affect the well-being or effectiveness of a product, it is obligatory to report this deviation. As per regulatory standards, such an event must be reported within 45 days upon discovery. The significance of these reports is emphasized by their function in upholding the utmost quality and standards in trials, as they can directly impact patient care and results.
Moreover, these reports serve a larger purpose, akin to the Suspicious Activity Reports (SARs) in the financial sector, which play a vital role in combating crimes by tracing the financial activities related to them. Similarly, careful documentation of serious adverse events assists in the continuous effort to guarantee the safety and effectiveness of new medical treatments.
In the context of clinical trials for rare diseases, where patients participate with the hope of advancing research that could benefit future generations, the timely and accurate reporting of serious adverse events is not just a regulatory requirement but a moral imperative. The data collected from these reports contribute to a more profound understanding of the investigational drug or device, ultimately guiding better treatment options and outcomes for patients.
Role of Investigators and Sponsors in SAE and SUSAR Reporting
The collaboration between investigators and sponsors is crucial in the reporting of Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs). Investigators bear the responsibility of swiftly identifying and documenting Sales with precision and thoroughness. Meanwhile, sponsors are tasked with the overarching management of the trial, entailing the gathering, examining, and relaying SAE and SUSAR reports to regulatory bodies and ethics committees. This intricate process not only demands rigorous adherence to legal and regulatory frameworks but also requires an understanding of the broader ethical and societal implications of the research. As highlighted by case studies, the ethical landscape is diverse, covering market incentives, intellectual property, and the overarching goal of improving the human condition through medical advancements.
The historical and international context of technology and research ethics further enriches our understanding of the evolving landscape in which SAE and SUSAR reporting operates. In the field of research that spans various sectors—academia, healthcare, government, and the private sector—each domain contributes to the mosaic of governance, enforcement, and ethical considerations that guide SAE reporting. Significantly, the FDA's Searchable Database for Postmarketing Requirements and Commitments serves as evidence of the openness and consistency in the monitoring of medical and nonmedical experiments, with updates provided every quarter.
In the world of clinical research, personal stories, like those shared by patients with transthyretin-mediated amyloidosis, shed light on the profoundly human aspect of participation in experiments. These stories serve as a reminder that every SAE and SUSAR report is a reflection of patients dealing with the challenges of treatment, often without the assurance of personal gain. Furthermore, the variability in trial transparency, as highlighted by industry experts, underscores the need for accessible trial protocols and comprehensive data sharing to ensure research integrity.
Each participant in the research ecosystem, whether a manufacturer, healthcare professional, or researcher, plays a crucial role in ensuring the well-being and effectiveness of the medical interventions at hand. With reports on security acting as a cornerstone in safeguarding public health, the contributions of all stakeholders are invaluable in the continuous pursuit of medical progress and patient well-being.
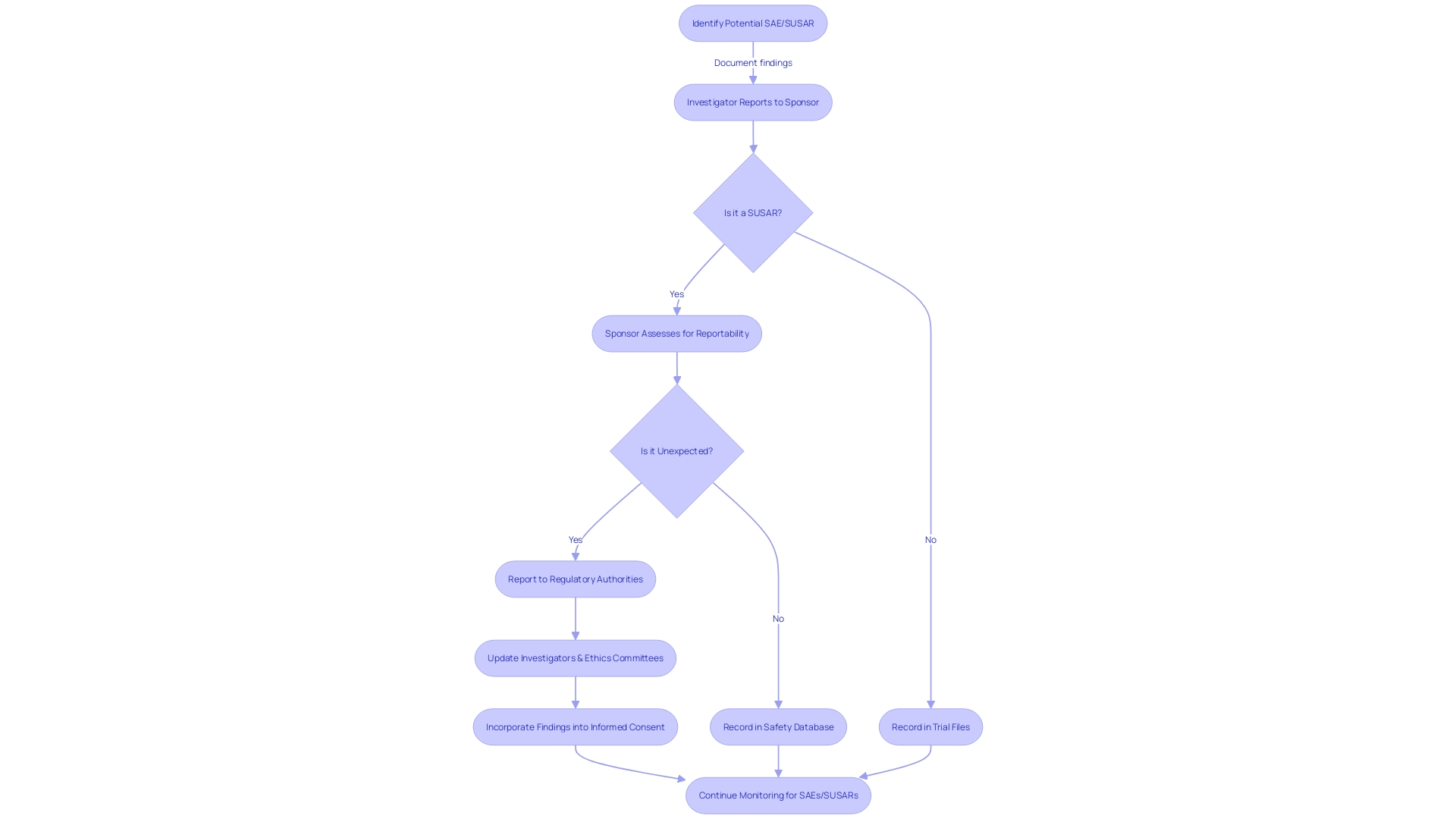
Annual Safety Reports (ASRs) and Continuous Safety Monitoring
Annual Reports (ASRs) are pivotal documents in clinical trials, encapsulating the information about the well-being of investigational drugs or medical devices over the trial's course. These reports are meticulously compiled to detail the occurrences of Serious Adverse Events (SAEs) and other pertinent risk data, thereby providing a snapshot of the investigational product's profile. Supported by continuous monitoring for participant well-being, which involves careful observation of relevant data and the implementation of strong risk management approaches, ASRs enable the continuous assessment of participant security. Crucially, they function as a mechanism for the early detection of any possible concerns regarding patient well-being that may arise throughout the examination, guaranteeing that patient welfare remains a top priority in medical research.
The U.S. Food and Drug Administration (FDA) emphasizes the significance of such monitoring for protection through guidance for sponsors on the operations of Data Monitoring Committees (DMCs). These committees have a vital function in monitoring and ensuring the integrity of ongoing experiments, emphasizing the importance of ongoing supervision. In the realm of medical devices, Post-Market Surveillance (PMS) extends this vigilance beyond pre-market phases. It encompasses a range of methodologies, from passive systems like spontaneous reporting to more proactive approaches, such as registries and electronic health records analysis, thus assuring the ongoing assessment of medical devices in real-life usage scenarios.
Moreover, the FDA's commitment to safeguarding public health is evident in its recent publication of the "Direct-to-Consumer Prescription Drug Advertisements" final rule. This rule mandates that drug advertisements on television and radio clearly and effectively communicate the major side effects and contraindications of the drug, ensuring that the information is accessible and understandable to consumers. This emphasis on transparency and clarity in communication parallels the objectives of ASRs and continuous monitoring in clinical trials, which collectively aim to maintain the highest standards of patient safety and inform public health decisions.
Conclusion
In conclusion, Serious Adverse Events (SAEs) are critical occurrences during clinical trials that require immediate investigation. Distinguishing between Adverse Events (AEs) and SAEs is crucial for patient safety and regulatory compliance. SAEs represent a narrower category that poses life-threatening conditions or requires immediate medical intervention.
The identification and categorization of SAEs follow a structured process guided by well-defined criteria. This meticulous approach ensures accurate classification and reporting, contributing to patient safety and regulatory compliance.
Reporting SAEs is a shared responsibility of investigators and sponsors. Timely and accurate reporting is essential for transparency and comprehensive assessment of patient safety, influencing the evaluation of new treatments' risks and benefits.
Suspected Unexpected Serious Adverse Reactions (SUSARs) play a significant role in safety monitoring during clinical trials. They uncover new safety concerns that may not have been previously recognized, contributing to improved patient care and informed decision-making.
Annual Safety Reports (ASRs) provide a snapshot of the investigational product's safety profile and facilitate early identification of potential safety issues. Continuous safety monitoring, including the oversight of Data Monitoring Committees (DMCs) and post-market surveillance, ensures ongoing assessment of participant safety.
The synergy between investigators and sponsors is pivotal in reporting SAEs and SUSARs. Their collaboration ensures swift identification and documentation, adhering to legal and regulatory frameworks, and upholding ethical considerations.
In summary, the meticulous reporting of SAEs and SUSARs, adherence to specific requirements and timelines, and the compilation of ASRs are crucial components of clinical trials. These processes prioritize patient safety, transparency, and informed decision-making, ultimately advancing medical knowledge and improving patient care.




