Introduction
Clinical trials rely on well-defined study endpoints to determine the effectiveness and safety of treatments. These measurable outcomes serve as objective indicators, answering specific research questions and guiding researchers in achieving their goals. The selection of appropriate endpoints is crucial, as it ensures that the outcomes truly reflect the research objectives.
Different types of endpoints, such as primary, secondary, composite, and safety outcomes, play a vital role in clinical trials. Primary endpoints reflect the most important outcomes, while secondary endpoints provide additional insights. Surrogate endpoints act as proxies when direct measurement of the primary outcome is challenging, and patient-reported outcomes capture the patient's perspective.
Choosing the right endpoints requires careful consideration of sensitivity, clinical significance, reliability, validity, sample size, and participant characteristics. The adjudication process by expert panels ensures data integrity and adherence to ethical guidelines. Standardized criteria are essential in endpoint assessment, enabling reliable comparisons, meta-analyses, and optimal decision-making.
Various therapeutic areas require specific endpoints, such as major adverse cardiovascular events in cardiovascular research or overall survival in oncology. Challenges in endpoint selection include the need for standardized measures, transparency in reporting, patient perspectives, and balancing risk-benefit trade-offs. By incorporating technological advancements, patient-centric measures, and standardized criteria, clinical trials can produce scientifically robust and meaningful outcomes for medical science and patient care.
What are Study Endpoints?
The success of clinical experiments depends on clearly defined study objectives, which are the measurable results used to assess the efficacy or safety of a treatment or intervention. These critical data points serve as objective indicators for evaluating whether the intervention has fulfilled its intended goals. The various kinds of study outcomes, like main, additional, combined, and safety results, are customized to address the specific questions posed at the start of the trial. For example, the PICO framework—Population, Intervention, Comparison, and Outcome—assists in shaping focused and answerable inquiries by ensuring that each component of the study is clearly defined. This framework is instrumental in guiding researchers to pinpoint the outcomes that directly relate to the inquiry at hand, as underscored in a recent review paper on depression measurement challenges published in Nature Reviews Psychology.
The significance of choosing suitable terminations cannot be emphasized enough; it is a procedure that necessitates meticulous consideration of what is truly being measured. Take, for example, the diverse ways in which depression is quantified—over 280 different measures exist, which may not consistently capture the same construct, potentially hindering the accumulation of scientific knowledge. This emphasizes the necessity for accuracy and uniformity in the selection of indicators that truly represent the objectives of the study. Trials expert and physician Frank David emphasizes the importance of such terms and metrics in his introduction to an updated report on trials, asserting their increased relevance in today's research landscape.
Recent progress in studies demonstrates the influence of carefully selected outcomes. A study on cervical cancer, for instance, compared the survival rates of patients treated with chemotherapy before radiotherapy against those receiving only the standard radiotherapy treatment. The evident outcome of patient survival without further cancer development revealed an increase from 64% to 73% with the addition of chemotherapy, showcasing the tangible benefits of this treatment approach. Such results not only provide information for medical decisions but also open the door for future scientific efforts, demonstrating the significant impact of established study goals in experimental investigations.
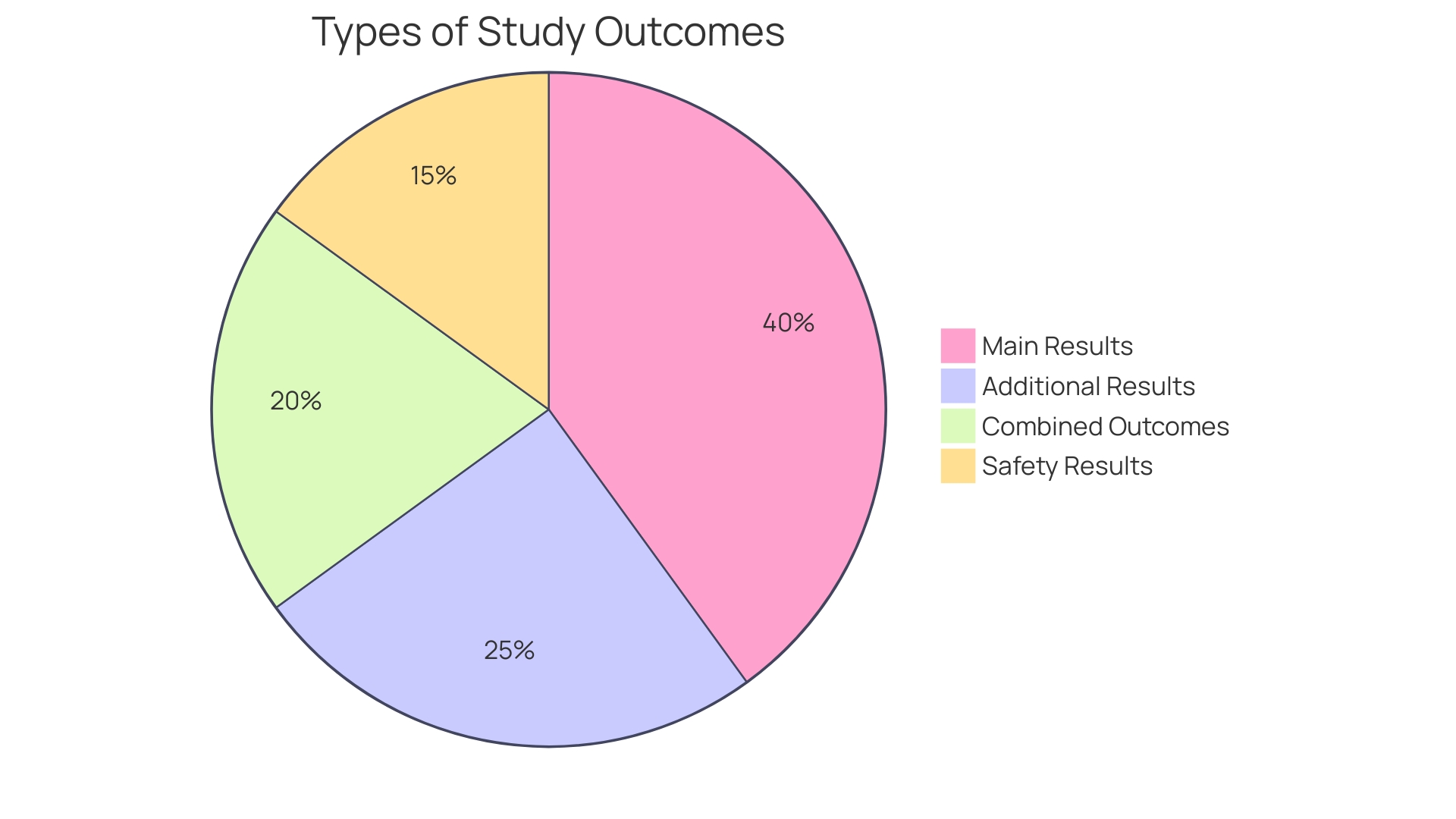
Types of Study Endpoints
Comprehending the different categories of conclusions in medical studies is vital for interpreting the outcomes and assessing the efficacy of a therapy. The main goals are crucial as they mirror the most significant results pertinent to the research query. Their selection is based on the trial's objectives and their results are pivotal in the study's overall interpretation.
Secondary outcomes, while not as crucial as primary results, offer extra insights into the intervention's effects. They help to paint a broader picture of the treatment's potential benefits or risks, contributing to a more comprehensive understanding of its impact.
Surrogate measures come into play when the main outcome is not feasible or practical to measure directly. They serve as substitutes, expected to correlate with the actual endpoint of interest, and are particularly useful in situations where direct measurement of the primary outcome is challenging.
Patient-reported outcomes (PROs) are self-reported measures from patients about their own health, quality of life, and well-being, which are vital for understanding the intervention's impact from the patient's perspective. Pros are increasingly acknowledged for their worth in capturing the patient's perspective in scientific investigation.
Lately, the FDA highlighted the significance of well-structured experiments, which guides their decision-making on product safety and effectiveness. This underscores the significance of well-defined inquiries and the choice of suitable endpoints. As the medical investigation scenery progresses, the approaches for instructing, implementing, and evaluating medical deduction skills are also advancing, guaranteeing that new experiments are designed with the latest insights in mind.
Comprehensive articles and expert commentary further underscore the importance of focused inquiry questions and answerable hypotheses. The PICO framework is a tool that aids researchers in defining these questions by focusing on the population, intervention, comparison, and outcome.
It's evident that end points have a crucial function in trials, directing the data gathered and the deductions made. Comprehending these diverse termination points enables a nuanced analysis of research results, ultimately aiding the progress of medical science and patient care.
Assessing Study Endpoints
Selecting the appropriate study goals in trials is a intricate task that requires thorough deliberation. Ensuring that the selected measures are sensitive enough to detect significant differences between treatment groups is crucial. They must also hold clinical significance for both patients and healthcare providers. For example, in the context of diabetes—a condition that affects more than 38 million Americans and has an annual cost of $412.9 billion—the outcomes must align with the significant physical and economic consequences of the disease.
When assessing the limits, scientists need to assess the dependability and accuracy of measurement instruments. With advancements in digital health, such as the use of AI to predict tumor margins in prostate cancer care, the precision of measurements can be greatly enhanced. These tools, however, must also be feasible for real-world application and cost-effective within the constraints of the study's budget.
Adequate sample size and participant characteristics are equally important. For instance, a digital health solution aiding in diabetic remission must be tested among diverse populations to account for the varied impact of diabetes across different racial and ethnic groups.
Furthermore, patient-related outcomes (Pros) are becoming increasingly relevant. They focus on the individual's lived experience, which is essential for personalized care. This shift towards Pros underscores the need for validated questionnaires, known as patient-related outcome measures (Proms), to assess the effects of treatments from the patient's perspective.
In summary, through the integration of a combination of technological progress, financial factors, and patient-focused measures, researchers can guarantee that the goals selected for trials are not only scientifically strong but also valuable to all parties involved. This multi-faceted approach is exemplified in the creation of the prostate cancer 'Report Card,' which presents survival rates, recurrence, and the impact of treatment on lifestyle and wellbeing, alongside personal experiences, thus offering a comprehensive view of treatment efficacy.
Endpoint Adjudication Process
The evaluation of endpoints in medical studies is a crucial stage towards the preservation of data integrity and dependability, especially when dealing with endpoints that are subjective or intricate in nature. A group of specialists, often with a vast amount of knowledge in practice and research, like those found at Voiant, the premier Independent Reading Center for ophthalmic imaging, carry out this essential process. Their task is to perform a meticulous, unbiased evaluation of outcomes based on predetermined and standardized criteria.
This independent review process is informed by ethical guidelines, such as those outlined in the Declaration of Helsinki and further elaborated upon in the Belmont Report, which stress the importance of respect for individuals, beneficence, and justice. Updated guidance from the Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard underscores the evolving application of these ethical principles.
The consensus reached by the panel is not just a procedural formality; it represents a commitment to scientific accuracy and consistency, ensuring that the results can stand up to scrutiny from regulatory authorities and the wider medical community. This is of utmost importance in our era where experimentation data not only guide medical practice but also inform regulatory decisions and healthcare policies.
As stated by Gamertsfelder, a clinical and policy researcher, and Osipenko, who leads a non-profit dedicated to improving clinical study quality and integrity, the human element is essential. Their insights remind us of the personal sacrifices made by participants in experiments, who endure invasive tests and new side effects, in the hope of advancing medical science for future generations. The adjudication process, therefore, is not just a technicality; it is a safeguard, ensuring that the contributions of these participants are honored with the highest standards of research integrity.
Importance of Standardized Criteria
The reliability of clinical study results depends on the thorough specification and assessment of research goals. Using standardized criteria is not just a procedural formality; it is a crucial practice that strengthens the dependability of assessments and guarantees consistency across diverse studies. Such standardization mitigates the risks of bias and variability which, if unchecked, could compromise the integrity of trial results. By utilizing a common framework for evaluation of the target, as guided by statistical decision theory and information economics, researchers can define a clear decision problem. This involves delineating a state dependent on the payoff, crafting a data-generating model that yields signals influencing the state's distribution, defining an action space for the decision-maker, and applying a scoring rule that measures the decision quality based on chosen actions and state realizations.
The application of these criteria enables the clear communication of information to participants, empowering rational decision-making based on the signals provided, such as data visualizations or model predictions. This structured approach ensures that any conclusions drawn regarding the efficacy of interventions are grounded in optimality, as understood by statistical decision theory.
Moreover, standardized criteria play a crucial role in enabling data integration for meta-analysis, enhancing the robustness of evaluations concerning an intervention's effects. In the context of diverse study designs and methodologies—ranging from classical frequentist to Bayesian approaches—the consistent application of such criteria is essential. Not only do Bayesian methodologies allow for more adaptable study designs and the potential for early discontinuation of ineffective treatments, but they also enable the seamless extension of studies without statistical penalties, further reinforcing the importance of standardized endpoint assessment criteria.
As highlighted by the International Society for Evaluation Education, the practical application of these principles can be seen in the Case Collaborative's efforts to provide context-rich evaluation experiences through case studies. Real-life implementation highlights the importance of practical wisdom in maneuvering the socio-political terrain of scientific investigation. In an environment where disparities in drug access and examination diversity persist, as evidenced by varying availability of approved drugs across Europe and the underrepresentation of pregnant women and children in examinations, the adherence to standardized criteria becomes even more critical for equitable and accurate outcomes.
The importance of standardizing depression measurement is also reflected in the difficulties, as shown by a Nature Reviews Psychology review paper. With over 280 different depression measures, the need for a unified approach to assess interventions becomes starkly apparent. Standardized criteria not only ensure comparability across studies but also address critical gaps in the field, fostering innovative concepts and methodologies that can significantly enhance the impact of clinical investigation.
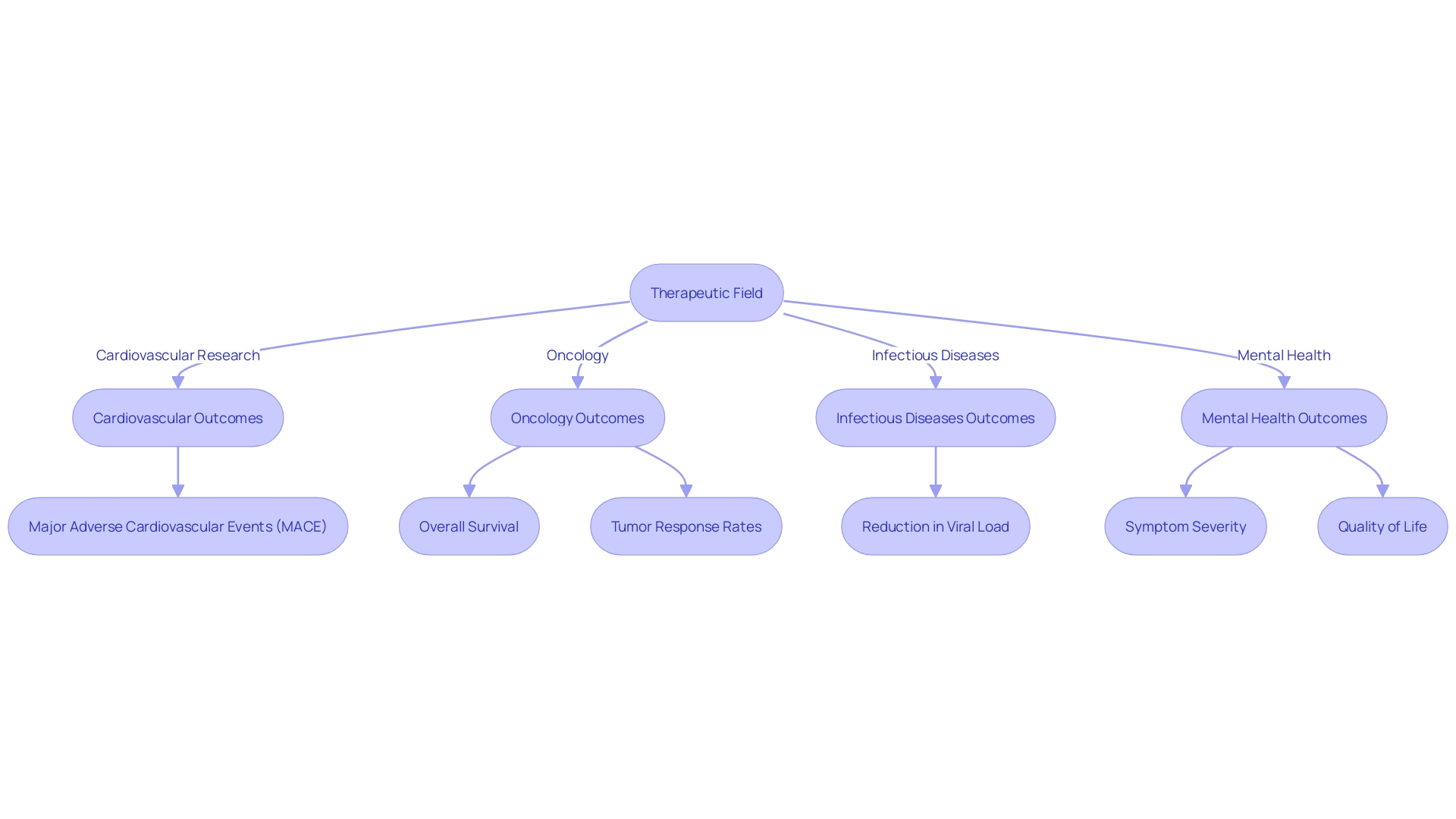
Examples of Study Endpoints by Therapeutic Area
Clinical trials in different therapeutic fields depend on meticulously chosen study markers to assess the effectiveness and safety of interventions. In cardiovascular research, investigators may focus on the incidence of Major Adverse Cardiovascular Events (MACE), such as heart attacks, strokes, or cardiovascular-related deaths. These outcomes are critical in evaluating the success of interventions aimed at reducing the morbidity and mortality associated with cardiovascular diseases. Recent advancements, like the investigation of Zilebesiran to deter high blood pressure, highlight the significance of identifying points of completion that can exhibit substantial clinical advantages.
In the field of oncology, the key measures are overall survival, progression-free survival, and tumor response rates. These measures are essential for understanding how a cancer treatment can extend life or delay the progression of the disease. For example, progress in therapies that focus on particular molecular pathways in cancer cells have transformed treatment options, emphasizing the requirement for measures that can capture these subtle effects.
For infectious diseases, measures such as time to improvement in symptoms, reduction in viral load, or prevention of infection are crucial. These criteria are particularly relevant as they reflect both the direct impact of an intervention on the pathogen and the clinical benefit to the patient. As the global health landscape evolves, the importance of these markers in guiding treatment strategies and public health policies becomes even more pronounced.
Mental health experiments frequently employ ratings of symptom severity, rates of remission, or measures of quality of life as indicators. Tools such as the Health Assessment Questionnaire - Disability Index (HAQ-DI) and the WHO Quality of Life assessment (WHOQOL) are employed to evaluate the multidimensional aspects of mental health disorders, emphasizing patient-centered outcomes and the broader impact on individuals' daily lives.
Ethical considerations remain paramount across all therapeutic areas, with guidelines urging researchers to declare any changes to methodology or reporting transparently. This involves obtaining approval from appropriate ethics committees, ensuring that the study design and goals are in line with regulatory and legislative mandates, and preserving the integrity of the research process.
As medical studies progress, the choice of pertinent and significant outcomes, guided by moral principles and influenced by the most recent scientific advancements, remains a fundamental aspect in the development of medical understanding and the enhancement of patient results.
Challenges in Choosing Study Endpoints
Choosing study endpoints for medical experiments requires a subtle strategy, considering the intricacy of illnesses and the complexities involved in measurement and analysis. With over 280 different measures for a single condition like depression, researchers face the daunting task of ensuring these various tools are assessing the same underlying constructs. This task becomes even more challenging when considering the diverse patient responses and the need for validated, feasible measurement techniques within a research setting. The FDA's push for efficient, well-designed medical research underscores the importance of clear, reliable data to inform decisions on medical product safety and effectiveness. As echoed in recent literature, the transparency in reporting composite outcomes and the balance of component events can shed light on treatment effectiveness and patient challenges across illnesses. Moreover, the consideration of patient perspectives is gaining ground in drug development, highlighting the necessity of relevant, non-redundant questionnaires to gauge improvement in conditions such as cancer. In the domain of non-inferiority experiments, the focus is moving towards a more transparent expression of experiment objectives, investigating not only efficacy but the risk-benefit trade-offs of new treatments. This clarity in objectives can lead to experiments that are more aligned with patient care and potentially more impactful in practice. With the FDA's continued efforts to harmonize regulations and protect clinical trial participants, combined with a rigorous, collaborative approach to endpoint selection, the goal is to enhance the integrity and utility of clinical research outcomes.
Conclusion
In conclusion, the selection of appropriate study endpoints is crucial in clinical trials as they serve as objective indicators for evaluating the effectiveness and safety of treatments. Primary endpoints reflect the most important outcomes, while secondary endpoints provide additional insights. Surrogate endpoints act as proxies when direct measurement of the primary outcome is challenging, and patient-reported outcomes capture the patient's perspective.
Choosing the right endpoints requires careful consideration of sensitivity, clinical significance, reliability, validity, sample size, and participant characteristics. The adjudication process by expert panels ensures data integrity and adherence to ethical guidelines. Standardized criteria are essential in endpoint assessment, enabling reliable comparisons, meta-analyses, and optimal decision-making.
Different therapeutic areas require specific endpoints, such as major adverse cardiovascular events in cardiovascular research or overall survival in oncology. Challenges in endpoint selection include the need for standardized measures, transparency in reporting, patient perspectives, and balancing risk-benefit trade-offs.
By incorporating technological advancements, patient-centric measures, and standardized criteria, clinical trials can produce scientifically robust and meaningful outcomes for medical science and patient care. The thoughtful selection of endpoints allows for the accurate evaluation of treatments and supports the advancement of medical knowledge and the improvement of patient outcomes. The adherence to standardized criteria and the involvement of expert panels ensure the integrity and reliability of clinical trial outcomes.




