Introduction
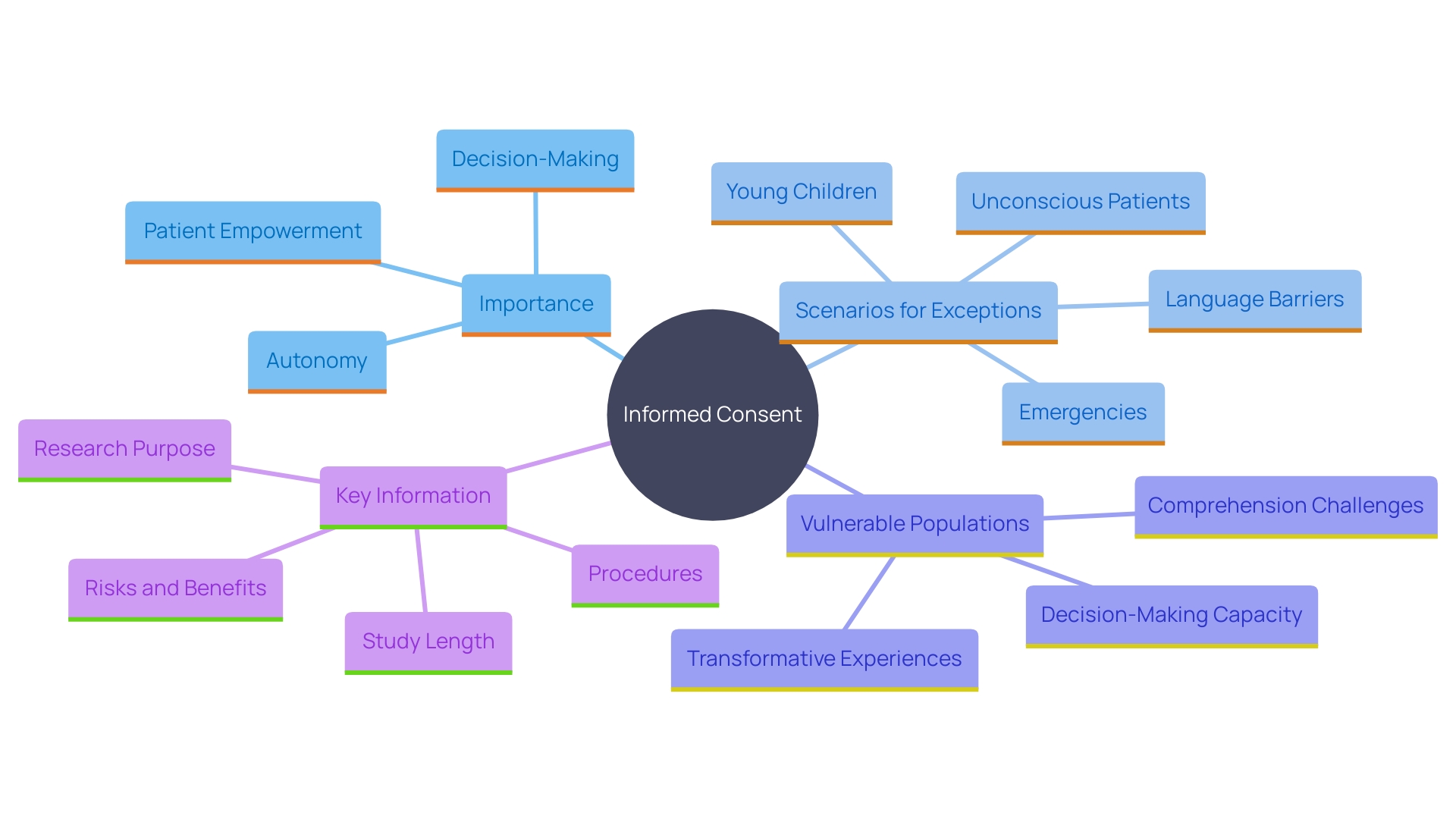
Informed consent stands as a cornerstone of ethical and legal standards in healthcare and research, underscoring the imperative of respecting patient autonomy. This principle is not merely a regulatory formality but a critical element that ensures patients are fully informed about their care decisions, fostering trust between patients and healthcare providers. Despite its importance, the evolution of informed consent documents into lengthy, complex texts has posed significant challenges.
Originally designed to aid prospective subjects in understanding research participation, these documents have become cumbersome, often exceeding twenty pages and filled with over 270 mandatory items. This complexity has made them difficult to create, update, review, and comprehend, creating barriers to clinical trial enrollment, particularly among underserved minority populations.
The intricacies of these documents, often written at a high reading level and heavily legalistic to comply with various laws, have led to widespread dissatisfaction among stakeholders. To address these issues, recent draft guidance suggests presenting key information clearly and concisely at the outset of informed consent documents. This approach aims to make the process more accessible and understandable, thereby improving the overall informed consent experience.
The following sections will delve into the essential elements of informed consent and explore effective strategies for overcoming the barriers that hinder its efficacy.
Ethical and Legal Foundations of Informed Consent
Informed agreement is a fundamental ethical principle in healthcare and studies, emphasizing respect for individual autonomy. It legally protects individuals' rights by ensuring they receive the essential information to make knowledgeable choices about their care. This principle not only promotes confidence between patients and healthcare providers but also complies with regulatory requirements necessitating awareness before treatment or study involvement.
However, the contemporary landscape of informed consent documents reveals significant challenges. Initially meant to help potential participants in comprehending involvement, these documents have transformed into intricate, cumbersome texts. Over the past two decades, their length has increased from three to four pages to often exceeding twenty pages, packed with over 270 mandatory items. This complexity has rendered them difficult to create, update, review, and comprehend for all stakeholders, including IRBs, physicians, clinical trial sponsors, research participants, and regulators.
The intricacy of these documents poses a barrier to clinical trial enrollment, particularly among underserved minority populations. They are frequently written at a high reading level and have become increasingly legalistic to comply with legislation, such as data privacy laws. This complexity has led to widespread dissatisfaction among stakeholders in drug development. For example, the National Organization for Rare Disorders (NORD) has praised new draft guidance that permits creative methods for obtaining agreement, such as videos, to make the process more accessible.
Tackling these matters, the draft guidance suggests presenting essential information clearly and briefly at the start of permission documents. This information should cover the study's purpose, potential risks and benefits, and the project's duration and procedures. By enhancing understanding and offering adaptability in executing these prerequisites, the guidance seeks to guarantee that informed agreement is available and clear for all individuals, thus enhancing the overall informed agreement procedure.
The 5 Essential Elements of Informed Consent
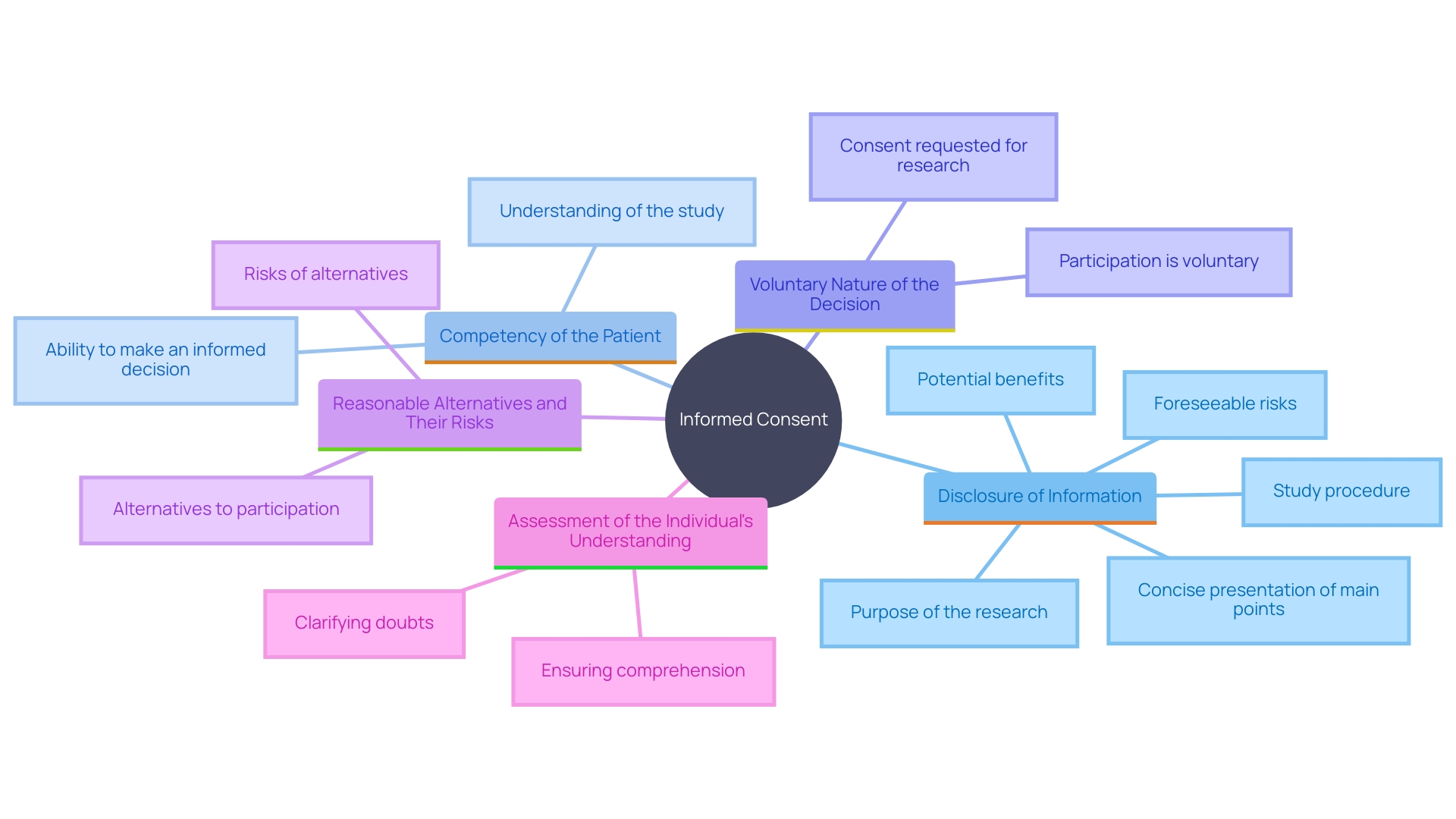
Informed consent in healthcare and studies is built upon five essential elements that must be clearly communicated to the patient. These elements include:
-
Disclosure of Information: This involves providing comprehensive and understandable information about the research or medical procedure. The primary goal is to assist the prospective subject in making a knowledgeable decision by presenting necessary information in a clear and concise manner, avoiding overwhelming lists of isolated facts.
-
Competency of the Patient: Ensuring that the patient is mentally capable of understanding the information presented and making a knowledgeable decision. This is crucial for the ethical and legal validity of the consent process.
-
Voluntary Nature of the Decision: The decision to participate must be made without any form of coercion or undue influence. Patients should feel free to make their choice based on their own values and preferences.
-
Reasonable Alternatives and Their Risks: Patients must be informed about all reasonable alternatives to the proposed intervention, including their respective risks and benefits. This enables individuals to consider their choices and select the one that most closely matches their personal health objectives.
-
Assessment of the Individual's Understanding: It is essential to evaluate whether the individual truly comprehends the information provided. This can involve asking the patient to explain the information in their own words or using other methods to confirm their understanding.
Each of these elements plays a vital role in ensuring that the agreement process is both ethical and legally sound. However, the complexity and length of informed consent documents have become a significant barrier. These documents, often exceeding 20 pages, are written at a high reading level and have become increasingly legalistic to comply with various regulations. This complexity presents difficulties for all parties involved, including IRBs, physicians, clinical trial sponsors, participants, and regulators. The primary purpose of these documents—to facilitate comprehension and assist in decision-making—has been overshadowed by their burdensome nature, making them difficult to create, update, review, read, and comprehend.
Disclosure of Information
Disclosure involves providing comprehensive information about the treatment or research study, including the purpose, procedures, risks, benefits, and potential alternatives. This information must be presented in a clear and understandable manner, allowing patients to grasp the implications of their decisions. Ensuring adequate disclosure is vital for knowledgeable decision-making. As per the recommendations from the National Organization for Rare Disorders (NORD), knowledgeable agreement should be customized to address the distinct requirements of participants, taking into account elements like language obstacles and health understanding abilities. The draft guidance suggests presenting key information at the beginning of the consent document, covering essential topics such as the aim of the study, possible risks and benefits, and the duration and procedures involved. Additionally, transparency in study methodology, such as openly declaring changes and seeking ethics committee approval, is crucial for maintaining trust and integrity in clinical trials. By ensuring all relevant information is accessible and comprehensible, individuals are better equipped to make knowledgeable decisions about their participation in research or treatment.
Competency of the Patient
Competency in individuals pertains to their capacity to grasp the provided information and make informed decisions based on that understanding. Healthcare providers need to evaluate if individuals possess the cognitive and emotional abilities to understand the consequences of their choices. This assessment is influenced by various factors such as age, mental health status, and the specific context of the situation. Research from the Rutgers Center for Health Services has shown that individuals with cognitive disabilities, like autism spectrum disorder and Down syndrome, often encounter barriers when accessing quality healthcare. They tend to report lower satisfaction levels compared to the general population, highlighting the importance of thorough competency evaluations. Ensuring that individuals can comprehend and participate in their care decisions is crucial, especially for those with cognitive impairments, to uphold the ethical principles of autonomy and beneficence.
Voluntary Nature of the Decision
Voluntary consent is a cornerstone of ethical medical practice, requiring that individuals make decisions free from coercion or undue influence. Empowering individuals to ask questions and seek clarifications is vital for aligning healthcare decisions with their personal values and preferences. This respect for individual autonomy fosters a trusting environment, essential for effective healthcare delivery. Research emphasizes that knowledgeable agreement safeguards an individual's right to make healthcare choices, ensuring autonomy and aligning care with personal values. The Joint Commission Journal on Quality and Safety highlights the significance of clear and concise communication in the consent process, aiding individual comprehension and decision-making.
Reasonable Alternatives and Their Risks
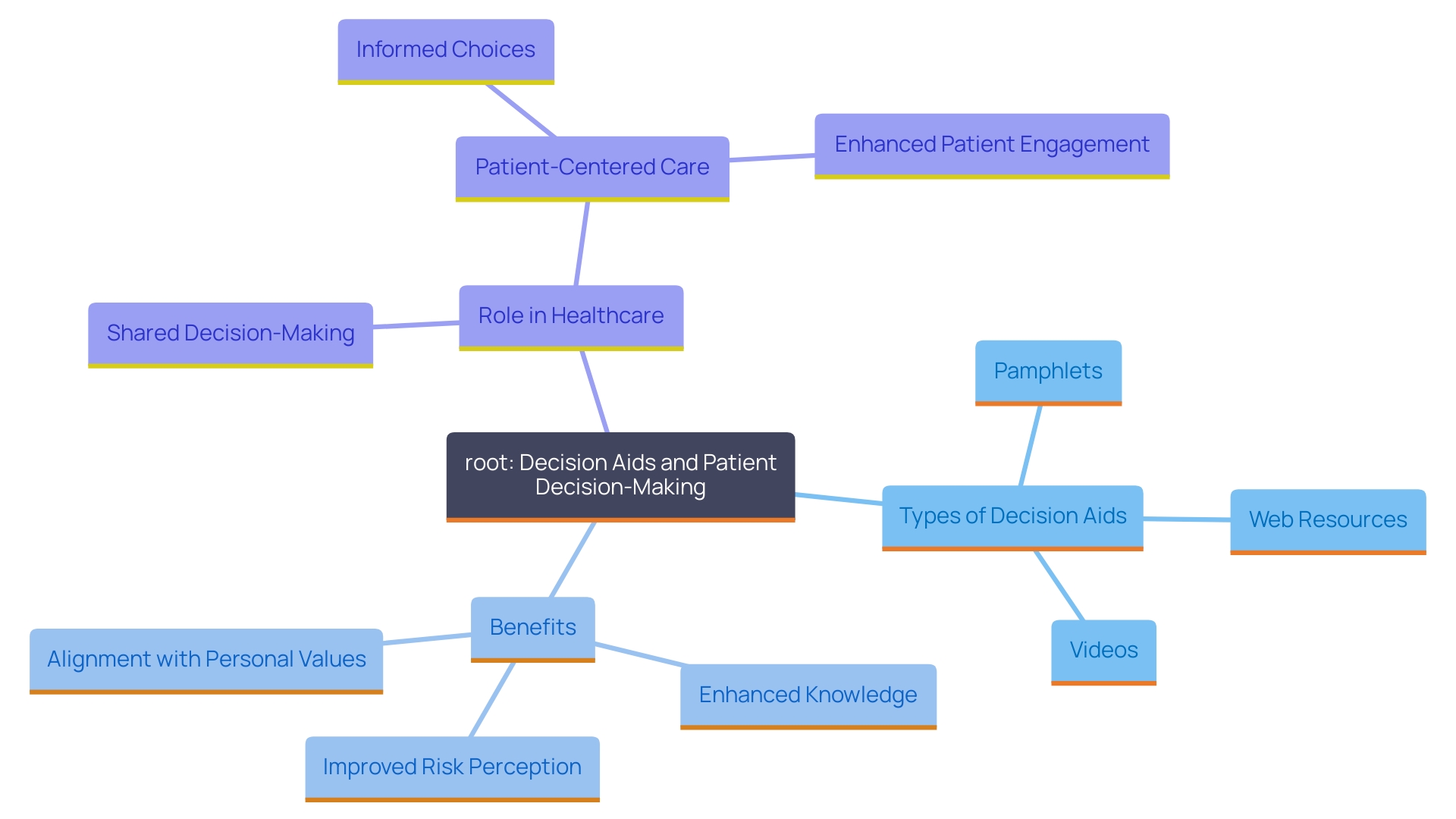
Patients should be informed about reasonable alternatives to the proposed treatment or study, along with associated risks and benefits. This facilitates a transparent decision-making process, enabling individuals to evaluate their options effectively and choose what best aligns with their health needs and personal circumstances. 'Decision aids, such as pamphlets, videos, or web-based resources, can significantly enhance this process by clearly presenting the healthcare decision to be made, outlining available options, and assisting individuals in understanding the potential outcomes.'. According to a 2024 update, decision aids have been shown to enhance individuals' knowledge, risk perceptions, and alignment of their choices with personal values, without increasing distress or regret. These tools support shared decision-making, a crucial aspect of patient-centered care and health system improvements. Research has shown that decision aids result in improved engagement and satisfaction with the decision-making process, emphasizing their significance in clinical practice.
Assessment of the Patient's Understanding
Evaluating individual comprehension is crucial to guarantee that they can make knowledgeable choices regarding their treatment. This can be effectively achieved by asking open-ended questions or encouraging individuals to summarize their understanding of the treatment or study. For example, the Declaration of Helsinki emphasizes the significance of continuous informed consent during studies, stressing the need for participant understanding. Participating in this conversation not only educates individuals but also enables them to make choices based on a clear comprehension of their alternatives. This approach aligns with the principles outlined in the Belmont Report, which emphasizes respect for persons and their autonomy. Moreover, recent initiatives, such as the Multi-Regional Clinical Trials Center's revised guidelines, promote the participation of healthcare partners in the research process, improving the relevance and practicality of clinical studies. By fostering clear communication and understanding, healthcare providers can significantly improve patient outcomes and adherence to treatment plans.
Documentation and Communication Strategies for Effective Informed Consent
Effective documentation is crucial for the informed consent process, ensuring transparency and thoroughness. Key information about the research, such as its purpose, risks, benefits, and procedures, should be presented clearly and concisely. This helps participants understand why they might or might not want to participate.
Communication strategies are vital in enhancing patient comprehension. Using plain language, visual aids, and teach-back methods can significantly improve understanding. The draft guidance encourages investigators to leverage these strategies, suggesting innovative approaches like multimedia elements and interactive glossaries, which simplify complex medical terms and processes.
For instance, digital approval formats, including audio and video, can cater to diverse demographics and address various barriers, such as language, vision, or hearing impairments. By making agreement information easy to access and interesting, researchers can ensure that participants fully understand the study's implications, ultimately encouraging knowledgeable and voluntary participation.
Exceptions to Informed Consent and Special Considerations
In specific circumstances, acquiring informed approval may be overlooked, such as during emergencies where immediate medical intervention is essential, or when individuals are unable to provide authorization due to incapacitation. It is crucial to handle these scenarios with the utmost care, ensuring that the patient's best interests are prioritized.
Special considerations are also essential for vulnerable populations, including children, the elderly, and those with disabilities. These groups require additional protections to ensure their rights and wellbeing are safeguarded throughout the agreement process. The National Organization for Rare Disorders (NORD) highlights the significance of providing knowledgeable agreement in accessible formats customized to participants' distinct requirements, taking into account aspects such as language barriers, sensory impairments, and health literacy levels. By taking these steps, healthcare professionals can ensure that all individuals, regardless of their circumstances, receive the respect and protection they deserve during the informed consent process.
Shared Decision Making and Informed Consent
'Collaborative decision-making, a concept established by the Presidential Commission in 1982, emphasizes teamwork between individuals and healthcare providers to ensure care focused on the individual.'. This approach necessitates that both parties have a comprehensive understanding of the individual's condition, treatment options, and potential outcomes, while also incorporating the individual's values, lifestyle, and preferences into the decision-making process.
'Jennifer Siedman, Director of Community Engagement at Courageous Parents Network (CPN), highlights the importance of addressing decision-making fatigue and understanding the individual's full experience, particularly in cases involving chronic and complex conditions.'. The diagnostic journey for rare illnesses frequently introduces new and diverse obstacles, making it essential for healthcare professionals to assist individuals and their families in navigating possible results and management options.
As Kunneman et al. assert, shared decision-making is a dialogue wherein clinicians and individuals collaboratively consider the individual's situation, integrating personal and clinical contexts to arrive at mutually accepted treatment decisions. This process empowers individuals, transforming them into active participants in their healthcare journey and leading to improved satisfaction and outcomes.
Overcoming Barriers to Informed Consent: Health Literacy, Cultural Sensitivity, and Language
Obstacles to effective informed agreement often arise from health literacy challenges, cultural differences, and language barriers. Tackling these problems requires customized communication approaches, cultural understanding, and resources like interpreters to ensure all patients can participate meaningfully in the agreement process. As stated by Dawn Corbett, NIH’s Inclusion Policy Officer, "To ensure that individuals from diverse backgrounds and those with disabilities are fully involved in the study, it is essential that they comprehend what is happening during the entire process." This emphasizes the significance of ongoing and transparent communication.
Successful knowledgeable agreement starts with providing essential details about the study in a straightforward and succinct way. This includes the purpose of the research, potential risks and benefits, and the study’s length and procedures. The National Organization for Rare Disorders (NORD) emphasizes the importance of providing informed agreement in accessible ways, such as videos, to accommodate participants' unique needs. Language barriers, hearing or vision impairments, and health literacy competencies are some factors that can make it challenging for participants to navigate and fully understand the consent process.
Melissa McGowan, Deputy Director of the Office of Clinical Research at the National Institute on Aging, emphasizes the significance of communication and language access in studies. She states, “When we are looking at our research portfolio…we really want to ensure that the work that we are doing, the work that we are funding, is going to be available for the broad spectrum of people in the United States who need those treatments, those prevention strategies.”
Incorporating these tailored communication strategies not only ensures compliance with regulations but also enhances the inclusivity and effectiveness of clinical research. By addressing these barriers, researchers can improve participant understanding and engagement, ultimately leading to more robust and generalizable research findings.
Conclusion
Informed consent is a cornerstone of ethical healthcare and research, essential for respecting patient autonomy. However, the complexity of consent documents has increased, often making them lengthy and difficult to navigate, particularly for underserved populations.
The five essential elements of informed consent—disclosure of information, patient competency, voluntary decision-making, reasonable alternatives with risks, and assessment of understanding—are vital for empowering patients. Yet, the intricacies of these documents can obstruct effective communication.
Recent guidance emphasizes the importance of presenting key information clearly at the outset of consent documents. Utilizing multimedia elements and simplified language can enhance accessibility, while special considerations for vulnerable populations can improve engagement.
Addressing barriers related to health literacy, cultural differences, and language is crucial for ensuring meaningful participation in the consent process. By adopting tailored communication strategies and fostering trust, the informed consent process can become more effective and ethical. Ultimately, a commitment to transparency and patient-centered care will lead to improved health outcomes and a more equitable healthcare system.




