Introduction
A 510(k) submission is a critical step in gaining FDA market entry for medical devices, requiring a detailed comparison to a legally marketed device known as the predicate. This article provides a comprehensive overview of the purpose, requirements, and key components of a 510(k) submission, emphasizing the need for meticulous research and adherence to FDA guidelines. It explores the process of identifying and selecting a suitable predicate device and delves into the preparation and submission process, highlighting the importance of accuracy and compliance.
The article also addresses the substantial equivalence discussion, ensuring patient safety and device quality, and offers insights into the FDA review process and timeline. Common challenges and tips for success are provided, along with a discussion of post-submission requirements and maintenance. By following these guidelines and understanding the intricacies of the 510(k) approval process, manufacturers can navigate the regulatory landscape and ensure the safety and efficacy of their medical devices.
What is a 510(k) Submission?
A 510(k) submission is essential for medical devices seeking market entry, serving as a critical step in demonstrating to the FDA that a new device is as safe and effective as an already legally marketed device, known as the predicate. This comparison to the predicate device underpins the FDA’s determination of substantial equivalence, a necessary benchmark for most Class II and some Class I devices, with certain exemptions. To ensure a successful 510(k) submission, a comprehensive understanding of the device's intended use, technology, and its users—including clinicians and patients—is paramount. This involves meticulous research into the current market landscape, reviewing competitor devices, and consulting an array of sources such as clinical studies and FDA's own databases. A comparative analysis is then developed, often in the form of a detailed table, juxtaposing the new device's features with those of the predicate to elucidate similarities and technological characteristics. Furthermore, scrutinizing the Summaries of Safety and Effectiveness available from the FDA can clarify the nuances that may impact the submission's outcome. It is a rigorous process that demands a strategic approach, where thorough preparation and adherence to the FDA's guidelines are keys to a successful 510(k) submission process.
Purpose and Requirements of a 510(k) Submission
Securing FDA clearance through a 510(k) submission is a pivotal step for medical device manufacturers. This process involves presenting comprehensive data to the FDA to demonstrate that a new device is as safe and effective as a legally marketed predicate device. A thorough comparative analysis is critical, and should encompass an examination of the intended users—clinicians, physicians, dentists, or patients—and a careful review of the device's instructions, warnings, and cautions.
A successful submission rests on the identification of a suitable predicate device with equivalent intended use and comparable technological characteristics. Collaborating with marketing teams to understand the competitive environment enhances this endeavor. Researching literature, existing clinical studies, and competitor marketing materials, such as websites and instructional brochures, is instrumental in developing a comparative table that underscores the similarities and distinctions between the new device and its predicate.
In addition to a cogent comparative analysis, the submission must include a detailed description of the new device, its operational principles, and any clinical test results that affirm its safety and efficacy. The FDA's publicly accessible 510(k) database, which includes Summaries of Safety and Effectiveness (SSEs), is a valuable resource for understanding the expectations for demonstrating substantial equivalence.
The FDA continuously updates its list of AI/ML-enabled devices and other medical equipment, providing transparency and insights into trends within the medical device field. As the regulatory landscape evolves, manufacturers must remain vigilant and informed to navigate the 510(k) submission process successfully.
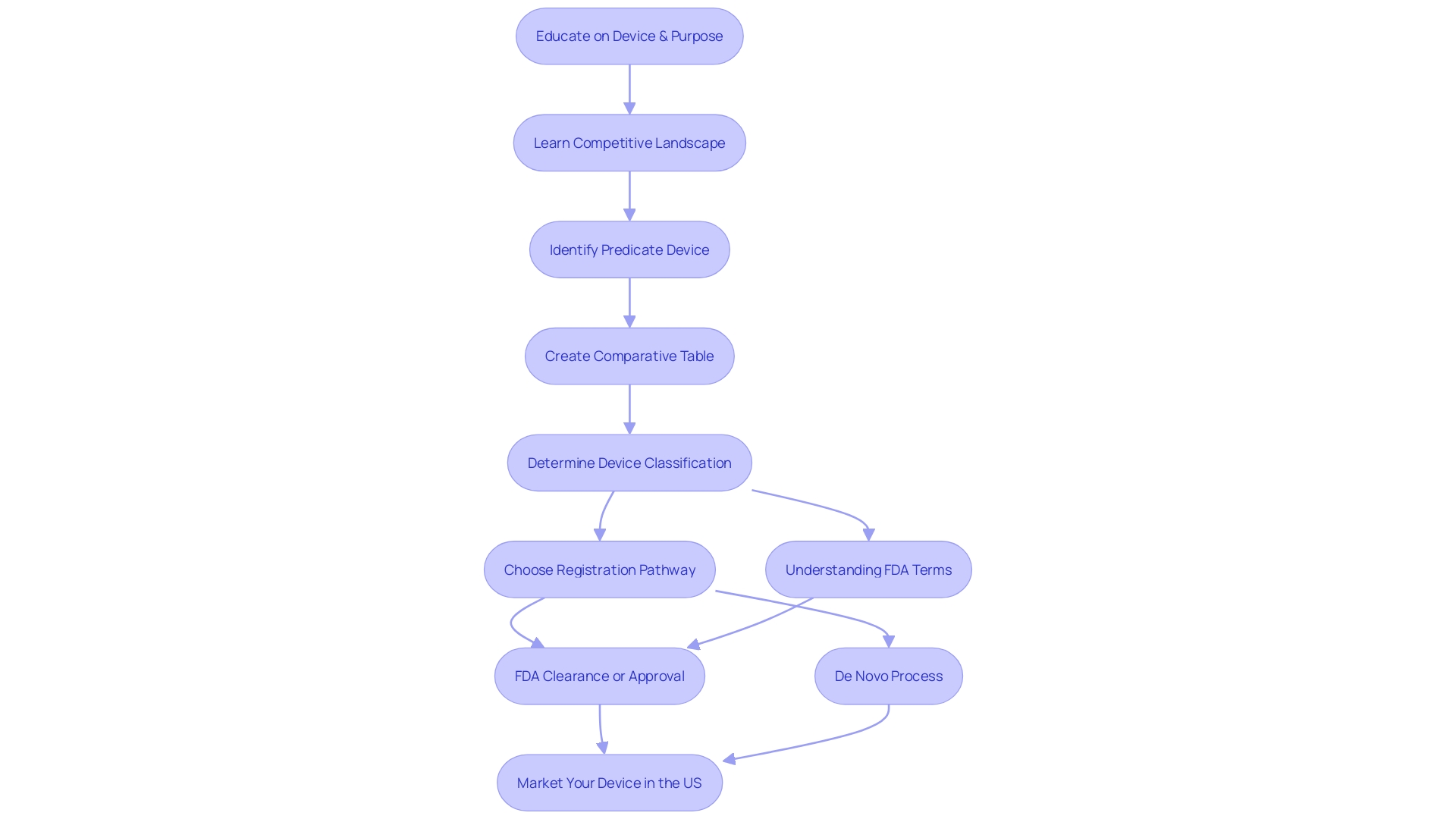
Key Components of a 510(k) Submission
The 510(k) submission process is a crucial pathway for medical devices seeking FDA clearance. It demands meticulous preparation of key components to demonstrate a device's safety and effectiveness. First, a comprehensive Device Description outlines the device's design, materials, and intended use, which sets the foundation for the FDA's assessment. The Indications for Use section must articulate the device's purpose and the patient demographic it serves.
A pivotal aspect of the submission is the Comparison to Predicate Device. This involves a detailed analysis of a similar, legally marketed device, revealing both resemblances and distinctions in design, materials, and functions. Providing clear Performance Data is also essential, offering evidence from bench testing, animal studies, or clinical trials.
Biocompatibility information, addressing the device's compatibility with biological systems, is a requirement. This often includes results from specific biocompatibility tests conducted. For devices with software or electrical components, thorough documentation must confirm their safety and effectiveness.
Labeling proposals are not to be overlooked, as they encompass instructions for use, along with any necessary warnings and precautions. Additionally, any Clinical Data that bolsters the device's claims of safety and effectiveness, such as clinical trials or literature reviews, should be included.
Understanding the competitive landscape is fundamental, as noted by experts who emphasize the importance of identifying potential predicate devices with similar intended uses and technological characteristics. Creating a comparative table can be an efficient strategy to organize this information.
The FDA's role in protecting public health underscores the importance of compliance with their guidelines. The FDA's resources, including guidance documents and the 510(k) database, are invaluable for applicants to understand and navigate the submission process effectively.
Finally, it is essential to recognize the implications of public comments on submissions. Comments are made part of the public record, and confidential information should be carefully managed to avoid unintended disclosure.
The success of a 510(k) submission hinges on a well-informed approach that considers all regulatory requirements and leverages available FDA resources to demonstrate a device's safety and equivalence to existing products.
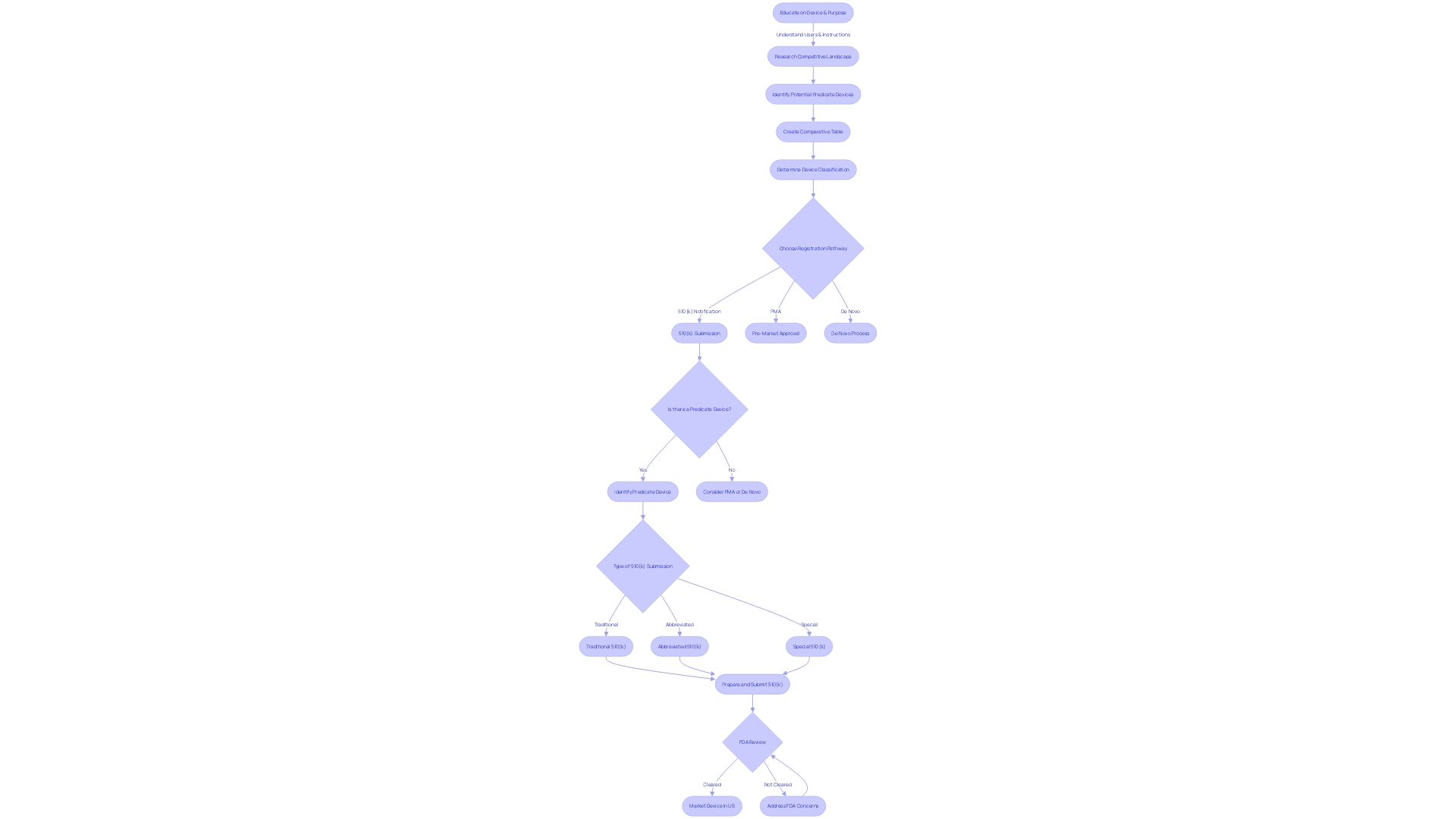
Identifying and Selecting a Predicate Device
A thorough understanding of the medical device in question is paramount for a successful 510(k) submission. It is crucial to grasp who the users are—ranging from clinicians and physicians to dentists and patients—and to familiarize oneself with the device's instructions, including any warnings and precautions. Collaborating with the marketing department can yield insights into the competitive landscape, which aids in pinpointing devices that could serve as suitable predicates due to their identical intended use and comparable technological characteristics.
Creating a comparative table becomes an invaluable step in this process. It involves examining various sources such as research literature, clinical studies, and the marketing materials of competitors. This table should compare the new device with potential predicates, focusing on technological features and intended use.
The FDA's Medical Device Database is a pivotal resource when searching for predicates. It provides access to Summaries of Safety and Effectiveness (SSEs), which are instrumental in assessing the similarities and differences between devices. It is important to ensure that the selected predicate is not only legally marketed but also currently registered with the FDA, thus confirming its status as a valid reference for comparison.
The documentary 'The Bleeding Edge' highlighted the significance of the 510(k) clearance process, illustrating that not all devices require clinical trials, underscoring the importance of carefully choosing a predicate that will not compromise patient safety.
The FDA continuously strives to refine the 510(k) process, as evidenced by the recent guidance document released on September 7, 2023. This document suggests best practices for selecting predicates, which include assessing long-term safety data—especially pertinent when considering older predicates.
The FDA expects 510(k) submissions to adhere to their guidelines and thoroughly understands the submission process. The challenge lies in compiling the necessary information within a specified time frame and ensuring it satisfies the FDA's criteria for a determination of substantial equivalence.

Preparing the 510(k) Submission
The process of preparing a 510(k) submission encompasses meticulous planning and a comprehensive understanding of the FDA's requirements. It begins with the collection of all pertinent information, such as device specifications, performance data, clinical studies, and labeling. This ensures that the submission will reflect an exhaustive knowledge of every aspect of the new device, a practice that is not only recommended but essential as per the FDA guidelines.
Following the information gathering, a crucial step is to conduct a detailed search for a predicate device within the FDA's Medical Device Database. Identifying a predicate device with the same intended use and comparable technological features is pivotal. This is where a comparative analysis becomes invaluable, and creating a comprehensive table to juxtapose the subject device against the predicate device can provide clarity on the similarities and differences.
Organizing the information in a clear, concise manner is the next step, with a focus on addressing all the required components as outlined by the FDA. This includes a thorough review of the submission to ensure both accuracy and completeness, especially for the supporting documentation.
Adherence to the FDA's guidance and regulations for formatting, content, and submission requirements cannot be overstated. This not only facilitates a smoother review process but also reflects the manufacturer's commitment to compliance and quality.
Moreover, it's important to consider the public nature of certain submission components and the FDA's instructions on submitting comments. Any confidential information must be carefully excluded from the public domain to protect both the manufacturer and the patients.
In sum, a well-documented 510(k) submission is the result of a systematic approach that incorporates a deep understanding of the device, its competitive landscape, and the FDA's regulatory framework. By following these structured steps, manufacturers enhance the likelihood of a favorable FDA review.
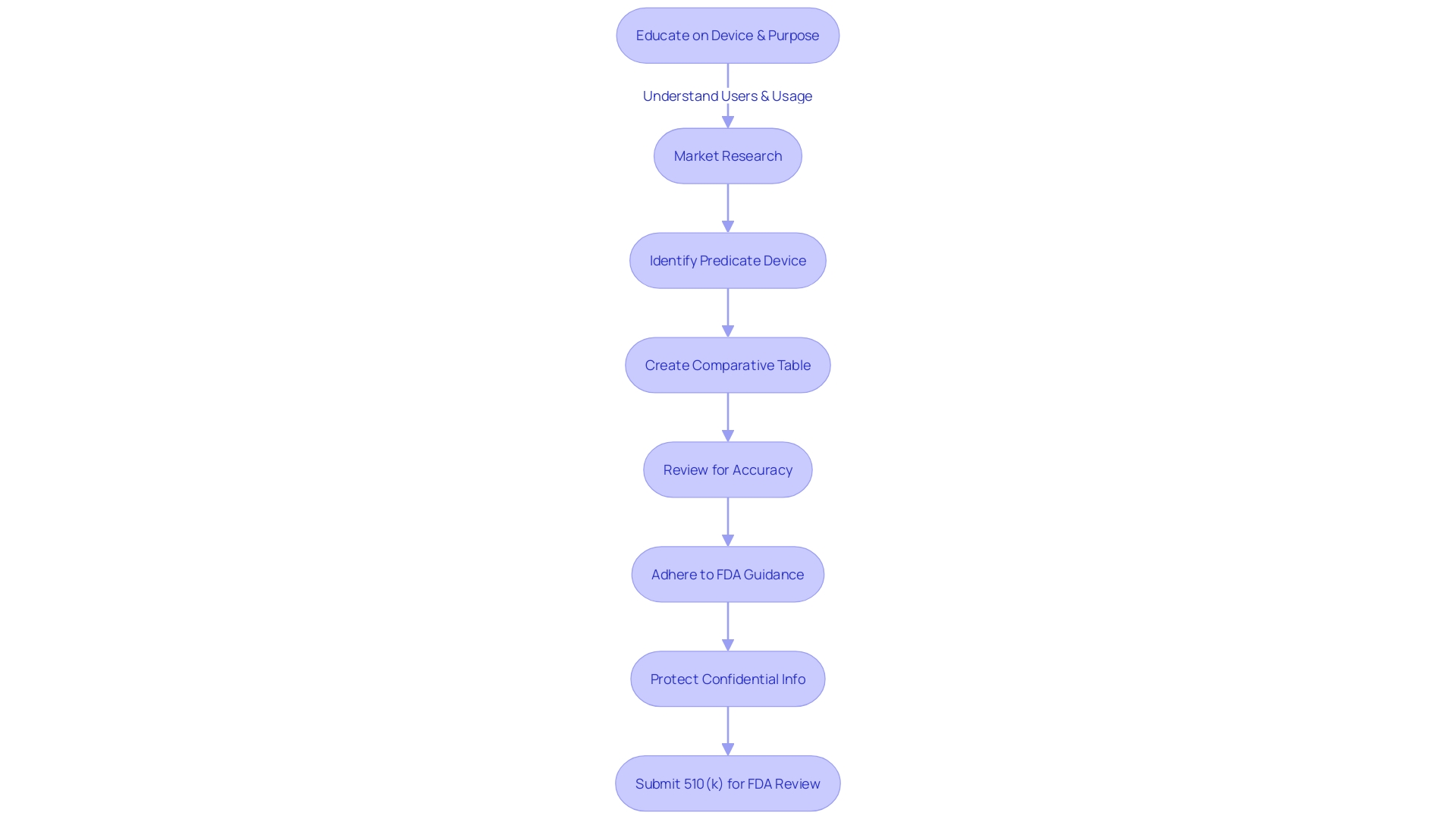
Cover Sheets and User Fee Forms
In preparing a 510(k) submission for FDA review, meticulous attention to detail is paramount, especially concerning the cover sheets and accompanying user fee forms. These foundational documents serve as a gateway for the FDA to assess the particulars of the medical device in question. Cover sheets must be diligently filled out to include the device's trade name, applicant's details, and a succinct yet thorough summary of what the submission entails.
The user fee forms represent a key financial aspect of the submission process. It's crucial to remit the appropriate fees, ensuring the submission can be processed without unnecessary delays. This fee structure is part of the FDA's commitment to safeguarding public health by evaluating the safety and effectiveness of medical devices rigorously.
Moreover, before even reaching the stage of submission, a comprehensive understanding of the device, its intended users, and the competitive landscape is a must. This involves delving into research literature, clinical studies, and direct comparisons with predicate devices. Creating a comparative table based on this research, which includes examining available Summaries of Safety and Effectiveness, can illuminate the similarities and differences between the new device and its predicate counterparts.
It's essential to remember that any comments or information submitted electronically to the FDA will become public record, so confidentiality should be preserved where necessary. This can include the exclusion of sensitive personal or business information from public documents.
The FDA regulates a broad spectrum of products to ensure public safety, and the 510(k) process is a critical part of this mission. The data on 510(k) submissions reveal the FDA's ongoing efforts to evaluate devices for market entry, ensuring they meet the high standards set out to protect and promote public health.
In summary, accuracy in the initial paperwork and a deep understanding of the device's context within the medical field are the first steps towards a successful 510(k) submission. These efforts facilitate the FDA's efficient review and contribute to the overarching goal of delivering safe and effective medical devices to the market.

Technical and Performance Information
The 510(k) submission process is a critical pathway to FDA clearance for new medical devices. A pivotal component of this process is the technical and performance information section, which must articulate the device's design, materials, and performance with precision and clarity. Essential elements to include are the device's intended use, detailed specifications, comprehensive engineering drawings, and the results of any performance testing.
Understanding the device's purpose and the users it serves—be they clinicians, physicians, dentists, or patients—is crucial. The inclusion of a thorough instruction manual, complete with warnings and cautions, is not only a regulatory requirement but also a best practice for user safety and efficacy. Collaboration with the Marketing department can provide valuable insights into the competitive landscape, helping to identify predicate devices that have analogous intended uses and technological characteristics. This comparative analysis is bolstered by a detailed review of available research literature, clinical studies, and marketing materials from both your device and its competitors.
Furthermore, constructing a comparative table is a strategic step that can facilitate the FDA's evaluation of your device against existing products. Reading Summaries of Safety and Effectiveness Data (SeEDs) from the FDA's 510(k) database can provide a benchmark for evaluating the similarities and differences between your device and its predicates, thereby supporting your claim of substantial equivalence.
The FDA's mission is to ensure the safety, effectiveness, and security of medical devices, among other public health responsibilities. Adhering to the FDA's guidance documents and understanding the submission process is paramount to a successful 510(k) submission. The challenge lies in compiling the requisite information within the allotted timeframe, ensuring that the submission meets the FDA's criteria for a decision of substantial equivalence.

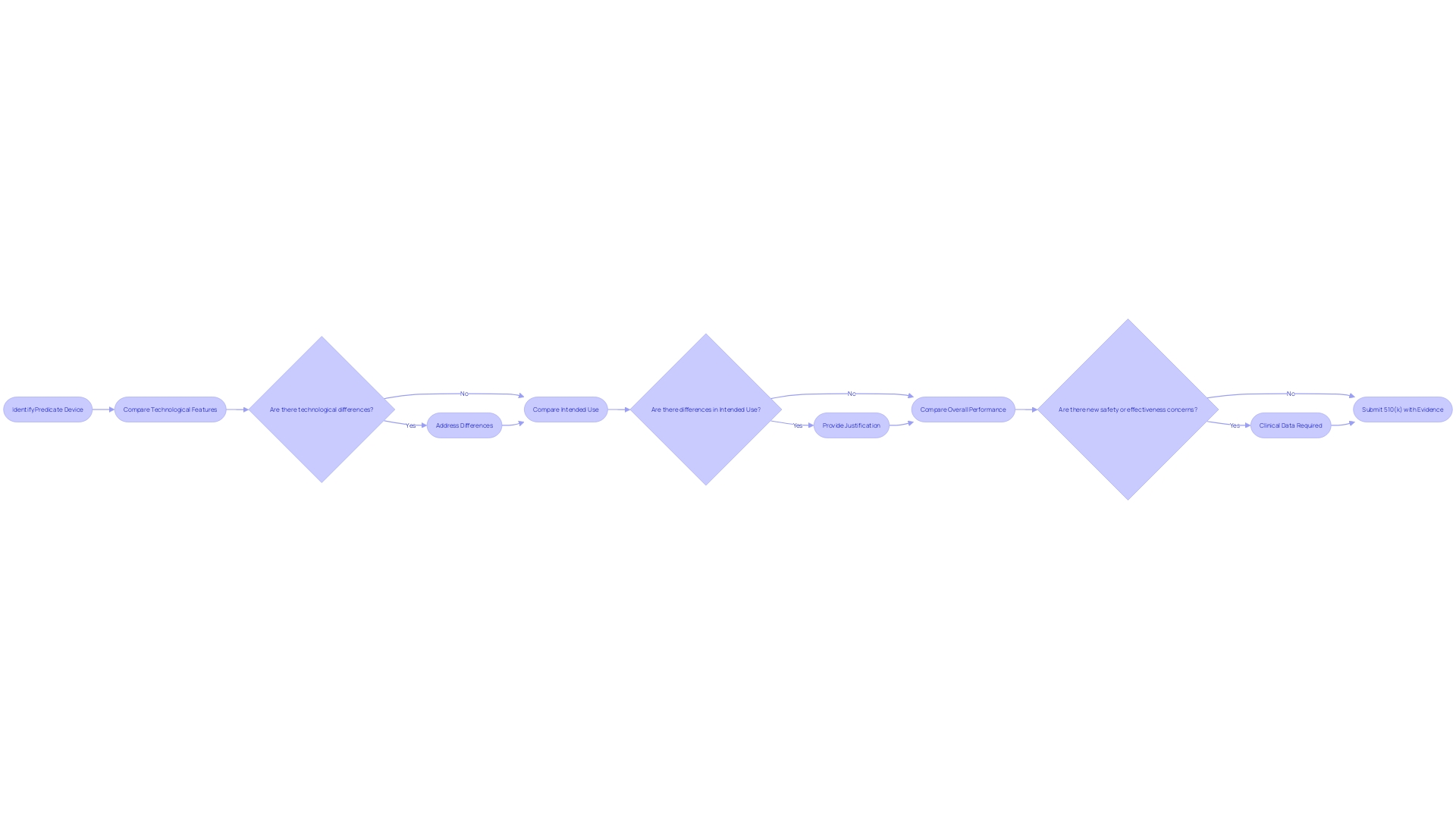
Substantial Equivalence Discussion
A crucial element of the 510(k) submission is the substantial equivalence analysis, where manufacturers are tasked with demonstrating that their new medical device is at least as safe and effective as an existing, legally marketed predicate device. This process necessitates a comprehensive comparison that assesses the new device against the predicate in terms of technological features, intended use, and overall performance.
Manufacturers must meticulously address any variances between the two devices, providing a clear rationale for why these differences do not introduce new safety or effectiveness concerns. This may include a range of evidence, such as clinical data, in circumstances outlined by the FDA's draft guidance. Particularly, clinical data may be required when there are discrepancies in the indications for use, technological differences that cannot be evaluated through non-clinical testing, or when a known risk associated with the predicate device is exacerbated in the new device.
The FDA has illustrated scenarios when clinical data may be necessary through their 510(k) Program Guidance. This includes instances where the new device presents different technological characteristics or where the intended use diverges from that of the predicate device. A thorough understanding of both the device under review and the predicate is essential, often involving review of research literature, clinical studies, and Summaries of Safety and Effectiveness from the FDA's database.
In the spirit of ensuring public health and safety, the FDA's mission extends to comprehensive reviews of medical devices, ensuring they meet stringent standards for safety and efficacy. Manufacturers must navigate this regulatory landscape with a clear understanding of FDA expectations and the submission process, ultimately supporting their claim of substantial equivalence with robust and reliable evidence.
Ensuring Patient Safety and Device Quality
Navigating the 510(k) FDA approval process is critical for medical device manufacturers aiming to ensure that their products meet stringent safety and efficacy standards. The journey involves a rigorous evaluation of the device, requiring manufacturers to provide substantial evidence that their device is equivalent to a previously cleared device, known as a predicate device. Such evidence includes comprehensive testing protocols.
Performance testing is a cornerstone of the 510(k) submission, with manufacturers required to comply with precise guidelines and standards. For instance, the case of the Impella Connect System exemplifies the scrutiny of software and hardware components in medical devices. The system, which provides critical cardiac support, showcases how software functions such as remote monitoring and alarm notifications are evaluated under the premarket authorization framework, emphasizing the importance of these features in patient care.
Moreover, instances like the HeartMate 3 Left Ventricular Assist System Implant Kit's recall for correction demonstrate the continuous obligation to uphold quality post-approval. The HeartMate 3, which serves as a vital support system for heart failure patients, illustrates the ongoing commitment to patient safety throughout a device's lifecycle.
The FDA's role extends beyond the initial clearance, ensuring the safety, effectiveness, and security of medical devices through continuous oversight. Regulatory pathways are classified based on the device's intended use and inherent risk, with Class I, II, and III designations dictating the level of scrutiny and type of submission required. Class III devices, which include life-supporting implants, undergo the most rigorous examination due to their critical nature.
To successfully navigate the complexities of the 510(k) approval, manufacturers must be well-versed in the distinctions between the terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted.' Each term reflects a different stage and classification in the regulatory process, with 'Cleared' referring to 510(k) submissions, 'Approved' to PMA, and 'Granted' to the De Novo pathway. Understanding these terms is paramount for compliance and market entry.
Manufacturers should also be aware of the potential need for clinical data to support their submission, especially when there are significant differences between the new and predicate devices or when non-clinical testing alone cannot demonstrate substantial equivalence. The FDA's draft guidance and recent efforts to modernize the 510(k) process highlight the agency's commitment to evolving standards that prioritize patient experiences and device safety.
In summary, a successful 510(k) submission hinges on demonstrating that a medical device is safe and effective, adhering to good manufacturing practices, and understanding the regulatory landscape. By prioritizing these aspects, manufacturers can enhance the likelihood of achieving FDA clearance and ensure their devices contribute positively to patient care.
Submission Process and Electronic Templates
Navigating the 510(k) submission process is critical for manufacturers aiming to market their medical devices in the United States. Proficiently utilizing the FDA's electronic templates and directives is essential for a successful submission. These tools are designed to ensure that manufacturers include all necessary information and adhere to the proper formatting requirements. A comprehensive understanding of the device, its users, and the competitive landscape is crucial. This involves researching clinical studies, regulatory literature, and existing market products to identify suitable predicate devices that share the same intended use and technological characteristics. Creating a comparative table based on this information can be instrumental in highlighting the similarities and differences between the new device and its predicate.
Moreover, the selection of a predicate device is a nuanced process that requires careful consideration of the FDA's classification levels and the appropriate regulatory pathway—whether it be 510(k), Pre-Market Approval (PMA), or De Novo process. This step is pivotal because each classification carries varying degrees of patient risk, and choosing the correct path is key to achieving FDA clearance, approval, or grant status for the device. This is affirmed by recent FDA initiatives aimed at refining the review process and establishing best practices for predicate selection, as indicated in draft guidance documents.
Manufacturers must also be diligent when submitting comments and information to the FDA, taking care to prevent the disclosure of confidential or sensitive information. The responsibility lies with the manufacturers to ensure that any public submissions do not inadvertently contain private data. For confidential materials, the FDA provides specific instructions for written/paper submissions to protect such information.
Understanding the nuances of the FDA's regulatory framework and submission process is essential for medical device manufacturers. By following these guidelines and harnessing the FDA's resources, manufacturers can enhance their chances of a timely and successful 510(k) submission.
FDA Review Process and Timeline
Upon submission of a 510(k) to the FDA, an intricate review process commences, designed to verify the device's safety and efficacy. This evaluation follows a multi-stage approach, starting with an administrative assessment to ensure complete and proper documentation. Following this, a substantive review is conducted, which delves into the specifics of the device's technology and performance. The final stage of decision-making is pivotal, as it determines whether the device will be brought to market.
It's essential to recognize that the review duration can fluctuate, influenced by the device's complexity and the thoroughness of the submission. The FDA may issue requests for additional data or clarifications, which necessitates manufacturers to be responsive and prepared. According to FDA guidance, while the agency's recommendations are not legally binding, they reflect the current thinking and best practices for submission preparation.
For a comprehensive understanding of the device and competitive landscape, manufacturers should meticulously research the intended use, users, and existing market offerings, including an analysis of potential predicate devices. This includes a thorough examination of research literature, clinical studies, and marketing materials. As per recent FDA guidelines, such as those outlined for direct-to-consumer prescription drug advertisements, clarity and consumer comprehension are prioritized.
In terms of regulatory trends, there is a movement towards more streamlined pathways and enhanced cooperation between agencies and industry stakeholders, a dynamic accelerated by the COVID-19 pandemic. These initiatives aim to shorten approval times, especially for devices that meet urgent medical needs, as seen in the realms of digital health and personalized medicine. It remains crucial for manufacturers to stay informed of these evolving regulatory landscapes to optimize their submission strategies and timelines.
Common Challenges and Tips for Success
Navigating the FDA's 510(k) approval process is a critical step for medical device manufacturers aiming to bring new products to the market. To address the complexities of regulatory requirements, manufacturers must diligently compare their new device to an existing, legally marketed predicate device, while providing comprehensive evidence that demonstrates safety and effectiveness. To facilitate a smooth and successful 510(k) submission, the following strategies are recommended:
-
Early Engagement: Initiating the preparation for 510(k) submissions early is crucial. This proactive approach allows manufacturers ample time to meticulously prepare documentation and respond to any requests for additional information from the FDA.
-
Consultation with Experts: Leveraging the expertise of regulatory professionals who are well-versed in the intricacies of the 510(k) process is invaluable. These specialists can provide guidance on compliance and enhance the quality of the submission.
-
Rigorous Testing: Conducting extensive testing is integral to substantiating the safety and effectiveness of the device. This includes biocompatibility and performance testing, which are pivotal in demonstrating that the device meets the required standards.
-
Regulatory Vigilance: Staying informed about the latest FDA regulations, guidance documents, and industry best practices is essential for compliance. Manufacturers need to be agile and ready to integrate new regulatory developments into their submission process.
By following these approaches, which are echoed by industry professionals and supported by FDA guidelines, manufacturers can bolster the probability of a successful 510(k) approval. This is evidenced by companies like PharmaSens, which achieved significant advancements in insulin pump technology and received ISO 13485 certification, underscoring the importance of thorough preparation and a user-centric design philosophy in medical device innovation. The FDA's dedication to patient welfare and data-driven decisions serves as a reminder that patient safety should remain the paramount objective throughout the submission process.
Post-Submission Requirements and Maintenance
The approval of a medical device by the FDA is not the final stage in its journey; rather, it marks the beginning of a critical ongoing process known as Post-Market Surveillance (PMS). This process is essential for monitoring the safety and effectiveness of medical devices after they have been introduced to the market. PMS extends beyond pre-market assessments and involves continuous evaluation and reporting to ensure devices perform as intended under real-world conditions.
A robust PMS system is imperative because it aids in promptly identifying and rectifying potential safety issues, thus enhancing device performance over time. Data collection methods used in PMS include passive systems like spontaneous reports from healthcare professionals and patients, and active surveillance such as registries and studies. Additionally, electronic health records and administrative databases are instrumental in facilitating the ongoing assessment of medical devices in practical settings.
The FDA has acknowledged the challenges in funding and patient identification for effective PMS, but is actively working on overcoming these obstacles. With over 1.7 million injuries and 83,000 deaths potentially linked to medical devices over a decade, the importance of stringent PMS practices becomes clear. Manufacturers must comply with FDA-imposed post-approval requirements, including adverse event monitoring and fulfilling any specific conditions set forth by the agency.
To ensure the safety and efficacy of medical devices, manufacturers are obliged to develop and implement procedures for monitoring device performance and to respond adequately to any safety concerns that may emerge. In doing so, they maintain the trust of patients and healthcare providers, ensuring that medical devices continue to serve their vital role in patient care.
Conclusion
A successful 510(k) submission is crucial for medical device manufacturers aiming to gain FDA market entry. This process requires meticulous research, adherence to FDA guidelines, and a comprehensive understanding of the device's intended use, technology, and users. The identification and selection of a suitable predicate device play a pivotal role in demonstrating substantial equivalence.
Key components of a 510(k) submission include a thorough device description, indications for use, comparison to the predicate device, performance data, biocompatibility information, labeling proposals, and clinical data. Manufacturers must also pay attention to cover sheets and user fee forms, ensuring accuracy and compliance. The substantial equivalence discussion is a critical element, requiring a comprehensive comparison and addressing any differences between the new device and the predicate.
Ensuring patient safety and device quality is of utmost importance, with performance testing and ongoing post-approval monitoring playing key roles. The FDA review process involves administrative assessment, substantive review, and decision-making, with the timeline varying based on the device's complexity. Navigating the submission process requires early engagement, consultation with experts, rigorous testing, and regulatory vigilance.
Post-submission, manufacturers must comply with post-market surveillance requirements to monitor device performance and address safety concerns. By following these guidelines and harnessing the FDA's resources, manufacturers can navigate the 510(k) approval process successfully, ensuring the safety and efficacy of their medical devices.




