Introduction
The 510(k) process stands as a vital pathway for medical device manufacturers seeking to gain FDA approval, offering a structured approach to demonstrate the safety and effectiveness of new devices. By establishing substantial equivalence to existing, legally marketed devices, known as predicate devices, this process not only streamlines regulatory compliance but also accelerates the introduction of innovative technologies into the healthcare market.
Experts in regulatory affairs, such as Ana Criado and Katherine Ruiz, emphasize the significance of understanding these intricate frameworks to navigate the complexities of device regulation effectively. As the landscape of medical device innovation continues to evolve, grasping the nuances of the 510(k) process is essential for stakeholders aiming to enhance patient outcomes while adhering to stringent safety standards.
Introduction to the 510(k) Process: A Key to Medical Device Approval
The 510k flowchart outlines the 510(k) procedure, which functions as a premarket submission to the FDA, intended to show that a medical instrument is safe and effective by establishing its substantial equivalence to an existing legally marketed instrument, referred to as a predicate instrument. This pathway is particularly crucial for manufacturers seeking to efficiently navigate the complexities of the regulatory landscape while ensuring adherence to established safety standards. Experts like Ana Criado, our Director of Regulatory Affairs, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, highlight the importance of understanding these regulatory frameworks.
Ana’s extensive background—spanning executive roles at Colombia’s INVIMA, academia, and consulting for major global firms—provides essential insights into the aspects of the 510(k) submission. Katherine Ruiz’s expertise further complements this understanding, particularly in navigating the regulatory requirements specific to the Colombian context. The case study titled 'When to Submit a 510(k) Premarket Notification' emphasizes that the 510k flowchart outlines an efficient procedure for medium-risk medical products, which is essential for manufacturers seeking to enter the U.S. market.
By comprehending the 510k flowchart, stakeholders—including manufacturers and Regulatory Affairs professionals—can facilitate the rapid introduction of new medical technologies, ultimately enhancing patient outcomes. The FDA recommends that a 510(k) should be submitted at least 90 days before marketing the product, reflecting the agency's commitment to thorough evaluation while supporting timely market access. Furthermore, A.S. observed the intricacies of the 510(k) pathway, especially concerning software as a medical application (Sand), emphasizing the importance of this route.
Furthermore, as the landscape of medical innovation evolves, the FDA’s consideration of the nature of algorithms in medical technologies is crucial for assessing safety and effectiveness. The 510k flowchart remains a pivotal component of the 510(k) process in medical product development, providing a streamlined route for bringing beneficial technologies to the healthcare market.
Navigating the 510(k) Flowchart: Step-by-Step Submission Guide
-
Determine the Classification: Begin by identifying the classification of your medical instrument. It is crucial to understand whether your equipment falls under Class I, II, or III, as this determines the appropriate submission pathway and regulatory requirements.
Identify Predicate Instruments: Locate a legally marketed product that is similar to yours and can serve as a predicate. This step is vital for demonstrating substantial equivalence, which is a core requirement of the 510k flowchart in the 510(k) process.
Prepare Documentation: Compile all necessary documentation, including detailed device descriptions, labeling, and performance testing results. Ensure that all information is presented clearly and comprehensively to facilitate a smooth evaluation. Given the expertise of leaders in Regulatory Affairs like Ana Criado, who has extensive experience in this area, including her significant contributions at INVIMA and her consulting roles with global companies such as General Electric and Omron Healthcare, it is beneficial to consult with professionals during this phase.
-
Submit the 510(k): Submit your 510(k) application to the FDA, making sure that all forms are filled out completely and accurately. Attention to detail in this submission can significantly impact the review timeline.
-
Respond to FDA Queries: Be prepared to address any questions or requests for additional information from the FDA during their review. Maintaining clear and respectful communication is essential for fostering positive relationships and avoiding misunderstandings. It is vital to maintain professionalism and thoughtfulness in all interactions with FDA reviewers, as this can aid in facilitating smoother communications. It is important to note that there is a 180-calendar day period for responding to such requests, emphasizing the need for timely engagement.
-
Obtain Clearance: Upon successful review, you will receive a clearance letter, allowing you to market your device. This stage marks a significant milestone in the regulatory pathway, enabling you to bring your product to the market effectively.
In the context of the 510(k) procedure, manufacturers may find that they can refer to the 510k flowchart to submit changes under a Special 510(k), provided they can justify that no additional performance data are necessary. As stated by the FDA,
In cases where manufacturers determine under their design control procedures that no additional verification or validation testing is necessary to evaluate a change that otherwise requires submission and clearance of a 510(k), manufacturers may submit these changes as a Special 510(k) with a clear rationale supporting their conclusion that no performance data are necessary.
A successful case illustrating this procedure involved a biomedical sensor company that effectively navigated their FDA premarket notification by utilizing a 510k flowchart.
By redirecting their regulatory strategy to the optimal 510(k) program and using the 510k flowchart to provide comprehensive documentation while engaging proactively with regulators, they enhanced their chances for market clearance. The insights from specialists such as Ana Criado, whose academic positions as a professor in biomedical engineering and her deep knowledge of health economics and regulatory frameworks significantly improve the submission, demonstrate how expert guidance and strategic communication within the 510k flowchart result in successful outcomes in the 510(k) submission.
Recent FDA Guidance on 510(k) Submissions: What You Need to Know
Recent guidance from the FDA highlights the increasing significance of incorporating real-world evidence and post-market data into the 510k flowchart for the approval procedure. This move aims to enhance the credibility of submissions and ensure that manufacturers remain compliant with evolving regulatory standards. Notably, the FDA has called for greater transparency in the submission process, pushing manufacturers to provide detailed summaries that clearly articulate the safety and effectiveness of their products.
Additionally, the introduction of new guidelines focused on cybersecurity measures reflects the FDA's proactive approach to addressing emerging risks associated with medical technology. As Brian J. Miller notes, the article examines potential policy enhancements to the third-party review program intended to increase the efficiency of FDA medical product regulation. It's important to note that the FDA faces challenges in hiring qualified technical staff due to high living costs and limited local job opportunities, which can impact the efficiency of the regulatory process.
Furthermore, the 21 Century Cures Act has significantly influenced the FDA's regulatory authority, particularly concerning software and AI technologies, by defining exempt functions from pre-market review. Staying informed about these updates is essential for manufacturers seeking to effectively navigate the complexities of the 510k flowchart. With equipment regulation activities constituting one-tenth of the FDA’s annual budget, understanding these recent developments is vital for enhancing approval rates and ensuring product safety.
In this context, leveraging comprehensive clinical trial management services such as those provided by bioaccess®, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
can significantly improve the success of clinical trials in Latin America. With over 20 years of expertise in the Medtech field, bioaccess® specializes in various studies, including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
This extensive experience equips clients with the knowledge and resources necessary to navigate regulatory challenges effectively.
Common Challenges in 510(k) Submissions and How to Overcome Them
-
Submitting incomplete or poorly organized documentation remains one of the most prevalent challenges illustrated in the 510k flowchart of the submission process. To mitigate this risk, it is essential to develop a comprehensive checklist of required documents. Ensure that all materials are meticulously compiled and thoroughly reviewed prior to submission. Ignoring this step can lead to substantial delays, as statistics show that addressing inconsistencies can significantly prolong the review timeline. Ana Criado's extensive experience in Regulatory Affairs, particularly her work with global companies, underscores the importance of thorough documentation in successful submissions.
-
Identifying Predicate Tools: Choosing suitable predicate tools can be a source of confusion that hinders timely submissions. Conduct detailed research on available predicates, ensuring that their equivalence to the new equipment is clearly documented. This diligence not only streamlines the submission process but also strengthens the case for substantial equivalence, minimizing the likelihood of FDA pushback. Ana's background as a consultant for major global firms equips her with insights on effectively identifying and justifying predicate instruments, drawing from her academic and professional expertise.
Regulatory Changes: The regulatory landscape surrounding medical devices is dynamic and frequently evolving. To stay ahead of potential changes, it is advisable to subscribe to FDA updates and actively participate in industry webinars. Engaging with these resources will help ensure that your submissions reflect the most current guidance and requirements. Industry experts like Ana Criado, Director of Regulatory Affairs and a consultant for major global companies, often emphasize the importance of staying informed about these changes, given her extensive background in Regulatory Affairs, including her role in cannabis regulation, which illustrates her adaptability to evolving compliance requirements.
-
FDA Queries: Navigating FDA queries can be intimidating, yet preparation is key. Anticipate the types of questions that the FDA may raise and prepare comprehensive responses in advance. This proactive approach helps sustain momentum in the submission procedure and demonstrates a commitment to compliance. As Jon Speer aptly notes,
FDA reviewers see probably dozens, maybe hundreds of submissions in a finite period of time. Some are better than others. Well, make yours stand out. Make yours easier to read.
Furthermore, the consequences of ignoring FDA guidance can be significant. A case study emphasizes that failure to follow these guidelines may lead to insufficient data or documentation, causing significant equivalence issues and unexpected compliance challenges during the review. Addressing these common challenges head-on is vital for creating a successful 510k flowchart submission. Additionally, it's important to note that Section 9 of the FDA 510(k) submission requires declarations of conformity with specific standards and summary reports demonstrating compliance, which is critical for ensuring thorough documentation.
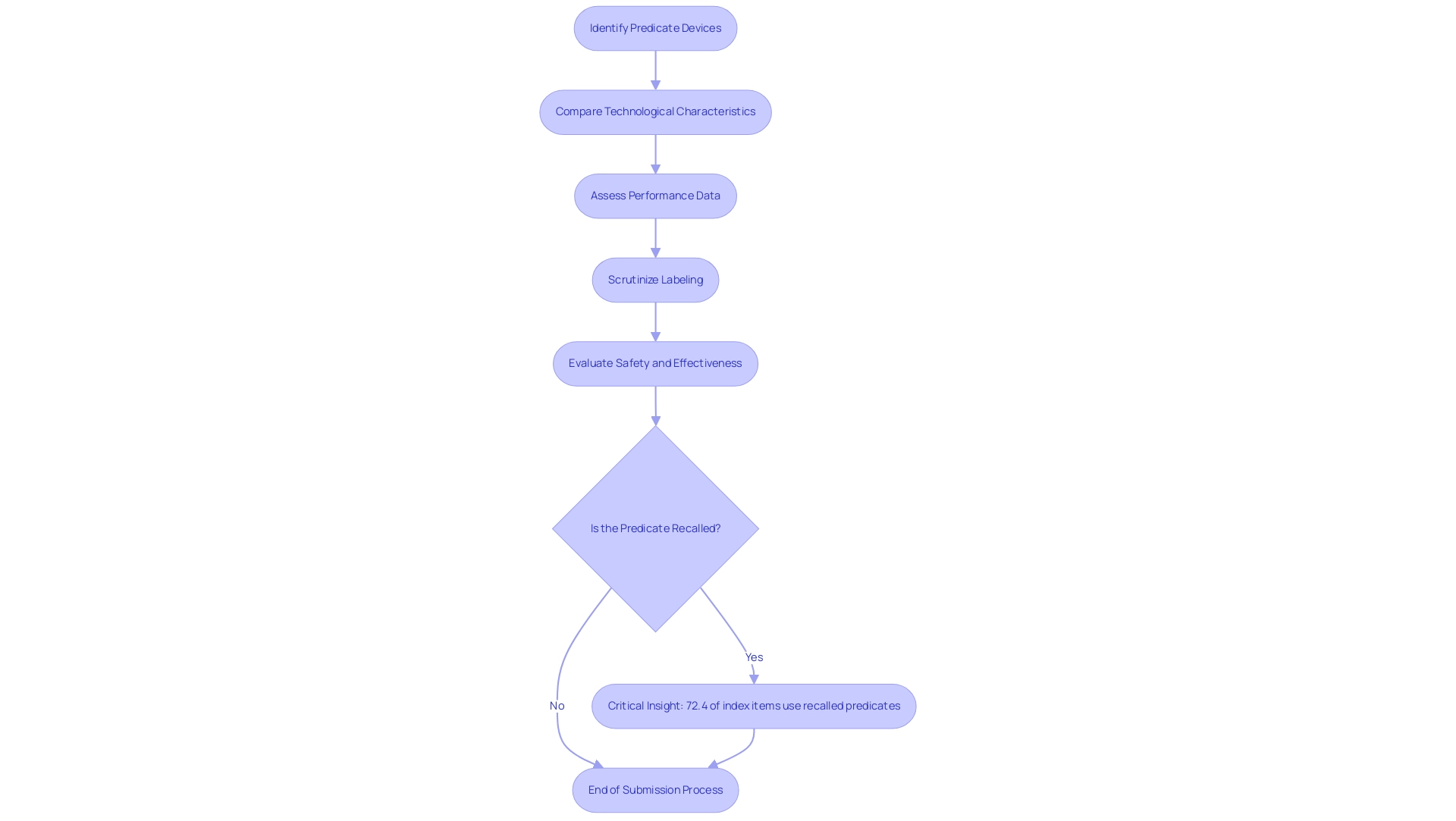
Understanding Predicate Devices: A Cornerstone of the 510(k) Process
Predicate instruments play an essential part in the 510k flowchart of the submission procedure as they are legally marketed standards that aid in demonstrating substantial equivalence. To effectively leverage a predicate system, manufacturers must carefully identify those with similar technological characteristics and intended uses. This involves a comprehensive comparison that not only assesses performance data but also scrutinizes labeling and any differences that may impact safety and effectiveness.
Notably, data indicates that 72.4% of index items utilize a recalled predicate, underscoring the importance of diligent selection in the approval process. Moreover, with a risk ratio for Class I recalls at 6.40, the potential implications of using recalled predicates highlight the critical need for careful selection. The median time between predicate authorization and index equipment clearance averages 4.0 years, emphasizing the need for strategic planning in development.
Grasping this relationship is essential to guaranteeing that new products can be efficiently introduced to the market while complying with stringent FDA regulations. As Dr. Harlan M. Krumholz noted, the complexities of the regulatory landscape necessitate a thorough approach to predicate selection. Moreover, recent research uncovers a lack of clarity in medical equipment regulation—demonstrated by the absence of public information regarding predicates for 17.3% of index items—demanding increased scrutiny in the selection method.
Insights from the case study titled 'Conclusion on FDA Guidance for Medical Devices' further illustrate the regulatory requirements for selecting appropriate predicate devices, underscoring that thorough identification and justification of these devices are essential for successful FDA approvals. Given the expertise of Ana Criado, Director of Regulatory Affairs and a leader in the field, along with Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, their insights could provide valuable guidance in navigating the complexities of the 510k flowchart process.
Conclusion
The 510(k) process is an essential mechanism that enables medical device manufacturers to obtain FDA approval by demonstrating substantial equivalence to existing devices. This pathway not only simplifies regulatory compliance but also promotes the timely introduction of innovative medical technologies into the healthcare market. Understanding the intricacies of this process is vital for stakeholders, including manufacturers and regulatory professionals, as it plays a crucial role in enhancing patient outcomes while maintaining high safety standards.
Navigating the 510(k) submission requires meticulous attention to detail, including the identification of predicate devices, comprehensive documentation, and proactive communication with the FDA. Common challenges, such as incomplete submissions and regulatory changes, can hinder the approval process. However, with thorough preparation and expert guidance, these obstacles can be effectively managed, ensuring a smoother pathway to market clearance.
Recent updates from the FDA highlight the increasing emphasis on real-world evidence and cybersecurity measures, reflecting the evolving landscape of medical device regulation. Staying informed about these developments is imperative for manufacturers aiming to comply with current standards and enhance their approval rates. As the medical device industry continues to innovate, leveraging the 510(k) process strategically will remain a key factor in bringing safe and effective technologies to patients in need.
Frequently Asked Questions
What is the purpose of the 510(k) procedure?
The 510(k) procedure is a premarket submission to the FDA intended to demonstrate that a medical instrument is safe and effective by establishing its substantial equivalence to an existing legally marketed instrument, known as a predicate instrument.
Why is the 510(k) pathway important for manufacturers?
The 510(k) pathway is crucial for manufacturers as it helps them efficiently navigate the complexities of the regulatory landscape while ensuring adherence to established safety standards, facilitating the rapid introduction of new medical technologies.
Who are some experts in Regulatory Affairs related to the 510(k) process?
Experts such as Ana Criado, the Director of Regulatory Affairs, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provide valuable insights into the 510(k) submission process.
What is the recommended timeline for submitting a 510(k)?
The FDA recommends that a 510(k) should be submitted at least 90 days before marketing the product to allow for thorough evaluation while supporting timely market access.
What are the initial steps in the 510(k) process?
The initial steps include determining the classification of the medical instrument (Class I, II, or III), identifying predicate instruments, preparing necessary documentation, and submitting the 510(k) application to the FDA.
What should manufacturers do after submitting the 510(k)?
After submission, manufacturers should be prepared to respond to any queries from the FDA, maintaining clear communication, and professionalism throughout the review process.
What happens upon successful review of a 510(k) submission?
Upon successful review, the manufacturer receives a clearance letter from the FDA, allowing them to market their device, marking a significant milestone in the regulatory pathway.
What is a Special 510(k)?
A Special 510(k) allows manufacturers to submit changes to their device without additional performance data, provided they can justify that no further verification or validation testing is necessary.
How can expert guidance impact the 510(k) submission process?
Expert guidance, such as that from professionals like Ana Criado, can significantly improve the submission process by providing strategic communication and comprehensive documentation, enhancing the chances for market clearance.




