Introduction
The 510(k) submission process is a critical regulatory mechanism employed by the FDA to oversee the introduction of moderate-risk medical devices into the U.S. market. This process involves affirming that a new device is "substantially equivalent" to a legally marketed device, known as a predicate device. The evaluation includes comparisons in intended use, technological characteristics, and safety and effectiveness parameters.
Understanding the specific classification of a medical device is crucial, as it determines the appropriate regulatory pathway. Recent scrutiny of the 510(k) process has emphasized the importance of thorough comparative analyses and evaluation of Summaries of Safety and Effectiveness (SSEs). Staying informed on FDA guidelines and developments is essential for regulatory professionals to ensure comprehensive compliance.
This article provides a step-by-step guide, eligibility criteria, key components of a submission, and insights into the substantial equivalence evaluation. By following best practices and engaging with the FDA, manufacturers can navigate the complexities of the 510(k) process and work towards achieving FDA clearance for their medical devices.
What is the 510(k) Pathway?
The 510(k) submission process is a pivotal regulatory mechanism employed by the FDA to oversee the introduction of moderate-risk medical devices into the U.S. market. The crux of this process is to affirm that a new device is "substantially equivalent" to a legally marketed device, known as a predicate device. This means that the new device must be as safe and effective as an existing device that has already been cleared by the FDA. This assessment of equivalence includes comparisons in intended use, technological characteristics, and safety and effectiveness parameters.
Understanding the specific classification of a medical device is crucial, as the FDA categorizes devices into three distinct levels based on the associated patient risk. Once classified, the manufacturer must navigate through the appropriate regulatory pathway, whether it's the 510(k) for Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. A device must be FDA Cleared, Approved, or Granted authorization through the De Novo process before it can be legally marketed in the U.S. It's imperative to grasp the nuances between these terms, as they are not interchangeable and carry significant regulatory implications.
In light of recent conversations and documentaries, such as 'The Bleeding Edge' released in 2018, there has been increased scrutiny of the 510(k) process due to concerns that not all devices undergo stringent clinical trials before approval. This has highlighted the importance of thorough comparative analysis and the evaluation of Summaries of Safety and Effectiveness (SSEs) during the 510(k) process. Engaging deeply with the subject device, its users, and the instructions for use is just as important as understanding the competitive landscape and identifying potential predicate devices through extensive research, including clinical studies and market comparisons.
As the FDA continues to update its guidelines and advisory committees on matters such as the Breakthrough Devices Program and digital health technologies, staying informed on these developments is essential for regulatory professionals. They must also ensure comprehensive responses to any FDA communications, such as Warning Letters, and provide thorough reasoning and documentation when addressing potential violations of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
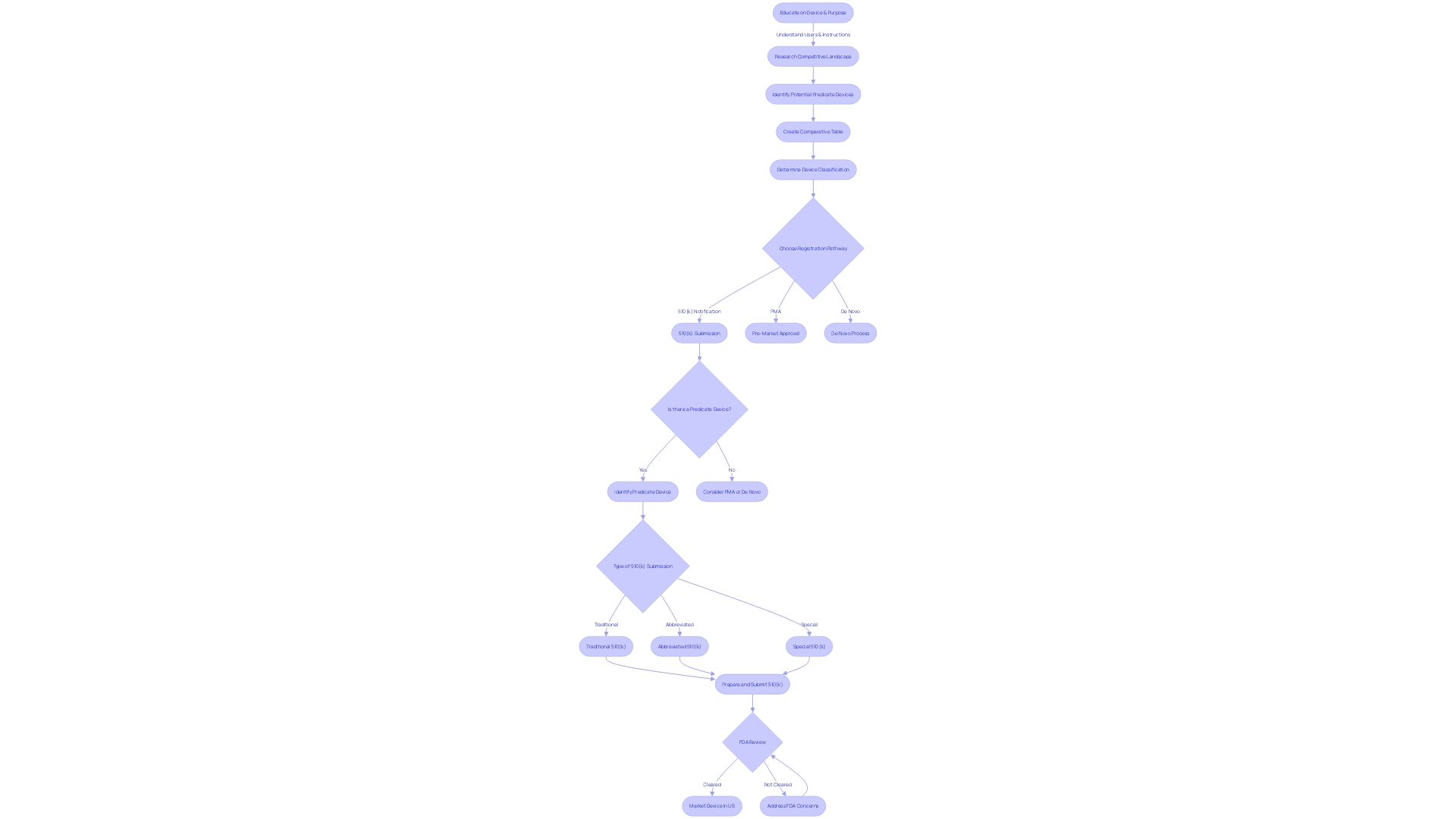
Eligibility Criteria for 510(k) Clearance
Navigating the 510(k) submission for medical device approval by the FDA requires a comprehensive understanding of the device's classification, intended use, and technological traits. The FDA categorizes medical devices into three distinct classifications based on the level of risk to patients. Prior to marketing a device in the United States, one must adhere to the appropriate regulatory pathway: Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process, following which the device may be FDA Cleared, Approved, or Granted.
For a successful 510(k) submission, it's crucial to conduct a thorough investigation of the device in question. This involves gaining insights into the device's users such as clinicians and patients, as well as its instructions for use, inclusive of any warnings. Collaboration with marketing teams can provide a broader view of the competitive landscape, allowing for the identification of potential predicate devices that share similar intended uses and technological characteristics. Creating a comparative table is a recommended step in this process.
FDA guidance documents serve as a valuable resource for understanding current thinking on various topics, although they do not impose legally enforceable duties. Instead, they offer recommendations unless they reference specific regulatory or statutory requirements. When preparing a submission, it's beneficial to consult the eCFR (Electronic Code of Federal Regulations), which presents regulations in a structured and user-friendly format.
Staying informed about FDA activities is also critical. The agency, part of the U.S. Department of Health and Human Services, is tasked with ensuring public health by overseeing the safety, efficacy, and security of medical devices, among other responsibilities. Recognizing the nuances between FDA terms such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted' is essential for regulatory professionals and contributes to a clear understanding of the regulatory environment. Moreover, special designations like 'orphan-drug' have specific implications, including exclusive approval under certain conditions which prevent subsequent sponsor approval for the same drug and indication for seven years, barring exceptions outlined by law.
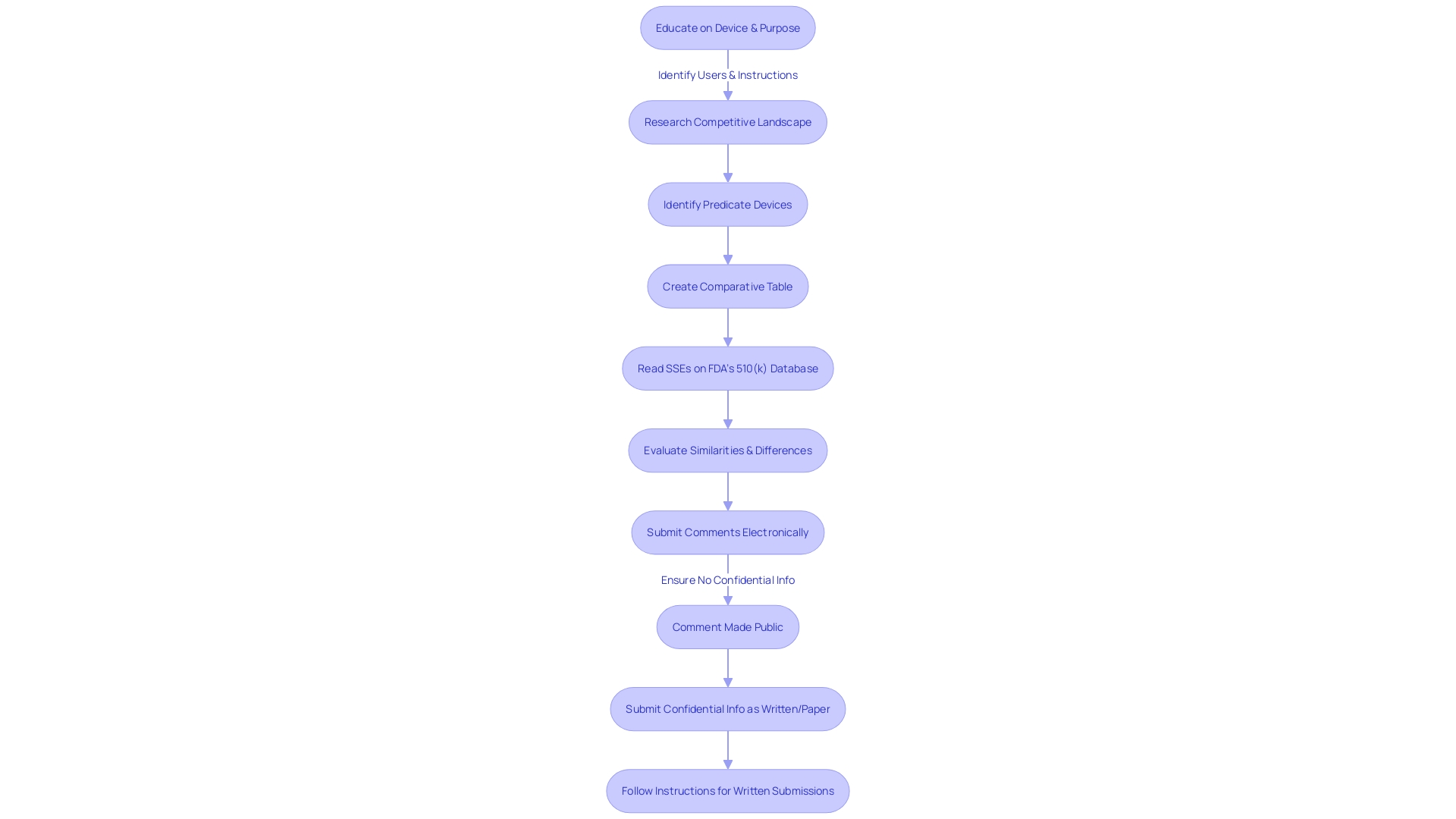
Key Components of a 510(k) Submission
To navigate the 510(k) submission process effectively, it is crucial to possess a profound understanding of the medical device in question, including its intended use, user base—ranging from clinicians to patients—and the specific instructions for use, which encompass all necessary warnings and precautions. Additionally, it is essential to collaborate with Marketing departments to comprehend the competitive landscape, as this will aid in identifying potential predicate devices that share a similar intended use and technological characteristics. A comparative table should be initiated to meticulously document these parallels.
A thorough review of research literature, clinical studies, and marketing materials such as websites, brochures, and labeling is vital in establishing the predicate device. It is advised to then delve into the Summaries of Safety and Effectiveness Data (SSED) accessible through the FDA's 510(k) database, to discern the similarities and differences between the subject device and the predicate.
The submission's success hinges on the comprehensive assembly of its components: the cover letter, a detailed device description, proposed labeling, performance data, and a comparison to the predicate device. Each element is integral to substantiating the safety and efficacy of the device. Prospective applicants are reminded to adhere to the FDA's guidance documents and familiarize themselves with the submission process to enhance the likelihood of a favorable review for substantial equivalence.
Moreover, it is imperative to be cognizant of the public nature of the comments submitted to the FDA. Comments, including attachments, submitted electronically become part of the public docket and are accessible to everyone. To safeguard sensitive information, it is incumbent upon the submitter to avoid including confidential data within the comments or opt for a written submission with specific instructions for handling confidential material.
An understanding of the broader context, such as the implications of safety standards and the potential economic and reputational repercussions, is also beneficial. This awareness can guide strategic decisions that balance regulatory compliance with public interest considerations.
Step-by-Step Guide to Preparing a 510(k) Submission
Crafting a 510(k) submission is a meticulous process that demands a strategic approach and thorough knowledge of FDA regulations. To ensure a successful submission, start by identifying a legally marketed predicate device that shares similar characteristics and intended use with your device. A thorough comparison to this predicate is foundational to your submission.
Next, determine the appropriate device classification by consulting the FDA's classification regulations. This classification will dictate the level of regulatory control and the requirements for your submission.
Conducting comprehensive testing on your device is crucial to collect performance data and demonstrate compliance with the relevant standards. This data will form a pivotal part of your submission, supporting your claims of safety and effectiveness.
The preparation of submission documentation is a multi-faceted task. It involves compiling a cover letter, detailed device description, labeling information, and all test data. Accuracy and clarity in this documentation are paramount, as it will be scrutinized by the FDA during their review process.
Furthermore, complete the necessary FDA forms with precision, including Form 3514 and Form 3601. Accuracy in these forms is essential to avoid any delays in the review process.
Finally, submit your 510(k) application to the FDA, ensuring all components are complete and well-organized. This will facilitate a smoother review process and increase the likelihood of a favorable outcome. For additional guidance, refer to the FDA's instructions for submitting comments and ensure your submission is devoid of any confidential information that should not be made public.
By following these steps diligently, you will be well-equipped to navigate the 510(k) submission process and work towards achieving FDA clearance for your medical device.

Comparing Your Device to a Predicate Device
The 510(k) submission process is a pivotal step for medical device manufacturers seeking market clearance in the United States. A critical component of this process is the comparison of the new device to an existing, legally marketed device known as the predicate. This involves a thorough analysis of intended use, technological characteristics, and performance data. The goal is to demonstrate substantial equivalence to the FDA, affirming that the new device is as safe and effective as the predicate.
To begin this intricate comparison, one must first ensure a comprehensive understanding of the subject device, its users, and its instructions for use, which may include any warnings or cautions. The next phase involves identifying a suitable predicate device. This requires an extensive review of competitor devices through research literature, clinical studies, and marketing materials to ascertain a device with the same intended use and similar technological attributes.
Once a potential predicate is identified, it's imperative to create a comparative table that outlines the similarities and differences in detail. Reading the Summaries of Safety and Effectiveness Data (Seeds) available in the FDA's 510(k) database can illuminate critical aspects of the predicate device, guiding the comparative analysis.
The FDA's modernization efforts for the 510(k) process, including public feedback on the use of older predicates, have culminated in a set of best practices outlined in draft guidance issued on September 7, 2023. These best practices emphasize the importance of selecting a predicate that not only has the same intended use but also possesses technological characteristics that do not introduce new safety and effectiveness questions.
In light of these guidelines, it is evident that the selection of a predicate device is not a mere formality but a strategic decision that can significantly impact the review process. The FDA's commitment to ensuring public safety while fostering innovation is reflected in their rigorous evaluation of 510(k) submissions, which often includes non-clinical testing and may require clinical data in certain scenarios.
Ultimately, the demonstration of substantial equivalence is a nuanced task that demands careful attention to detail and adherence to FDA guidelines. It is an essential step in bringing new medical devices to the market that are both innovative and secure for public use.
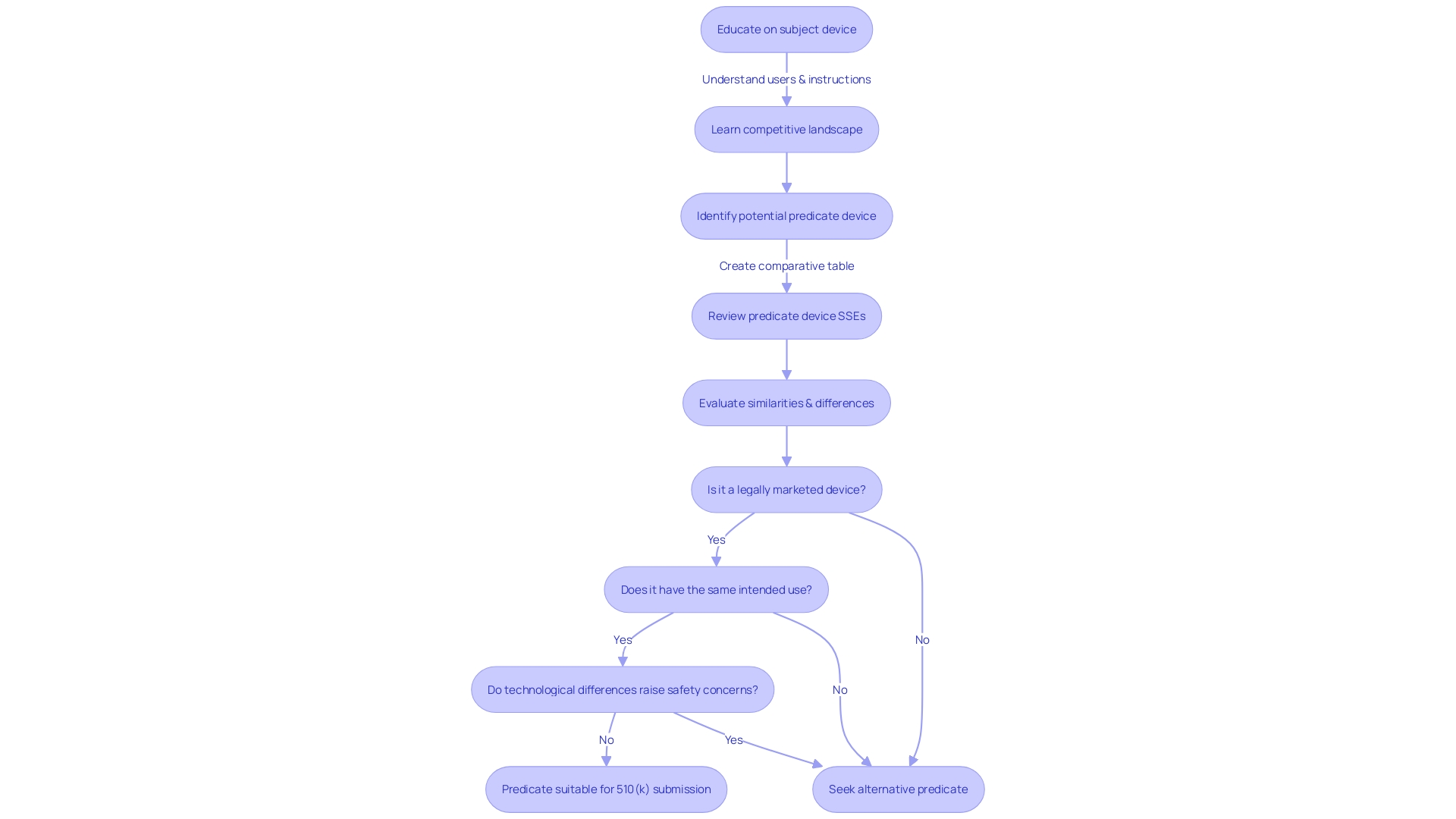
Substantial Equivalence Evaluation
The process of assessing substantial equivalence is a pivotal aspect of the 510(k) submission, requiring a meticulous comparison of the new medical device to a legally marketed predecessor, known as the predicate device. During this evaluation, the FDA scrutinizes various elements such as the intended use, technological attributes, and overall performance of the device to ascertain its equivalence. It's not merely a matter of checking off similarities; the FDA places emphasis on both the safety and effectiveness of the device in context to the predicate. For instance, even if there are differences in technological characteristics, they should not raise new questions of safety or effectiveness. This evaluation is not conducted in isolation; public comments and feedback play a role in the decision-making process, reflecting the FDA's commitment to transparency and stakeholder engagement. As such, stakeholders are advised to provide feedback while ensuring the confidentiality of sensitive information. The outcome of this comprehensive evaluation has a significant impact on whether the FDA will grant 510(k) clearance, which is essential for the device to enter the U.S. market. The FDA's guidance on this matter, while non-binding, offers insight into current thinking and recommended practices for a successful submission. In the ever-evolving landscape of medical device regulation, staying abreast of such guidelines is crucial for manufacturers aiming to navigate the 510(k) process effectively.
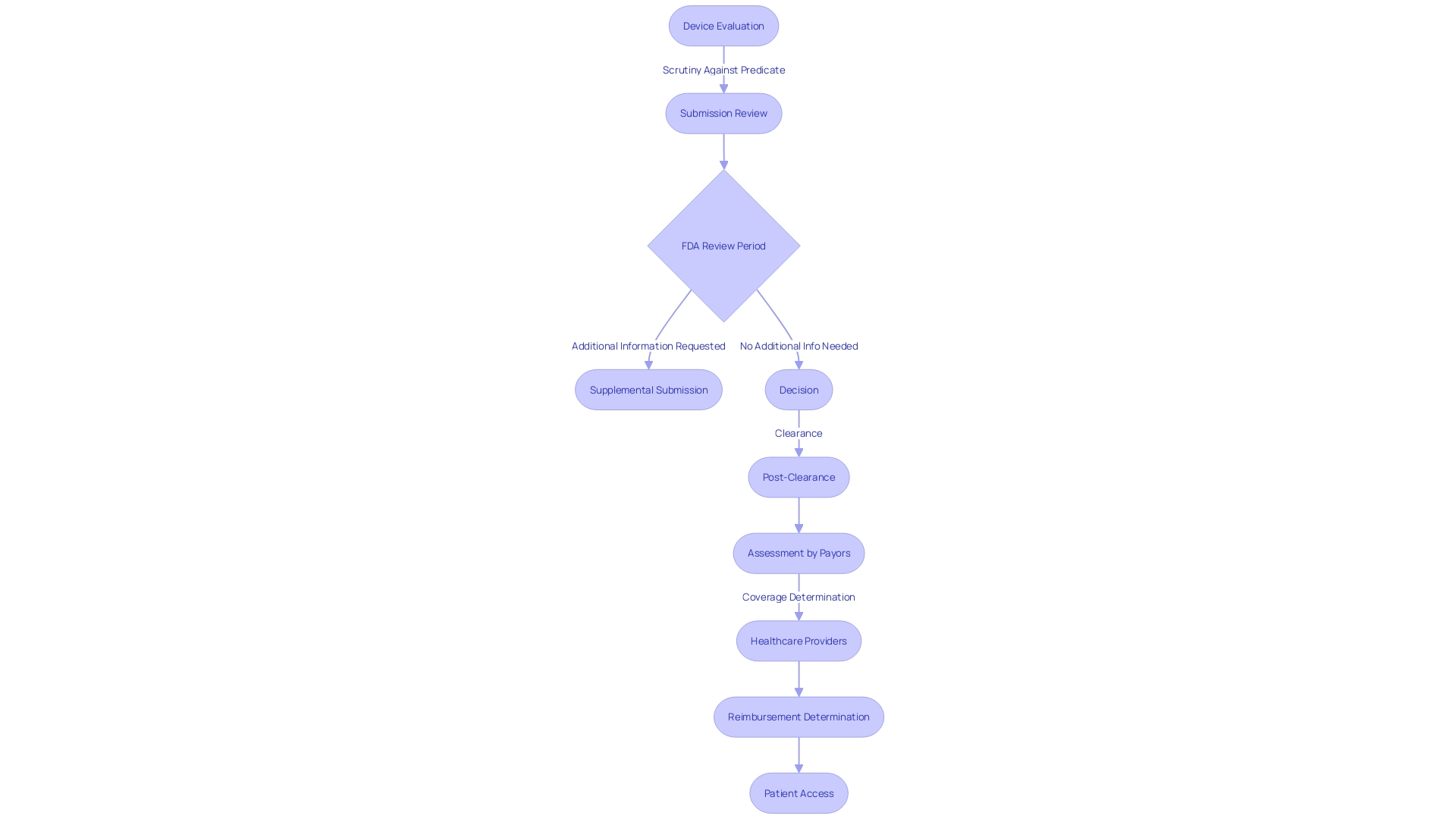
FDA Review Process and Timeline
The 510(k) submission review by the FDA is a pivotal step in bringing medical devices to the U.S. market, ensuring they meet safety and effectiveness standards. This evaluation process scrutinizes the device against a predicate, a previously cleared device with similar characteristics, which streamlines the approval pathway for class one and two devices. Although the average review period extends over several months, the timeline can fluctuate depending on the device's complexity and the submission's comprehensiveness. During this phase, manufacturers must be vigilant and responsive, as the FDA may necessitate supplementary information to address specific concerns.
The depth of the review is contingent on the risk category of the device: class three devices, which include critical life-sustaining equipment like pacemakers, undergo a more stringent review due to their significant role in patient care. This meticulous process is indicative of the FDA's commitment to public health, as seen in their recent initiatives to enhance clarity and transparency in direct-to-consumer drug advertisements, emphasizing the presentation of information in a consumer-friendly manner.
Post-clearance, the journey of a medical device continues as other stakeholders, including payors and healthcare providers, undertake their own assessments to determine coverage and reimbursement. The data that satisfied FDA requirements may not always align with the criteria set by entities like CMS or private health plans, which could potentially lead to a delay or denial in device coverage and accessibility for patients.
For medical device manufacturers, thorough research on the subject device, including its users, competitive landscape, and regulatory history, is indispensable. By analyzing existing research literature, clinical studies, and the FDA's 510(k) database, manufacturers can create a detailed comparative table to support their submission. This proactive approach, along with active engagement with the FDA, can help navigate the complexities of the approval process, ultimately facilitating patient access to essential medical devices.
Common Challenges and Best Practices
Navigating the 510(k) submission process for medical device approval can be intricate. A comprehensive understanding of both the device in question and FDA expectations is imperative. Applicants should delve into a thorough investigation of the device's users, such as clinicians and patients, and meticulously review instructions for use, including all warnings and cautions. Alignment with Marketing teams can provide valuable insights into the competitive landscape, allowing for the identification of potential predicate devices that share similar intended use and technological characteristics. This comparative analysis is integral to creating a robust submission document.
As noted by industry experts, the creation of a comparative table that outlines the similarities and differences between the subject device and the chosen predicate is a step that cannot be overlooked. To enhance this comparison, applicants should utilize resources such as research literature, clinical studies, and FDA's 510(k) database, which includes Summaries of Safety and Effectiveness (SSEs) for previously cleared devices.
The FDA, tasked with safeguarding public health, requires that submissions do not contain proprietary or confidential information that applicants do not wish to be made public. Therefore, it is the applicant's responsibility to ensure that the submission is free of such sensitive data before electronic submission. In the event confidential information must be submitted, it should be done through a separate written and paper process as specified by the FDA.
Drawing from the experience of professionals in the field, like Chris, a biomedical engineer with over a decade of experience in medical device clinical studies, it becomes clear that proactive communication with the FDA is crucial. Keeping an open dialogue throughout the 510(k) process can clarify questions and avoid potential pitfalls.
Statistics suggest that while the use of certain medical technologies is on the rise, the complexity of the 510(k) process remains a critical hurdle for many. With the goal of demonstrating substantial equivalence to a legally marketed device, applicants must navigate this process with diligence and precision. By doing so, they can significantly enhance their likelihood of achieving successful 510(k) clearance.
Conclusion
The 510(k) submission process is crucial for introducing moderate-risk medical devices into the U.S. market. Thorough comparative analyses and evaluation of Summaries of Safety and Effectiveness (SSEs) are essential in light of recent scrutiny. Staying informed on FDA guidelines and developments is vital for regulatory compliance.
Understanding the device's classification, intended use, and technological traits is crucial for a successful 510(k) submission. Collaboration with marketing teams and thorough investigation of the device and potential predicate devices are key. Adhering to FDA guidance and staying informed on FDA activities ensure a clear understanding of the regulatory environment.
Key components of a 510(k) submission include a cover letter, detailed device description, proposed labeling, performance data, and a comparison to the predicate device. Safeguarding sensitive information and following FDA guidelines for public comments are important considerations.
The step-by-step guide involves identifying a predicate device, determining the appropriate device classification, conducting comprehensive testing, preparing submission documentation, completing FDA forms accurately, and submitting the application to the FDA. Diligence in following these steps increases the likelihood of FDA clearance.
Comparing the new device to a predicate device is critical, as it demonstrates substantial equivalence. Adherence to FDA guidelines and staying updated on developments are essential. The substantial equivalence evaluation requires a meticulous comparison and stakeholder engagement through public comments and feedback.
In conclusion, the 510(k) submission process is a regulated pathway for introducing medical devices. Following best practices, engaging with the FDA, and staying informed on guidelines and developments enable manufacturers to navigate the process and work towards FDA clearance. This ensures the safety and effectiveness of medical devices for patients and healthcare providers.




