Introduction
The 510(k) submission process is a crucial step in bringing new medical devices to market. It involves demonstrating that the device is "substantially equivalent" to an existing one, known as the predicate device, which has already been legally marketed. This comprehensive process requires meticulous research and comparison to identify a suitable predicate device and create a comparative table to demonstrate equivalence.
The FDA classifies medical devices into three levels of risk, determining the appropriate pathway for registration. Recent industry developments highlight the importance of this process in addressing specific medical needs and enhancing patient outcomes. The FDA monitors the medical device market vigilantly to ensure patient safety, emphasizing the significance of a comprehensive 510(k) submission.
With safety concerns growing, securing funding and identifying device users remain challenges, but the 510(k) process serves as both a regulatory hurdle and a pathway for innovation. By understanding the clinical landscape, user needs, and technological aspects, medical device manufacturers can contribute to improving patient care and saving lives.
What is a 510(k) Submission?
Obtaining FDA approval through a 510(k) submission is an important stage in introducing new healthcare equipment to the market. This procedure involves proving that a forthcoming healthcare apparatus is 'substantially similar' to a preexisting one, referred to as the reference apparatus, which has already been lawfully promoted. Attaining significant similarity means the new apparatus has the identical intended purpose and technological traits with its forerunner.
For creators of healthcare equipment, navigating the 510(k) procedure is both a test and a chance to tackle particular healthcare requirements through technological advancements. As demonstrated by Dr. Hunter's journey in MedTech innovation, comprehending the clinical setting and patient requirements is the basis for creating medical equipment that can make a tangible impact. This thorough comprehension extends to the instructions for use, warnings, and cautions of the equipment, which are vital elements of the submission.
The procedure entails careful examination and comparison, frequently commencing with an extensive assessment of literature, clinical studies, and competitor analysis to pinpoint an appropriate precedent instrument. Creating a comparative table becomes an essential tool for demonstrating equivalence. The FDA categorizes healthcare equipment into three tiers according to the danger they present to patients, which determines the suitable route for registration, whether it is a 510(k) submission, Pre-Market Approval (PMA), or the De Novo procedure.
Recent industry developments underscore the importance of this process. For instance, VersaWrap's design aims to enhance patient outcomes across all demographics by focusing on simplicity and intuitiveness. Likewise, progress in the early identification of long-term illnesses, like kidney disease, by organizations such as Everly Health Solutions, highlight the importance of instruments that facilitate early detection and management of diseases.
The FDA closely monitors the market for healthcare equipment carefully to guarantee patient safety, with regulations in place for tracking, recall of flawed equipment, and timely risk notifications. This underscores the significance of a comprehensive 510(k) submission that not only meets regulatory standards but also advances the quality of patient care.
As safety concerns continue to grow, with over 1.7 million injuries and 83,000 deaths potentially linked to faulty healthcare equipment in a decade, the FDA is escalating its surveillance efforts. Nevertheless, obtaining the required funding and identifying users of the equipment continue to be obstacles.
Ultimately, the 510(k) procedure is more than a regulatory hurdle; it's a pathway that safeguards public health while fostering innovation in the healthcare technology industry. It demands a profound dedication to comprehending the clinical environment, user requirements, and the technological elements that contribute to the creation of instruments that can improve and preserve lives.
Medical Device Classes and 510(k) Eligibility
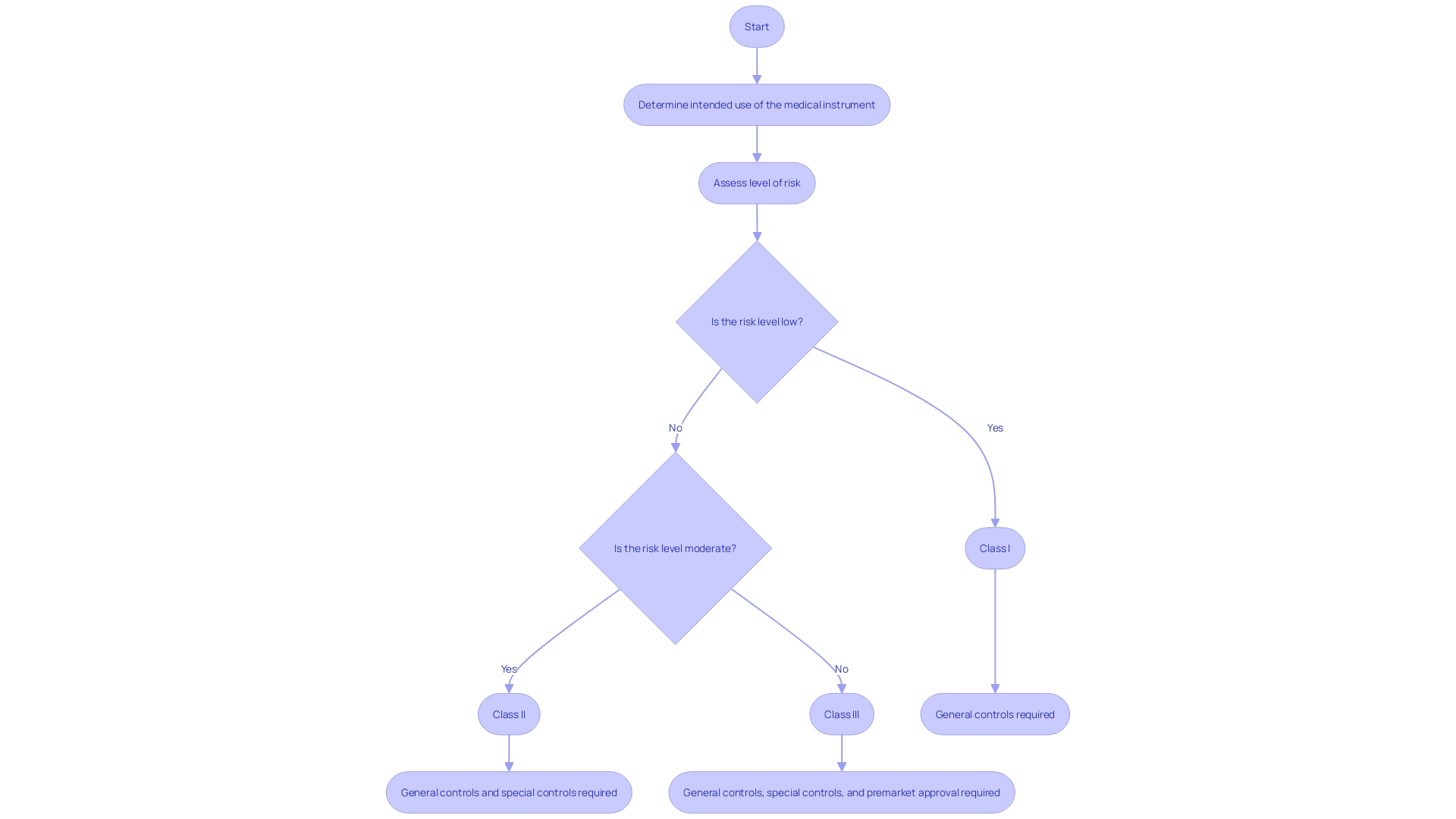
Understanding medical instrument classifications is crucial when navigating the 510(k) approval process, as these classifications have a direct influence on eligibility for the 510(k) pathway. Medical instruments are methodically classified into Class I, II, or III, representing the degree of hazard they present and the regulatory supervision required to guarantee safety and efficiency. Class I instruments, which pose the lowest risk, such as basic surgical tools like scalpels, typically require less regulatory oversight. On the other hand, the level of risk associated with a particular apparatus may increase with more specialized applications, potentially reclassifying a Class I apparatus to a higher class. For example, a scalpel utilized for corneal incision is classified as a Class III instrument because of the heightened risk and consequently, requires thorough examination.
The 510(k) pathway, a critical process for medical equipment approval, requires manufacturers to demonstrate that their product is 'substantially equivalent' to a legally marketed reference item. This involves a thorough comparison of the proposed apparatus's intended use, technological characteristics, and performance with an existing tool that is already approved for market. The FDA's Division of Standards and Conformity Assessment ensures that devices adhere to these stringent standards, and based on recent draft guidance, clinical data may be required to validate substantial equivalence in certain scenarios. These scenarios could include differences in indications for use or technological characteristics between the new product and its predicate, or when non-clinical testing alone cannot determine substantial equivalence.
The 510(k) procedure is constantly developing to improve safety and efficacy standards. For example, the FDA's multipronged effort to modernize the 510(k) program includes the introduction of new scenarios where clinical data might be necessary. This ensures that items entering the market are not only functionally equivalent to their predecessors but also maintain the highest levels of safety in light of any newly identified risks. The importance of this procedure is emphasized by data indicating that in a recent 10-year span, more than 1.7 million injuries and 83,000 deaths in the U.S. could be attributed to defective healthcare equipment, highlighting the crucial role of strict regulatory supervision to protect public health.
Choosing the Right Predicate Device
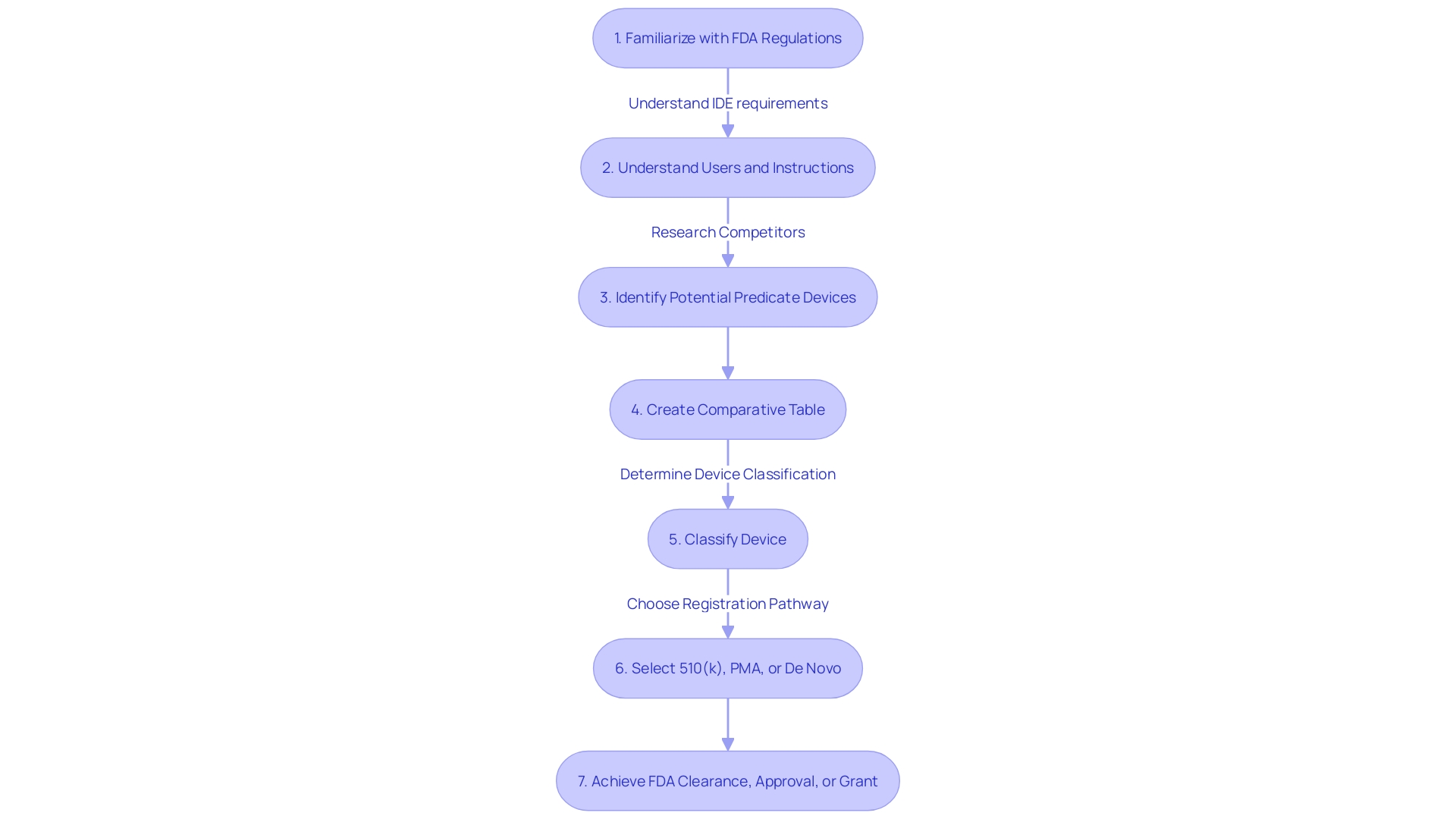
Choosing a suitable predicate apparatus is vital for the 510(k) medical equipment approval procedure, where showing significant similarity to an already legally marketed tool is crucial. Start by thoroughly understanding the users, instructions, warnings, and cautions associated with the category of the equipment. Collaborate with the marketing team to analyze the competitive landscape, examining research literature, clinical studies, and competitor information to identify potential predicates. Ensure the potential predicate is legally marketed and registered with the FDA, sharing the same intended use and technological characteristics without introducing new safety and effectiveness concerns. Utilize a comparative table to contrast your apparatus with the predicate to demonstrate substantial equivalence. The FDA suggests choosing predicates cleared through established methods such as FDA-recognized consensus standards, guidance documents, or a qualified medical tool for development. For 510(k) implants, the distinctive characteristics of the equipment, designed for continuous implantation for over 30 days, necessitates specific performance testing, content, and labeling considerations, emphasizing patient experience data to improve safety. Public comments and peer-reviewed scientific methods also have an impact on the decision-making procedure, acknowledging the significance of transparency and accountability in the regulatory environment.
Types of 510(k) Submissions: Traditional, Abbreviated, and Special
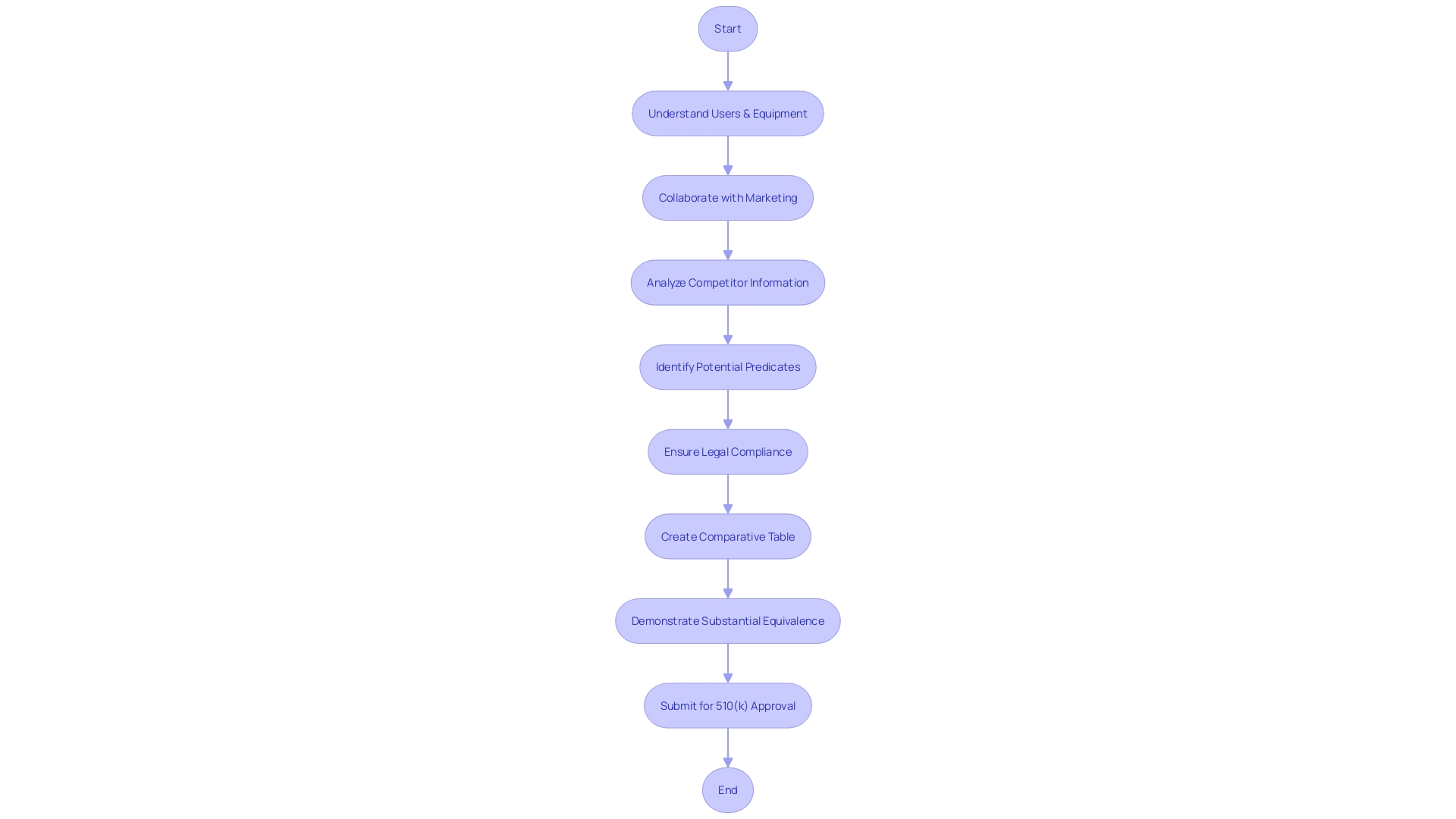
The FDA’s 510(k) clearance is a pivotal process for bringing medical equipment to market. It includes various forms of submissions—conventional, shortened, and distinctive—each customized to the distinct characteristics of the gadgets in question. The traditional 510(k) is the conventional route, suitable for items that can be compared to existing ones, known as 'predicate devices.' This comparison ensures that the new product is as safe and effective as the reference. For manufacturers with a strong understanding of the competitive landscape and a thorough comparative analysis of the reference devices, this pathway provides a structured framework for demonstrating substantial equivalence.
On the other hand, the abbreviated 510(k) relies on the use of guidance documents and recognized standards to demonstrate compliance, streamlining the process for well-understood instrument types. This pathway allows manufacturers to bypass some of the more cumbersome comparative analyses by relying on established benchmarks.
The special 510(k) is designed for minor changes to an already cleared product. It’s particularly advantageous for companies like PharmaSens, which continually innovate within their product lines. Like the other kinds, a thorough comprehension of the tool's use and the regulatory structure is crucial. For example, PharmaSens' recent progress in insulin pump technology, which received ISO 13485 certification, demonstrate the successful navigation of this procedure.
Each type of submission has its own merits and challenges. The traditional route, while comprehensive, can be resource-intensive. The abbreviated path offers efficiency but requires up-to-date knowledge of applicable guidelines. The special 510(k) provides a quick turnaround for modifications but is limited to minor changes. It’s crucial for companies to carefully weigh these factors and choose the most appropriate pathway, as a misstep can lead to delays or increased scrutiny from the FDA. Ensuring adherence to FDA regulations is essential for ensuring patient safety and lawful operations in the field of medical equipment.
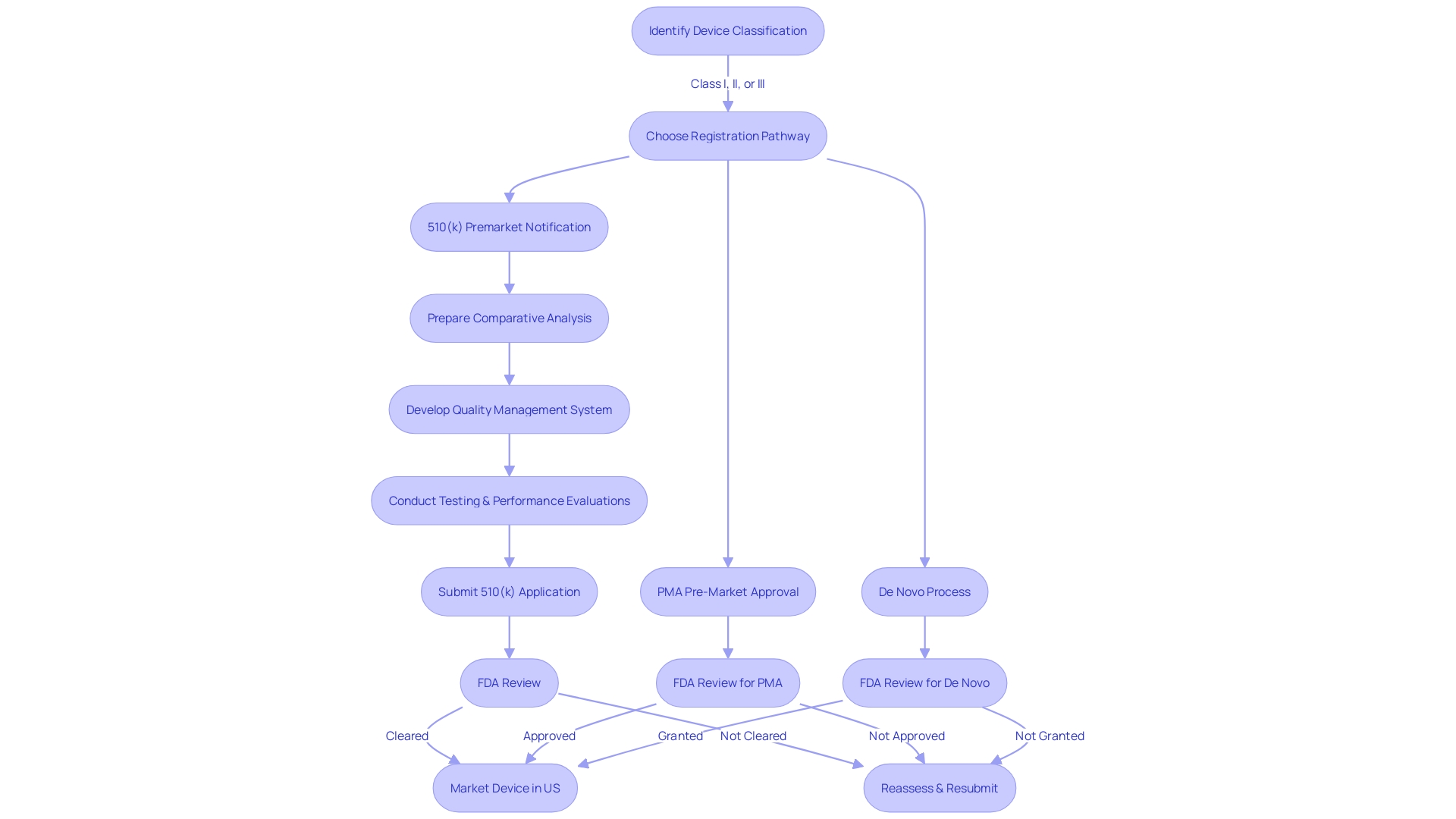
Key Steps in the 510(k) Process
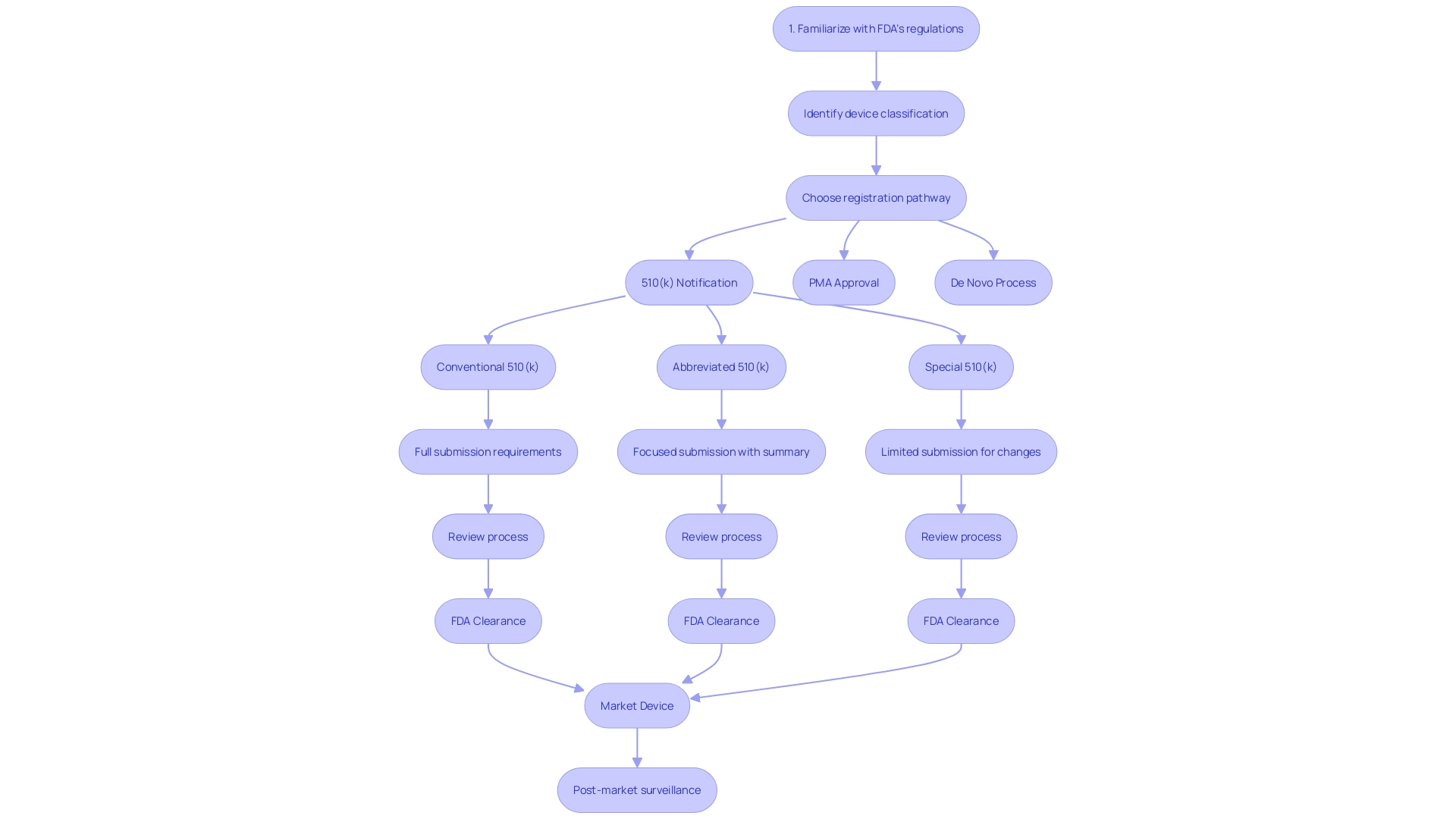
Comprehending the complexities of the 510(k) process is crucial for medical instrument manufacturers to effectively navigate FDA regulations. The journey starts by accurately categorizing the equipment according to FDA's three-tiered system, which is based on the level of risk posed to patients. Once categorized, a thorough comparative analysis of the apparatus against similar, previously sanctioned apparatuses (predecessor apparatuses) is crucial. This involves an exhaustive exploration of the competitive landscape, clinical literature, and existing technologies to establish substantial equivalence.
Throughout the application preparation, a thorough quality management system must be in place to guarantee the consistent manufacture, safety, and effectiveness. This is aligned with FDA's Quality System Regulation, Part 820, and its harmonization efforts with ISO 13485, reflecting the agency's commitment to quality and international regulatory standards.
The next phase entails rigorous testing and performance evaluations tailored to the device's intended use and technological attributes. Complete transparency in the documentation is crucial, as all materials submitted to the FDA become publicly accessible. It's this level of scrutiny and detail that allows for the FDA's CDER to ensure that new healthcare products meet the stringent requirements necessary for approval, ultimately enhancing patient care and introducing new treatment options to the health care system.
For industry leaders like Medtronic, maintaining a global presence and adhering to such strict regulatory procedures is part of their mission to alleviate pain, restore health, and extend life. Their dedication to innovation is evident in their diverse portfolio which addresses a multitude of health conditions and reflects their commitment to transforming lives through advanced technology.
The 510(k) pathway, although intricate, provides a means for healthcare products to efficiently enter the market, as long as they meet the FDA's stringent criteria for safety and efficacy. By thoroughly understanding and adhering to these steps, manufacturers can navigate potential challenges and contribute to the advancement of medical technology and patient care.
Device Testing and Performance Evaluation
The 510(k) approval process requires rigorous testing and performance evaluation to demonstrate the safety and effectiveness of the product. Manufacturers should fully understand the users of the equipment, such as clinicians, physicians, dentists, patients, etc., and pay attention to all instructions, warnings, and cautions. A comparative analysis of competitor products, considering research literature, clinical studies, and marketing materials, is essential to identify a suitable predicate item that shares the same intended use and technological characteristics.
Clinical data, bench testing, and other evaluation methods constitute the foundation of this procedure. Although not all gadgets necessitate clinical trials, as emphasized in the 2018 documentary 'The Bleeding Edge,' the FDA's 510(k) clearance procedure enables specific gadgets to be expedited if they closely resemble an already approved product.
Biomedical professionals, like Chris from Greenlight Guru with 13 years of experience, have a crucial role in managing clinical and post-market studies, ensuring the products under review meet the strict requirements established by the FDA, which are described in documents such as the FDA-2024-D-4165 draft guidance on analytical chemistry testing for biocompatibility assessment.
The testing landscape for healthcare equipment is dynamic, as evidenced by the remarks of UL Solutions' Mary Joyce on Michigan's thriving healthcare equipment sector. Moreover, Everly Health's initiatives highlight the significance of early disease detection, a goal that groundbreaking healthcare tools frequently fulfill.
To align with FDA regulations and standards, manufacturers should heed the FDA's recommendations on establishing a strategy for biocompatibility assessment, which may include chemical characterization. The FDA's division of Standards and Conformity Assessment promotes innovation and standardization in technologies, with a focus on reaching out and achieving global harmonization, ensuring patient access to new and safe healthcare equipment. Such standardization is defined succinctly by ISO/IEC Guide 2.
Manufacturers must not only accurately describe their product in accordance with FDA classification but also substantiate its substantial equivalence to existing products. It is imperative to conduct these evaluations with due diligence, maintaining transparency and adhering to federal government guidelines to safeguard confidential information.
To summarize, the 510(k) procedure is a complex assessment that requires a thorough comprehension of the product, meticulous comparison with current technologies, and strict compliance with regulatory standards to guarantee the introduction of secure and efficient products into the market.
Building a Quality Management System (QMS)
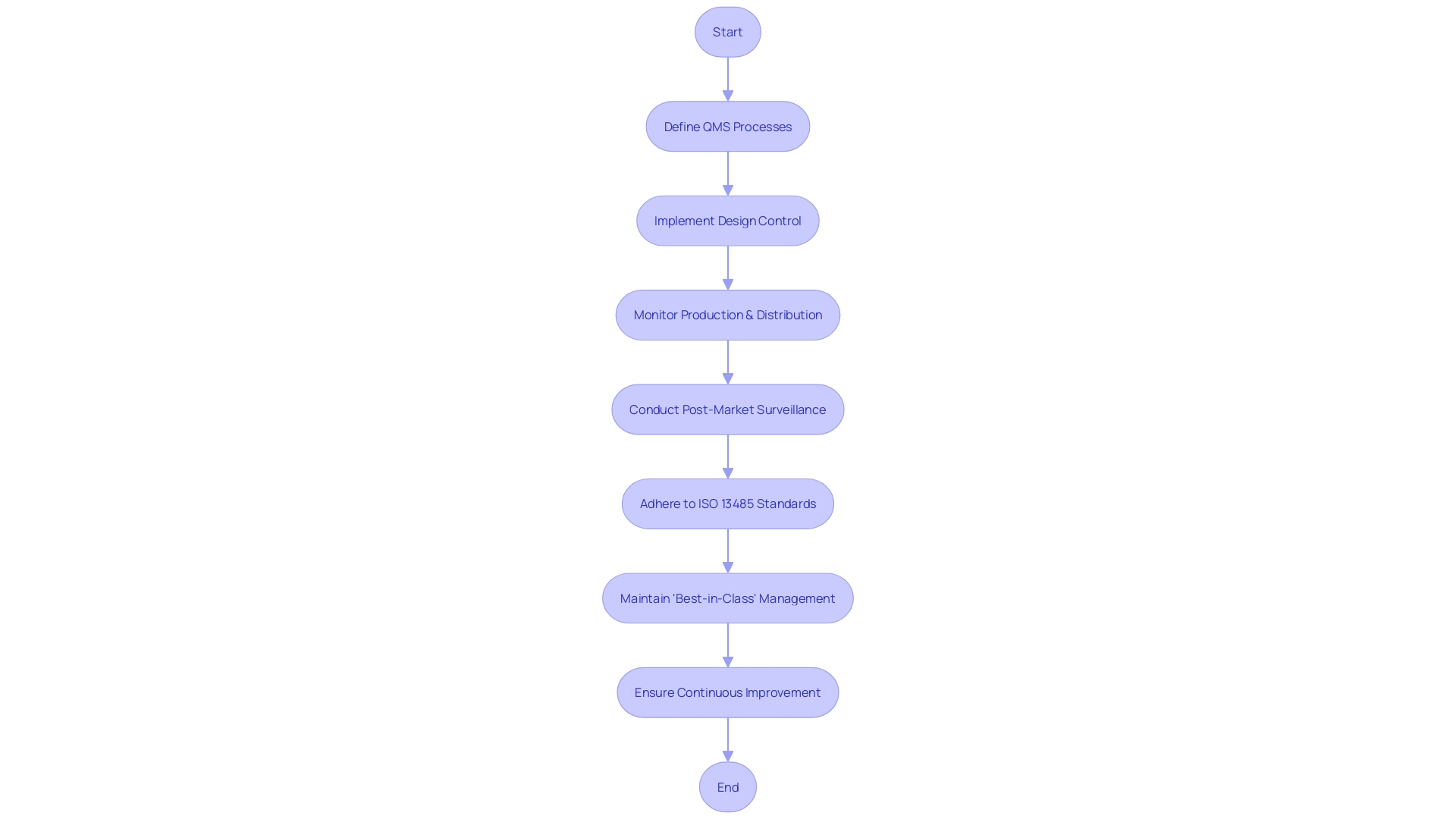
A Quality Management System (QMS) is the foundation of manufacturing for healthcare equipment, playing a crucial role in ensuring products are secure and efficient. It is not only about adhering to regulations; it involves a systematic approach to quality assurance throughout the product lifecycle. From design control that verifies the product meets requirements and addresses risks, to consistent monitoring during production, distribution, and post-market surveillance, the QMS is integral to delivering healthcare instruments that improve patient outcomes.
The medical industry is diverse, with products ranging from simple consumer items to complex systems like MRI machines and pacemakers. This diversity requires a QMS that is adaptable to various human and equipment factors. Such systems are crucial in the 510(k) approval process, as they ensure products comply with stringent standards for market entry.
Industry leaders who prioritize quality management are more likely to achieve high-quality outcomes and competitive market positions. According to Greenlight Guru's State of the MedTech Industry Report, which surveyed over 500 professionals, companies that are "very well equipped" to meet their quality goals exhibit clear advantages. This includes adherence to ISO 13485, which enhances product quality by demanding consistent quality monitoring. Such standards not only reduce the risk of product defects but also build customer trust.
As healthcare equipment technology flourishes, areas like Michigan are acknowledged for their expertise and production capabilities. Implementing a robust QMS enables companies to stand out in this competitive landscape. A digital QMS, in particular, can integrate seamlessly with existing systems, allowing for more efficient and streamlined quality control measures.
In summary, establishing and maintaining a robust quality management system (QMS) is crucial for medical device manufacturers, ensuring that they not only meet regulatory requirements but also deliver high-quality products that can save lives and improve patient care.
Assembling the 510(k) Application
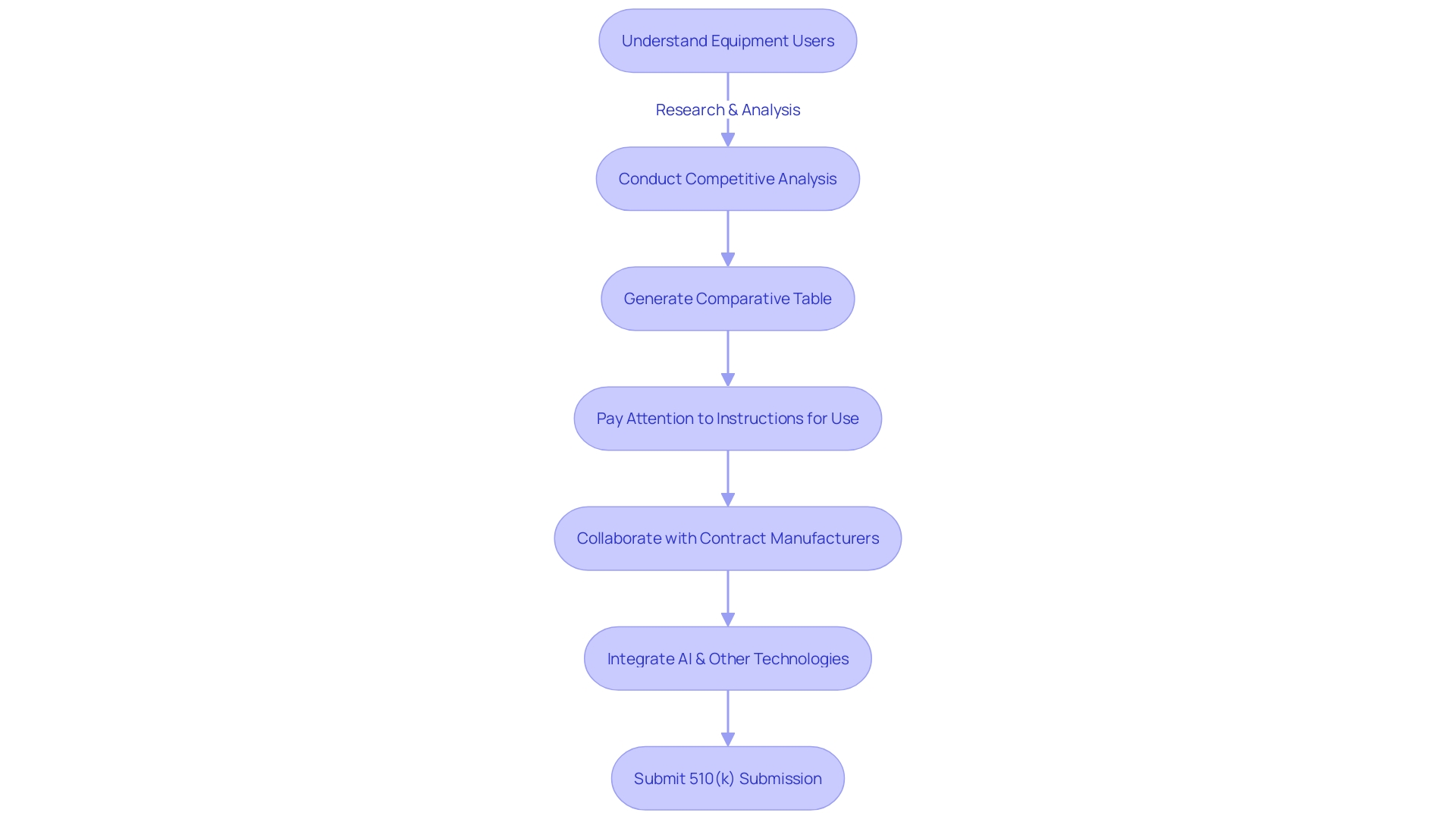
Compiling a thorough and structured 510(k) submission is imperative for approval. Your application should encompass comprehensive equipment descriptions, accurate labeling, detailed manufacturing information, and robust clinical data. Begin with a thorough understanding of your equipment's users, such as clinicians and patients, and the competitive landscape. This includes research literature, clinical studies, and direct comparisons with predicate instruments that share intended use and technological characteristics. Involve marketing teams to help with this competitive analysis and generate a comparative table to clearly illustrate the resemblances and distinctions to prior devices.
In preparing your submission, pay careful attention to the instructions for use, including warnings and cautions. The FDA's draft guidance for implanted products, which remain in the body for more than 30 days, outlines the expectations for performance testing, content, and labeling. This information is critical in making your submission comprehensive and patient-centric, as the FDA prioritizes patient experience in safety.
Keep in mind, the creation of a healthcare apparatus does not conclude with its design; collaborating efficiently with Contract Manufacturers is essential. The transition from prototype to mass production requires a significant effort to ensure that each unit is consistent and of high quality. This is a vital step that should not be underestimated, as highlighted by experts in the industry.
Maintaining an organized and clear application is essential, as regulatory submissions can be extensive, sometimes encompassing thousands of pages detailing every aspect of the medical device. The integration of AI and other technologies is transforming this procedure, making it more accessible and less burdensome. For instance, Artos and Aidy are initiatives that emerged from the necessity to streamline the regulatory submissions, demonstrating the potential of innovative solutions in this field.
By diligently addressing each of these aspects, your 510(k) application will be well-prepared, increasing its chances of successful clearance. As you go through this journey, bear in mind the significance of quality and clarity to ultimately enhance health outcomes for all.
Review and Approval Process
The FDA's 510(k) evaluation system is essential in guaranteeing the safety and efficacy of medical instruments prior to their arrival on the market. Upon submission of a 510(k) application, the FDA embarks on a meticulous review process that begins with an administrative review, ensuring all necessary components are present. This is followed by a substantive review, where FDA reviewers scrutinize the product against specific criteria such as safety, effectiveness, and security, drawing upon a wealth of scientific and regulatory expertise.
The FDA evaluates the intended use, technological characteristics, and any potential differences from similar devices. For instance, when differences in indications for use or technology are observed, clinical data may be required to establish substantial equivalence, beyond non-clinical testing like bench performance or biocompatibility testing. During this stage, the FDA may identify situations requiring clinical data to support claims of substantial equivalence with a predicate instrument.
The final determination of a 510(k) application can culminate in several outcomes. Approval signifies that the product is safe and effective for its intended use and is substantially equivalent to a legally marketed predicate. Conversely, the FDA may request additional information to address specific questions that arise during the review. In certain scenarios, the application may be rejected if the equipment does not meet the required criteria for substantial equivalence. It is crucial for applicants to be ready with thorough responses to any FDA inquiries, as these can quickly address concerns and facilitate the procedure.
Recent improvements in the 510(k) program, like the preliminary advice on the utilization of clinical data, demonstrate the FDA’s dedication to developing the procedure to improve safety and effectiveness of medical instruments. Candidates are encouraged to cultivate a profound comprehension of the product's usage, cautions, and competitive environment, which can be crucial in navigating the approval procedure and responding efficiently to FDA's inquiries.
Timeline and Communication with FDA Reviewers
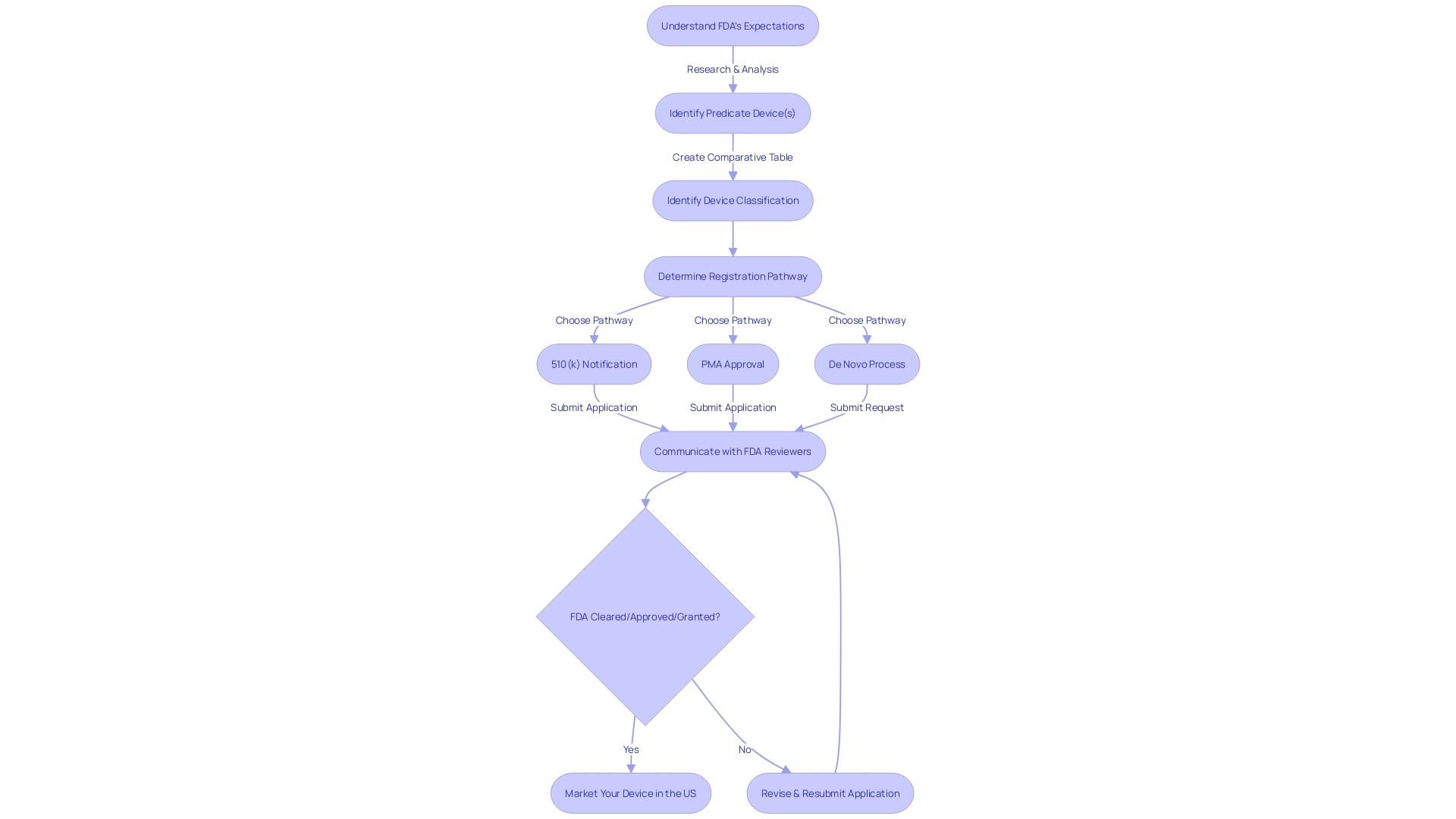
Navigating the 510(k) approval process requires a clear understanding of the FDA's expectations and a strategy for maintaining effective communication with FDA reviewers. The timeline for the review can vary, but it's essential to establish and nurture a relationship with the reviewers early on. Etienne Nichols, a seasoned Medical Device Expert, highlights the significance of comprehending the users, competitive environment, and potential precedent devices. By creating a comparative table, you can facilitate a smoother review by clearly demonstrating how your item compares with existing ones.
In addition, the FDA encourages transparency and responsibility in submissions. As expressed by industry professionals, it is important to outline how you intend to examine any concerns with your equipment, which may include interviews, document evaluations, and analytical tools such as 5-whys or fishbone diagrams. This level of detail in your communication reassures the FDA of your commitment to compliance and patient safety.
The FDA's mission to safeguard public health underlines the importance of your submission being complete, accurate, and without confidential information. With advancements in health care and regulatory science, the FDA's Center for Drug Evaluation and Research (CDER) stands as a cornerstone for clarity and guidance in product development. Their thorough assessments guarantee that pioneering healthcare equipment meets the required criteria to offer fresh therapeutic alternatives securely.
By heeding the advice of industry professionals and adhering to the FDA's guidance, you can improve the likelihood of a favorable review. Keep in mind, proactive and detailed communication throughout the procedure is crucial for successfully navigating the 510(k) pathway.

Common Challenges and Best Practices
Navigating the 510(k) medical equipment approval process requires a strategic approach that is both informed and meticulous. A successful 510(k) submission hinges on demonstrating substantial equivalence to a reference product, which can present unique challenges. For example, if the object being examined varies in its intended use or technological characteristics from an existing reference, clinical data may become crucial to establishing equivalence. As mentioned in the recent FDA guidance, non-clinical testing alone might not be enough, and the appearance of new risks linked to the previous model could require extra clinical evidence.
For medical equipment professionals like Chris, a Solutions Engineer with over a decade of experience in the field, the significance of in-depth knowledge of the device's users, instructions, and warnings cannot be overstressed. Moreover, engaging with marketing teams to understand the competitive landscape is crucial. This requires examining a range of resources, from research literature to product brochures, to identify a suitable predicate item. Constructing a comparative chart can be a valuable instrument in this procedure, aiding a transparent demonstration of the resemblances and distinctions between the new apparatus and its precursor.
Clinical data plays a significant role not only in demonstrating substantial equivalence but also in ensuring patient safety. Recent statistics highlight the seriousness of this obligation, with more than 1.7 million injuries and 83,000 deaths in the U.S. in a ten-year period associated with healthcare equipment. FDA's ongoing efforts to improve surveillance systems highlight the need for thoroughness in every 510(k) submission.
Managing the 510(k) approval also involves keeping up with regulatory updates and industry trends, which can be obtained from authoritative sources such as the Global Medical Device Podcast by Greenlight Guru. As these industry experts emphasize, submissions must exclude confidential information and focus on providing clear, public-facing data. By following these best practices, medical device companies can navigate the 510(k) process with greater confidence and efficiency, ultimately enhancing the quality and safety of medical devices brought to market.
Conclusion
In conclusion, the 510(k) submission process is a crucial step in bringing new medical devices to market. It involves demonstrating substantial equivalence to an existing legally marketed device through meticulous research, comparison, and the creation of a comparative table. The FDA's classification of medical devices into risk levels determines the appropriate pathway for registration.
Recent industry developments highlight the importance of the 510(k) process in addressing specific medical needs and enhancing patient outcomes. The FDA's vigilant monitoring of the medical device market emphasizes the significance of a comprehensive 510(k) submission in ensuring patient safety.
Navigating the 510(k) process requires a comprehensive understanding of medical device classifications and selecting an appropriate predicate device. Compliance with FDA regulations is crucial for patient safety and legal operation in the medical device sector.
Understanding the key steps in the 510(k) process is vital, including accurate device classification, establishing a quality management system, rigorous testing and performance evaluations, and maintaining transparency in documentation. By following these steps, manufacturers can navigate challenges and contribute to medical technology advancement.
Device testing and performance evaluation play a crucial role, requiring thorough comprehension of users, comparative analysis of competitor devices, and adherence to FDA regulations and standards. Building a robust Quality Management System is integral to ensuring device safety and effectiveness.
In summary, the 510(k) submission process is a complex evaluation that demands a comprehensive understanding, careful comparison, and adherence to regulatory standards. By following best practices and navigating challenges, medical device companies can contribute to medical technology advancement and patient care improvement.
Take the first step towards advancing medical technology today!




