Introduction
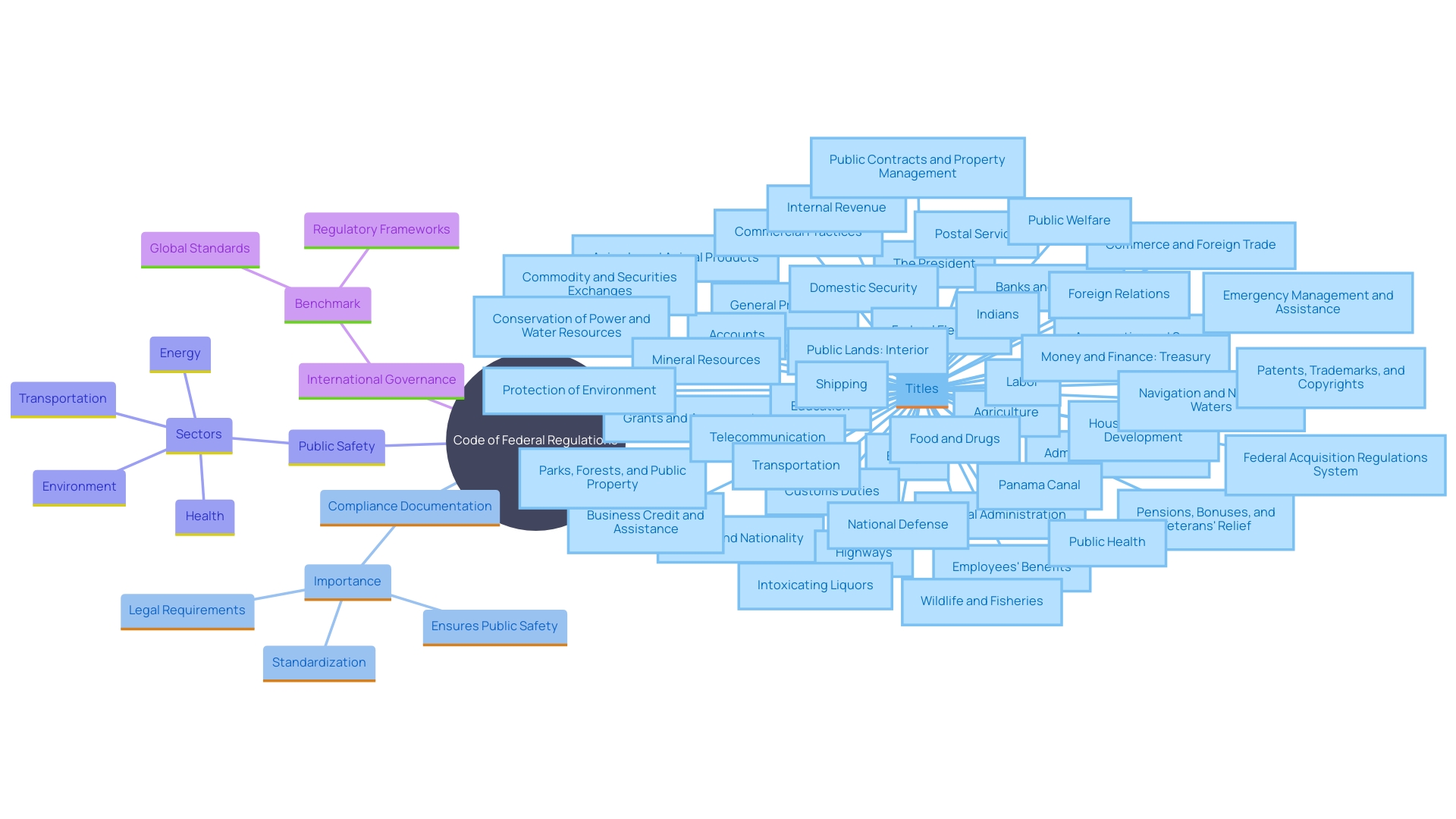
The Code of Federal Regulations (CFR) stands as a pivotal resource in the regulatory landscape of the United States, encapsulating the general and permanent rules issued by federal executive departments and agencies. This comprehensive compilation translates federal laws into actionable regulations, playing a fundamental role in ensuring compliance and enforcement across a myriad of sectors. Organized into 50 titles, each dedicated to a specific area of regulation, the CFR aids professionals in navigating complex legal frameworks, making it indispensable for those involved in regulatory affairs.
The significance of the CFR extends beyond its role in regulatory documentation — it is crucial for maintaining public safety and efficacy in sectors such as pharmaceuticals, medical devices, and food safety. The CFR's structured approach not only enhances transparency and accountability but also serves as a benchmark for international regulatory standards, fostering global collaboration and compliance. Understanding the CFR is essential for fostering a shared vocabulary and effective teamwork within regulatory environments, ensuring that legal and regulatory requirements are met comprehensively.
What is the Code of Federal Regulations (CFR)?
The Code of Federal Regulations (CFR) is an essential compendium of the general and permanent rules issued by the executive departments and agencies of the United States federal government. It plays a crucial role in translating federal laws into actionable guidelines that ensure compliance and enforcement. Organized into 50 titles, each dedicated to a specific area of federal regulation, the CFR facilitates the navigation of complex legal landscapes for professionals across various industries.
The CFR is essential for compliance documentation, which includes the papers submitted to health authorities to support research, development, and marketing applications, along with post-marketing activities. These documents are essential to fulfilling the legal and compliance requirements governing medicinal products. Therefore, understanding the CFR is foundational for anyone involved in compliance matters, enabling them to create a shared vocabulary and foster collaboration within their teams.
Moreover, the CFR is a critical resource for ensuring public safety and efficacy in numerous sectors, including human and veterinary drugs, medical devices, and food safety, as emphasized by the FDA. 'This governing structure assists in preserving clarity, responsibility, and adherence, which are essential for the integrity of scientific research and public health initiatives.'.
In the context of international comparisons, the CFR sets a benchmark for governance standards, offering a structured approach that other nations may look to when developing their frameworks. This international perspective is vital as global collaboration and compliance become increasingly significant in the management of emerging technologies and public health strategies.
Structure of the CFR
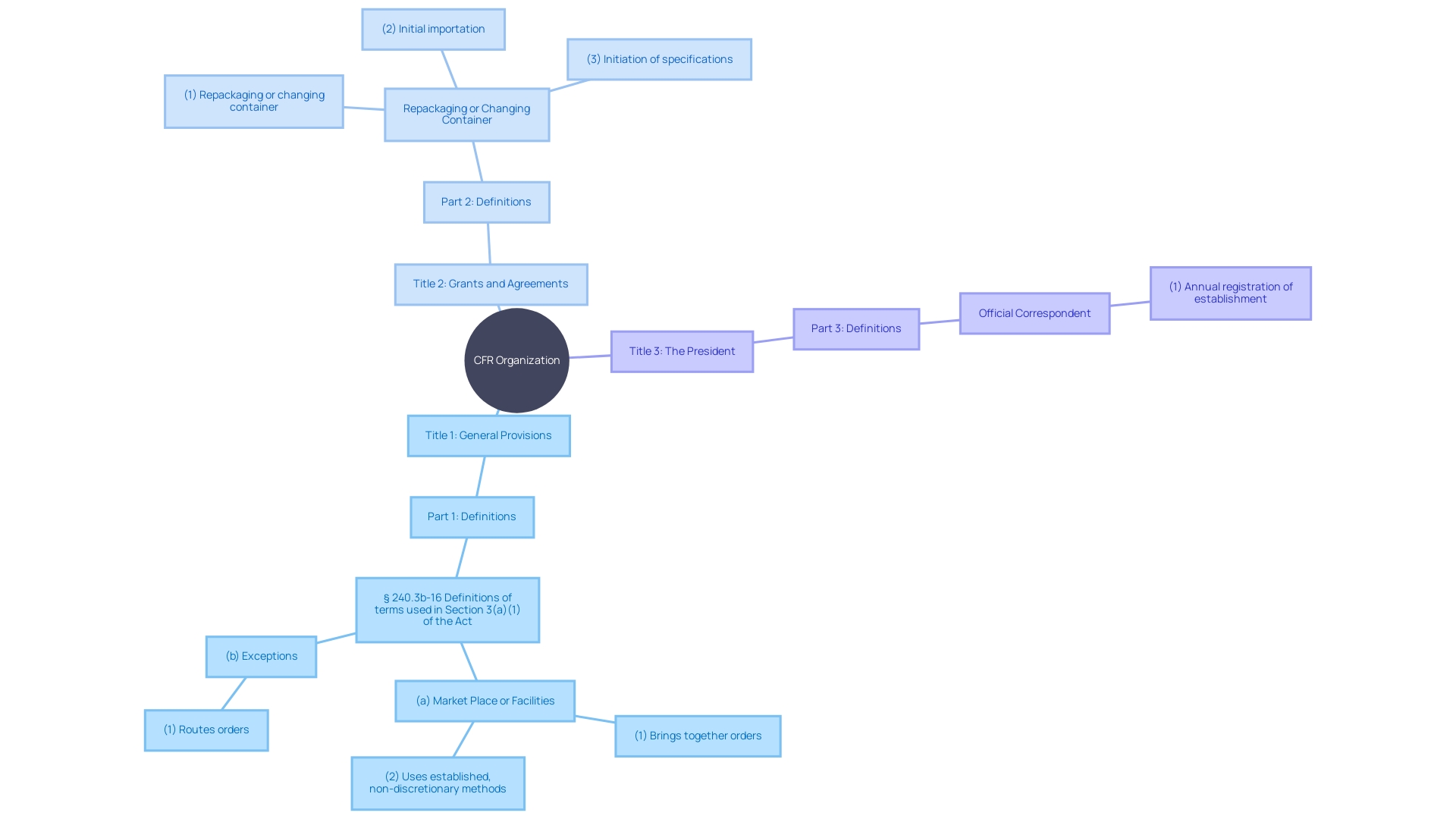
The Code of Federal Regulations (CFR) is meticulously organized into titles, parts, sections, and paragraphs to ensure clarity and accessibility. Each title zeroes in on a specific subject area, ranging from labor to food and drugs, as well as environmental protection. This structure is essential for navigating the extensive collection of compliance information. Within each title, parts explore more detailed rules and guidelines. 'The smallest division, sections, encapsulates specific governing language.'.
This hierarchical framework underpins the systematic organization of the CFR. Patrick McLaughlin, a senior research fellow at the Mercatus Center, emphasizes the sheer volume of federal regulations, which would take about three years to read in full-time capacity. This highlights the necessity of a well-structured and navigable governance framework. Moreover, the eCFR, a continuously updated online version, enhances accessibility, although it is not an official legal edition. This emphasizes the significance of clarity and simplicity in legal language to aid adherence and understanding.
'The careful arrangement of the CFR not only aids in compliance with regulations but also helps alleviate the economic downturn caused by the buildup of rules, as McLaughlin's research indicates.'. Thus, a clear and systematic structure is pivotal for both regulatory efficiency and economic vitality.
Understanding CFR Citations
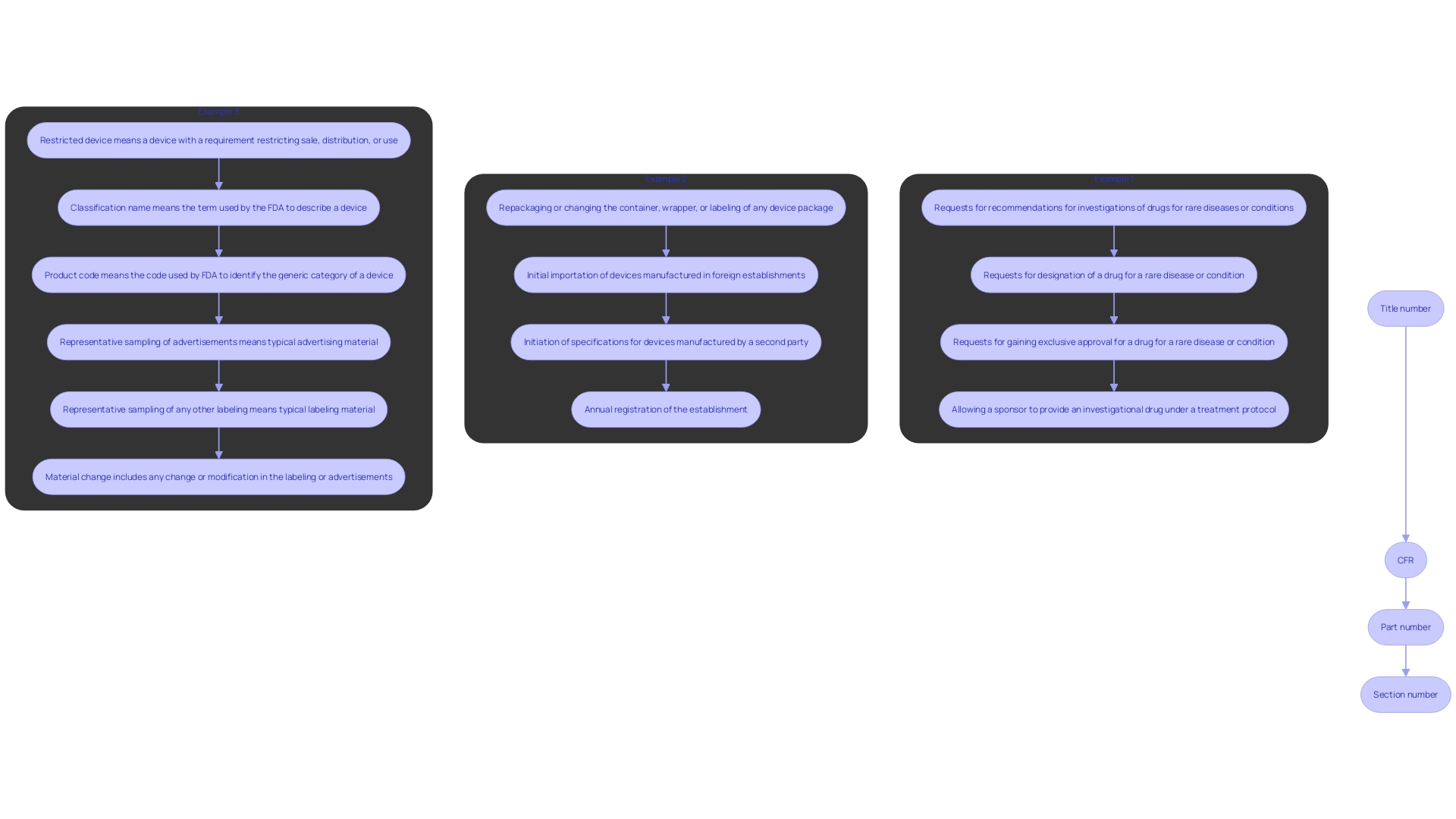
CFR citations play a crucial role in accurately referencing specific rules. A standard citation follows a precise format: Title number, CFR, part number, section number (e.g., 21 CFR 117.1). This structured system ensures users can pinpoint the exact location of a rule within the CFR, eliminating any ambiguity and facilitating quick access to the necessary information. For instance, when the FDA approved the heart monitor embedded in the Apple Watch in 2018, it was essential to reference specific CFR guidelines to ensure compliance and clarity in official documentation.
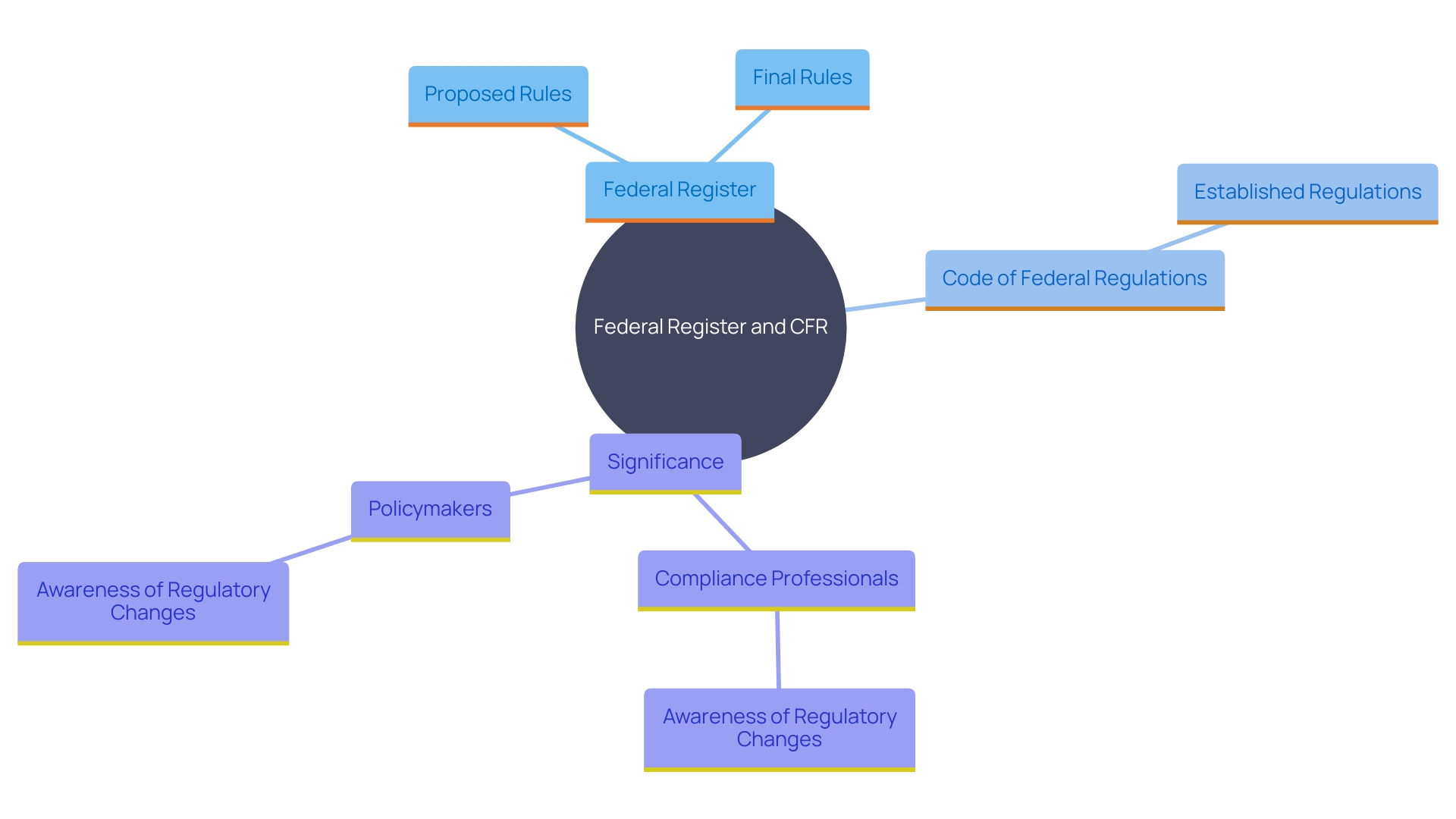
Difference Between the Federal Register and the CFR
The Federal Register is the government's daily publication that issues proposed rules, final rules, and notices from federal agencies. It acts as a vital instrument for the public to remain aware of new and changing guidelines. For instance, the Federal Trade Commission recently issued a rule on the use of consumer reviews and testimonials, adding significant paperwork but ultimately providing $15.5 billion in cost savings.
In contrast, the Code of Federal Regulations (CFR) is a comprehensive codification of these rules, organized into an easily accessible format and updated annually. This distinction between the Federal Register's dynamic updates and the CFR's stable structure is critical for professionals in the field. The CFR offers a reliable reference point for established regulations, which is indispensable for legal compliance and public health safeguarding.
Comprehending the complexities and ongoing development of these governing frameworks is essential. As pointed out by compliance experts, staying informed about changes in regulations is essential for professionals and policymakers to promote evidence-based decision-making and adjust to industry demands.
Key Components of CFR Titles and Parts
The Code of Federal Regulations (CFR) is meticulously structured to facilitate ease of navigation through its extensive legal content. Each title in the CFR is divided into various parts, which may further be subdivided into subparts and sections. Titles are numerically designated and thematically organized, ensuring a coherent categorization of rules. For instance, Title 21 is dedicated to food and drugs, encompassing numerous parts that address specific regulatory concerns in these areas. Likewise, Title 40 emphasizes environmental protection, offering comprehensive guidelines intended to preserve the environment. This structured approach enables users to efficiently find pertinent rules and comprehend the specific legal requirements in complex areas of law, thereby promoting compliance and informed decision-making.
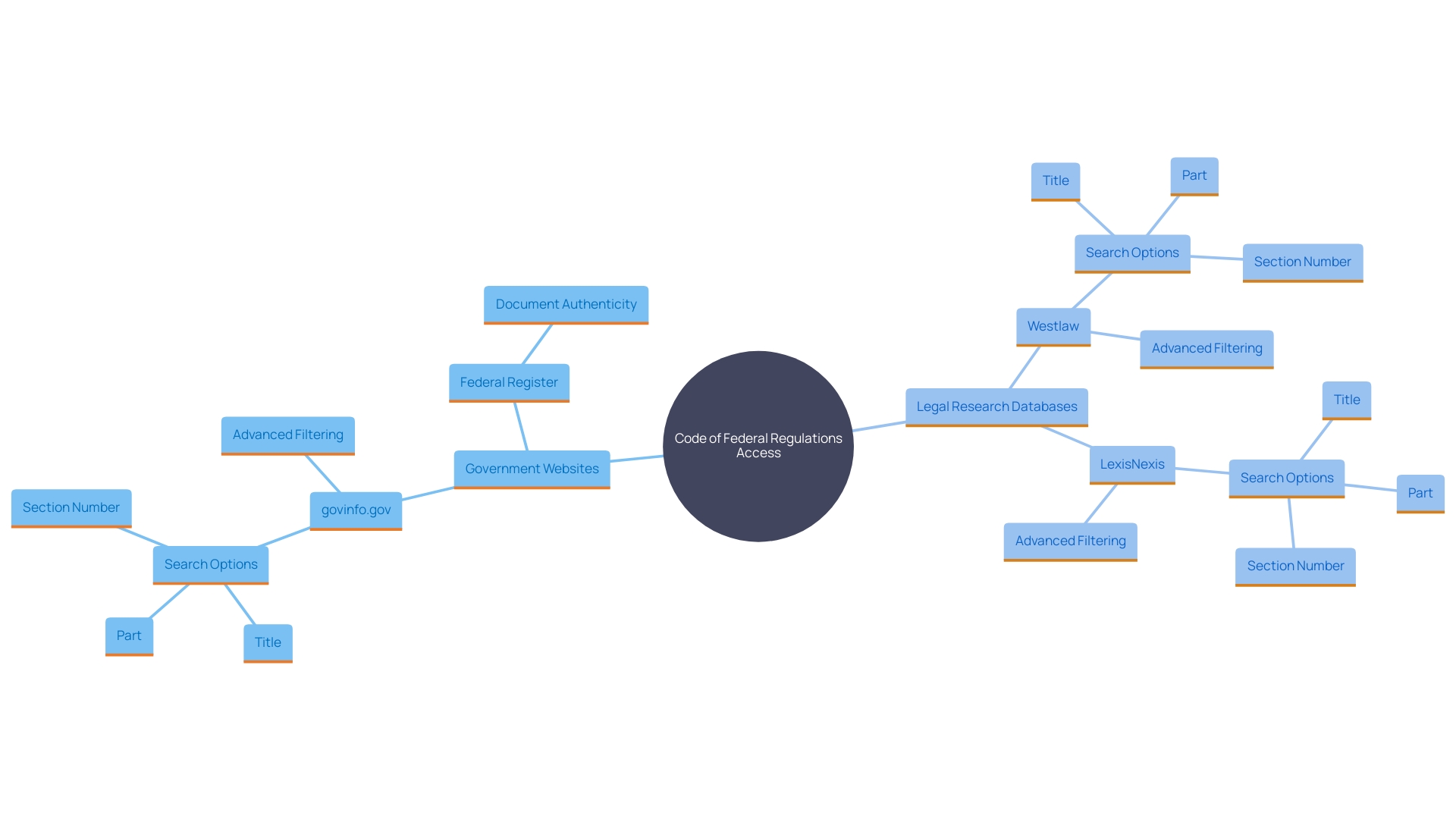
Accessing and Searching the CFR
The Code of Federal Regulations (CFR) can be accessed through multiple platforms, including government websites and legal research databases. Users can efficiently search for specific rules by title, part, or section number. Advanced search options on many online databases also allow filtering by keywords or regulatory agency, making the process more streamlined. Notably, the Federal Register provides XML renditions of published documents, with links to the official PDFs on govinfo.gov, ensuring authenticity and reliability. This level of accessibility is crucial for individuals and organizations aiming to comply with federal regulations effectively.
Conclusion
The Code of Federal Regulations (CFR) serves as a cornerstone of the regulatory framework in the United States, translating federal laws into actionable regulations that facilitate compliance across various sectors. Its organization into 50 titles allows professionals to navigate complex legal landscapes efficiently, ensuring that essential documents meet the rigorous standards set by federal agencies. The CFR not only promotes public safety and efficacy in critical areas such as pharmaceuticals and food safety but also establishes a benchmark for international regulatory practices.
The structured hierarchy of the CFR, with its titles, parts, sections, and citations, underscores the importance of clarity and accessibility in regulatory documentation. This organization aids in compliance efforts and helps mitigate the economic impacts of regulatory accumulation. Tools such as the eCFR enhance user access to updated regulations, reinforcing the need for a systematic approach to regulatory information.
Understanding the distinctions between the CFR and the Federal Register is crucial for regulatory professionals. While the Federal Register provides dynamic updates on proposed and final rules, the CFR offers a stable reference point for established regulations. This differentiation is vital for maintaining legal compliance and adapting to evolving industry needs.
Overall, a comprehensive grasp of the CFR and its components is essential for fostering effective regulatory practices and ensuring the integrity of public health initiatives.




