Introduction
Clinical trials are a crucial part of advancing medical research and bringing new treatments to the public. However, conducting these trials can be an intricate and expensive process.
In this article, we will delve into the factors that influence clinical trial costs and explore a case study that breaks down the expenses in a phase III trial. We will also discuss the importance of data collection and analysis in clinical trials, as well as analyze the dynamics of cost management. By understanding the complexities and challenges involved in clinical trial costs, companies can strategically allocate resources and optimize trial efficacy.
Understanding Clinical Trial Costs
Clinical trial companies face the delicate task of balancing meticulous study planning with the inevitable variability inherent in groundbreaking research, such as digital therapeutics. This emerging field utilizes software applications across devices like smartphones, VR headsets, and wearable sensors to manage conditions from chronic illnesses to neurological disorders.
Despite promising studies suggesting that digital therapeutics could decrease patient costs, their widespread adoption and integration into clinical trials are hampered by factors like their relative newness and limited insurance coverage. Moreover, the complexity of global clinical trials is exemplified by situations where patients, such as a rural Pennsylvania with an ultra-rare condition, must navigate international travel for a trial in Turkey.
The logistical quagmire of acquiring visas, transcending language barriers, and arranging travel is but one aspect of the myriad challenges clinical trial companies must account for when planning and budgeting. Contract research organizations advise that many decisions impacting the trial costs—from site selection to patient logistics—are set years in advance. As highlighted by industry experts, thorough due diligence during the planning stages can lead to more resilient decision-making, ensuring each 'link' in the clinical trial 'chain' is fortified against future uncertainties. Indeed, an estimated 80% of decisions made years prior could be more efficacious with a proactive and detail-oriented approach, thus optimizing resources and budget management for clinical trial companies.
Factors Influencing Clinical Trial Costs
The diverse factors influencing clinical trial costs necessitate a finely-tuned strategy, one that scrutinizes your company's structure and market approach. Larger trials entail an extensive recruitment effort, sustained monitoring procedures, and an expansive data management system. Furthermore, protracted trial periods demand additional logistical and infrastructural support.
Equally crucial is a clear understanding of the objectives and priorities. For instance, prioritizing thorough data over speed could alter the timeline and budget. It's essential to interrogate potential vendors about how their technology fits within your organizational framework and the degree of oversight required.
A poignant case illustrating the extent of logistical considerations involves a patient required to travel internationally for a trial, showcasing the need for intricate planning down to visa procurement and language barriers. As the FDA necessitates rigorous clinical trial phases to ascertain efficacy and safety, the role of participants becomes critically important, drawing from individuals of varying demographics. Each phase, from the initial safety focus to the subsequent efficacy tests, cumulates in Phase 3, the definitive stage for FDA approval pursuits.
Navigating through these trials requires participants to understand their role in advancing medical research. Drawing from insights about clinical trials, it's evident they are not just about new drugs but encompass advancing technologies, surgical methods, and overall care quality enhancements. With the prime goal of clinical trials being to examine health outcomes, they stand as a fundamental bridge between innovative research and the public availability of new treatments.
Case Study: Breakdown of Costs in a Clinical Trial
Delving into the economic aspects of conducting a phase III clinical trial for a novel oncology medication, it becomes evident that the financial commitments are multifaceted. Taking a closer look at a study with 500 participants over three years, the complexity heightens when this spans several international borders. Not only do conventional expenses such as site charges, participant recruitment, drug provision, and data oversight contribute to the financial outlay, but additional aspects also come into play.
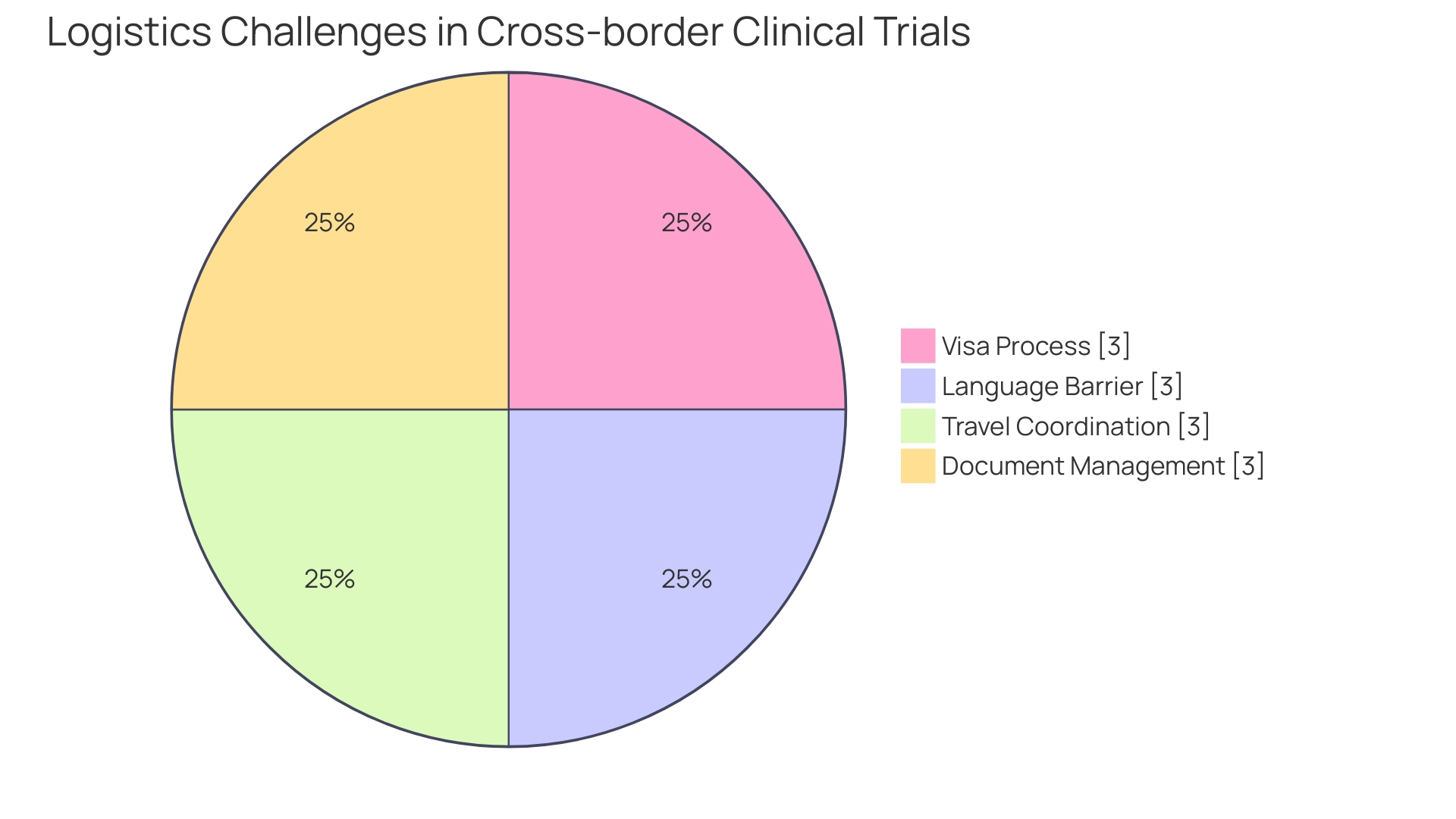
For instance, the costs of patient travel, particularly in cases where individuals - like a patient from rural Pennsylvania facing a rare disease with no approved treatments - must navigate the daunting process of international travel to a trial site in Turkey. The challenges compound as they confront issues like visa procurement, language barriers in paperwork, and the coordination of journey details. These examples underscore the intricate layers of expenses that impact the overall budget of a clinical trial, highlighting the need for clinical trial companies to strategically manage and optimize spending across all facets.
Data Collection and Analysis
The robustness of data collection and analysis is pivotal to the success of clinical trials, particularly when evaluating new treatments' safety and efficacy. With the advent of a pragmatic approach to trials, such as those leveraging Electronic Health Records (EHR-sourced trials), there exists a promise for improved operational efficiency.
However, the challenge lies in harmonizing trial sites' infrastructure to meet study objectives, as noted by Raman et al. in their breakthrough research.
Moreover, navigating the complexities of such trials adds to the costs, which include setting up effective data collection systems, meticulously training personnel across various sites, and preserving the integrity of the extensive data accrued. Specialized software, alongside professional expertise, is requisites for the meticulous analysis of the data, which inevitably contributes to the expenses incurred in clinical trials. This process intensifies when considering global trials, where logistics like cross-border travel for patients in remote locations, as in the illustrated scenario of a patient from rural Pennsylvania seeking treatment in Turkey, can become intricate and costly.
Cost Dynamics: A Detailed Analysis
Analyzing the cost structure of clinical trials demands attention to individual components that can impact financial efficiency. Among these, site fees, recruitment, medication management, data handling, monitoring, regulatory compliance, and overheads play critical roles in shaping the budget.
For example, advanced digital therapeutics, which span software-based treatments delivered through devices like smartphones and virtual reality, offer significant savings in patient management and outcome tracking. Such innovative solutions, however, face hurdles like inadequate insurance coverage and uncharted grounds in clinical testing.
Optimizing trial costs also involves tackling logistical complexities for participants, like those faced by a Pennsylvanian needing to travel abroad for an ultra-rare disease trial. The burden of securing visas, navigating foreign administration, and coordinating travel is substantial. According to industry insights, many clinical decisions made years prior could be optimized with more thorough planning and advisement, underscoring the immense value of strategic foresight in managing trials. By addressing these pivotal factors, clinical trial companies can refine resource allocation and enhance overall trial efficacy.
Conclusion
In conclusion, conducting clinical trials involves numerous complexities and challenges that have a direct impact on the overall costs. Factors such as the logistics of international travel for patients, the need for meticulous planning and budgeting, and the influence of trial objectives and priorities all contribute to the financial outlay of clinical trial companies.
Additionally, data collection and analysis play a crucial role in determining the success of these trials. The robustness of data collection systems and the expertise required for meticulous analysis contribute to the overall expenses incurred.
When conducting global trials, the complexities of cross-border travel and remote patient locations further add to the intricacies and costs involved. It is essential for clinical trial companies to strategically manage and optimize spending across all facets.
This includes addressing the individual components that impact financial efficiency, such as site fees, recruitment, medication management, data handling, monitoring, regulatory compliance, and overheads. By optimizing trial costs and addressing logistical complexities for participants, clinical trial companies can refine resource allocation and enhance overall trial efficacy. Overall, understanding the factors influencing clinical trial costs and adopting a proactive and detail-oriented approach can lead to more efficient decision-making and resource allocation. By navigating the complexities of clinical trial costs, companies can strategically allocate resources, optimize trial efficacy, and ultimately advance medical research by bringing new treatments to the public.
Contact bioaccess™ today to optimize your clinical trial costs and enhance overall trial efficacy.




